Abstract
Background
Aberrant vascular smooth muscle cell (VSMC) proliferation and cerebral endothelial cell (CEC) dysfunction contribute significantly in the pathogenesis of cardiovascular diseases. Therefore, inhibition of these cellular events would be by candidate agents for treating these diseases. In the present study, the mechanism of anti-proliferative and anti-inflammatory effects of andrographolides, a novel nuclear factor-κB inhibitor, was investigated in VSMC and CEC cells.
Methods
VSMCs and CECs were isolated from rat artery and mouse brain, respectively, and cultured before experimentation. The effect of andro on platelet-derived growth factor-BB (PDGF-BB) induced VSMC cell proliferation was evaluated by cell number, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of extracellular signal regulated kinase 1/2 (ERK1/2), proliferating cell nuclear antigen (PCNA), and the effects on lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) and, cyclooxygenase-2 (COX2) were detected by Western blotting.
Results
Andro significantly inhibited PDGF-BB (10 ng/ml) induced cell proliferation in a concentration (20-100 μM) dependent manner, which may be due to reducing the expression of ERK1/2, and by inhibiting the expression of PCNA. Andro also remarkably diminished LPS-induced iNOS and COX2 expression.
Conclusions
The results of this study suggested that the effects of andro against VSMCs proliferation and CECs dysfunction may represent a promising approach for treatment of vascular diseases.
Keywords: Andrographolide, CECs, COX2/iNOS, ERK/PCNA, LPS, PDGF-BB, VSMCs
INTRODUCTION
Cardiovascular diseases remain a major cause of death worldwide. Aberrant vascular smooth muscle cell (VSMC) proliferation and migration, as induced by vascular injury, has been shown to play a critical role in the pathogenesis of cardiovascular diseases including atherosclerosis, and pulmonary artery hypertension.1 Under normal circumstances, smooth muscle cells are maintained in a quiescent and nonmigratory state. However, their proliferation and migration are markedly increased in response to various growth factors and cytokines; one of the principal regulators of mitogenesis in VSMCs is platelet-derived growth factor (PDGF), which is produced by activated macrophages and endothelial cells. Among the three PDGF isoforms, platelet-derived growth factor-BB (PDGF-BB) is the most potent proliferative factor.2 The binding of PDGF to its receptor, PDGFR, leads to the phosphorylation of multiple tyrosine residues of the receptor, followed by the activation of phospholipase C-γ1, extracellular signal-regulated kinase (ERK)1/2, and phosphatidylinositol 3-kinase (PI3K)/Akt pathways, resulting in cell cycle progression.3
Endothelial cell dysfunction is an important factor in the pathogenesis of cardiovascular diseases such as atherosclerosis, stroke, diabetes, subarachnoid hemorrhage, and hypertension.4 It has been well-established that endothelium is a regulator of vasculogenesis, vascular tone, inflammation, and thrombosis.5 Lipopolysaccharide (LPS), a product of bacterial infection, is known to induce inflammatory cytokines and impairs the blood-brain barrier system. In endothelial cells, superoxide (O2) and nitric oxide (NO) are produced spontaneously from activated cyclooxygenase-2 (COX2) and inducible nitric oxide synthase (iNOS). Thus, the identification of novel molecular mechanisms, and particularly novel inhibitors controlling the PGDF-BB-dependent VSMC proliferation and LPS-induced endothelial dysfunction, are of considerable scientific and therapeutic interest.
Andrographolide, a novel novel nuclear factor-κB (NF-κB) inhibitor, is the most active and important constituent part of the medicinal plant Andrographis paniculata (andro),6 which has long been used as a herbal medicine to prevent and treat upper respiratory tract infections, diarrhea, rheumatoid arthritis, and laryngitis in Asia and Scandinavia.11 Recently, this plant was used in cooking as a valuable health food, and the extract is used as a nutritional supplement for preventing inflammatory diseases in Taiwan. Several studies demonstrated that andro possesses anticancer, antioxidant and hepatoprotective activities.7 It was shown to reduce total cholesterol in mice8 and inhibit inducible nitric oxide synthase expression and subsequent NO production in LPS-stimulated macrophages.9 Andro is widely used clinically for treating acute bacillary dysentery.10 Anti-HIV effects of andro have been reported by Calabrese et al.11 and Uttekar et al.12 Veeresham et al.13 suggested that andro provides a potential for use in galactosemic as well as diabetes mellitus patients. Our recent studies showed that andro inhibited collagen-stimulated platelets via increasing cyclic GMP/PKG, followed by inhibition of the p38 MAPK/HO•-NF-κB-ERK2 cascade.14,15 Our previous work also established that andro enhances the dephosphorylation of NF-κB subunit p65 Ser536 by activating protein phosphatase 2A in VSMCs.16 Even though, our very recent studies demonstrated that andro induced platelet apoptosis through the caspase-8-dependent extrinsic apoptotic pathway,17 and the clinical and experimental pharmacology of andro have been thoroughly summarized18 there is relatively no information about the effect of andro against VSMCs proliferation and cerebral endothelial cell (CEC) dysfunction. Thus, the present study was designed to have a two pronged approach: (a) to evaluate the effect of andro in PDGF-BB induced VSMCs proliferation; (b) and to determine whether andro alleviates LPS induced CEC dysfunction via inhibiting iNOS and COX2 expression.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin (0.25%), L-glutamine, penicillin/streptomycin, and fetal bovine serum (FBS), were purchased from Gibco (Gaithersburg, MD, USA). Andrographolide (≥ 98%), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) were from Sigma-Aldrich (St. Louis, MO, USA). Anti-phospho-ERK1/2 mAb, anti-PCNA mAb and anti-COX-2 were from Cell Signaling (Beverly, MA, USA); the anti-α-tubulin mAb was from NeoMarkers (Fremont, CA, USA). iNOS (NOX2) was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). The hybond-polyvinylidene difluoride membrane, enhanced chemiluminescence (ECL) western blotting detection reagent and analysis system, horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin G (IgG) were from Amersham (Buckinghamshire, UK). Andrographolide was dissolved in 0.1% dimethyl sulfoxide and stored at 4 °C until used.
Rat aortic smooth muscle cells primary culture
Male Wistar rats (250-300 g) were purchased from BioLASCO (Taipei, Taiwan) and VSMCs were enzymatically dispersed. Thoracic aortas from Wistar rats were removed and stripped of endothelium and adventitia. VSMCs were obtained by a modification of the combined collagenase and elastase digestion method.19 These cells were grown in DMEM supplemented with 20 mM HEPES, 10% FBS, 1% penicillin/streptomycin, and 2 mM glutamine at 37 °C in a humidified atmosphere of 5% CO2. The growth medium was changed every 2-3 days until cells had reached confluence. The growth medium was removed, and the monolayer was rinsed with phosphate-buffered saline (PBS). A trypsin-EDTA solution was added, and the monolayer was incubated at 37 °C for 2 min. Thereafter, the culture dishes were observed under a phase-contrast microscope until the cells had detached. Cells were removed with 10 ml of DMEM and centrifuged at 900 × g for 7 min. The pellet was resuspended in DMEM in a culture dish, and cells from passages 4-8 were used in all experiments.
Isolation of mouse brain microvascular endothelial cells
C57/BL6 mice (30-45 g) were used for this study. Mouse CECs (Figure 3A) were prepared according to the established methods by Wu et al.,20 with slight modifications. Briefly, the gray matter of fresh mouse brains was homogenized and filtered, and the resulting fraction was then sequentially digested with 4 mg/mL collagenase B for 2 h and 1 mg/mL collagenase/dispase (Roche Molecular Biochemicals, Indianapolis, IN, USA) for 2 h, followed by centrifugation in a 40% Percoll solution. The second band containing the microvessels was collected and washed before plating onto collagen-coated dishes. CECs migrating from isolated microvessels were maintained in DMEM, with high glucose and L-glutamine supplemented with 10% FBS, cell cultures (between passages 4 and 15) were grown to 85%-95% confluence before use. Mouse primary CECs were uniformly positive for factor VIII, vimentin, and characteristic bradykinin receptors (at 95% endothelial cell purity).
Cell viability assay
The viability of VSMCs upon treatment of andro was measured by a colorimetric MTT assay. Briefly, VSMCs (2 × 104 cells/well) were seeded on 24-well plates and cultured in DMEM containing 10% FBS for 24 h. VSMCs were pretreated with andro at concentrations of (20-50 μM) or an isovolumetric solvent control (0.1% DMSO) for 24 or 48 h. The cell number was measured using a colorimetric assay based on the ability of mitochondria in viable cells to reduce MTT as previously described.21 The cell number index was calculated as the absorbance of treated cells/control cells × 100%.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis
Western blot analyses were performed as previously described.19 Lysates from each sample were mixed with 6 × sample buffer (0.35 M Tris, 10% w/v SDS, 30% v/v glycerol, 0.6 M DTT, and 0.012% w/v bromophenol blue, pH 6.8) and heated to 95 °C for 5 min. Proteins were separated by electrophoresis and transferred onto polyvinylidene difluoride membranes for pERK1/2, PCNA, COX2 and iNOS. The membranes were then blocked with 5% nonfat milk in TBS-0.1% Tween 20, and sequentially incubated with primary antibodies and HRP-conjugated secondary antibodies, followed by enhanced ECL detection (Amersham Biosciences). BIO-PROFIL Bio-1D light analytical software (Vilber Lourmat, Marue La Vallee, France) was used for the quantitative densitometric analysis. Data of specific protein levels are presented as relative multiples in relation to the control.
Statistical analyses
The experimental results are expressed as the mean ± SEM and are accompanied by the number of observations. For analysis of the results, a one-way analysis of variance test was performed using Sigma Stat v3.5 software. When group comparisons showed a significant difference, the Student-Newman-Keuls test was used. A p value of < 0.05 was considered to be statistically significant.
RESULTS
Andro inhibited PDGF-BB induced proliferation in rat VSMCs without affecting cell viability
To determine whether andro inhibits PDGF-BB-stimulated VSMC proliferation, we assessed the anti-proliferative effects of andro by direct cell counting. VSMCs were pre-incubated in the presence of andro (20 and 50 μM) in serum-depleted medium for 24 h and then stimulated with 10 ng/ml PDGF-BB for 24 h. Pre-treatment with andro suppressed the PDGF-BB-stimulated cell numbers in a concentration-dependent manner (Figure 1A). Moreover, an MTT assay revealed that such concentration-dependent andro counteracted the PDGF-BB effect after 48 h of treatment, with cell proliferation remaining lower than that detected in cells cultured in the absence of the growth factor. The inhibition of PDGF-BB-stimulated (185.67 ± 24.64) cell proliferation by andro at 20, 50 and 100 μM was approximately 113.47 ± 7.54%, 98.40 ± 8.76 and 55.27 ± 15.05 (Figure 1B).
Figure 1.
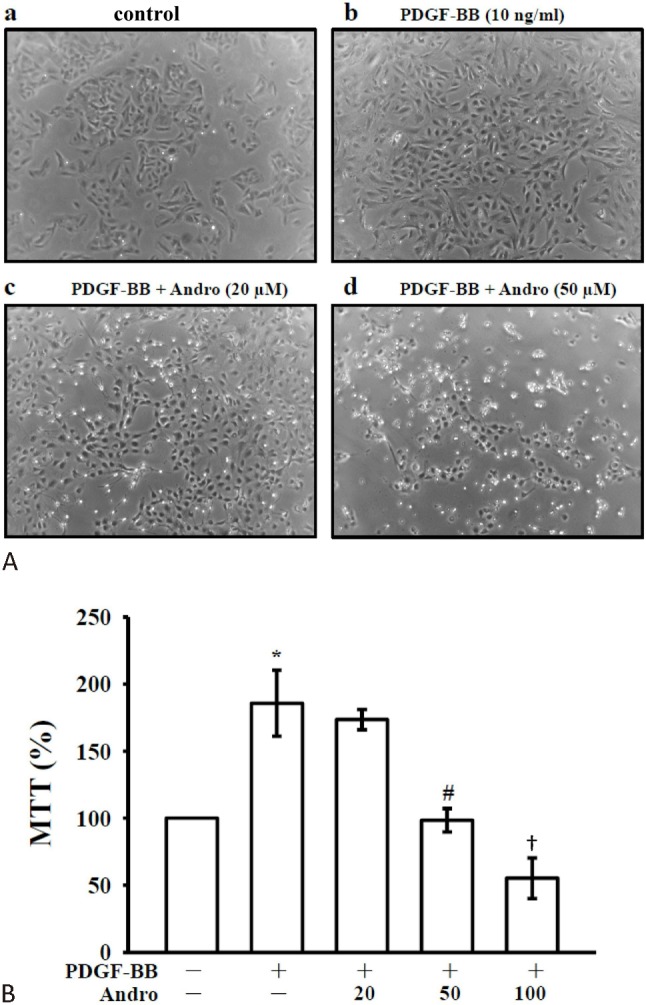
Effects of andrographolide on cell proliferation in vascular smooth muscle cells (VSMCs) stimulated by platelet-derived growth factor-BB (PDGF-BB). (A) VSMCs (2 × 104 cells/well) were treated with PBS (resting) or pretreated with andro (20 and 50 mM), or an isovolumetric solvent control (0.1% DMSO), followed by the addition of PDGF-BB (10 ng/ml). Cell numbers were evaluated by an MTT assay as described under Materials and Methods. (B) VSMCs (2 × 105 cells/dish) were treated with PBS (resting) or pretreated with solvent control (0.1% DMSO) or 20-100 μM andro, followed by the addition of PDGF-BB (10 ng/ml). Compiled statistical data are shown on the right. * p < 0.01 compared to the resting (PBS treatment) group; # p < 0.01; † p < 0.001, compared to the PDGF-BB group. Data are presented as the mean ± SEM (N = 5).
Andro suppressed the expression of proliferating cell nuclear antigen (PCNA) in PDGF-BB-treated VSMCs
Inhibition of VSMC proliferation in response to PDGF could be achieved at physiological andro concentrations that did not impair cell viability. The effect of andro on VSMC proliferation was further confirmed by the expression of a well-known cell proliferation marker, PCNA. As shown in Figure 2A, PDGF-BB stimulation significantly increased the expression of PCNA. It is noteworthy that andro was able to down regulate the expression of PCNA in a concentration dependent manner.
Figure 2.
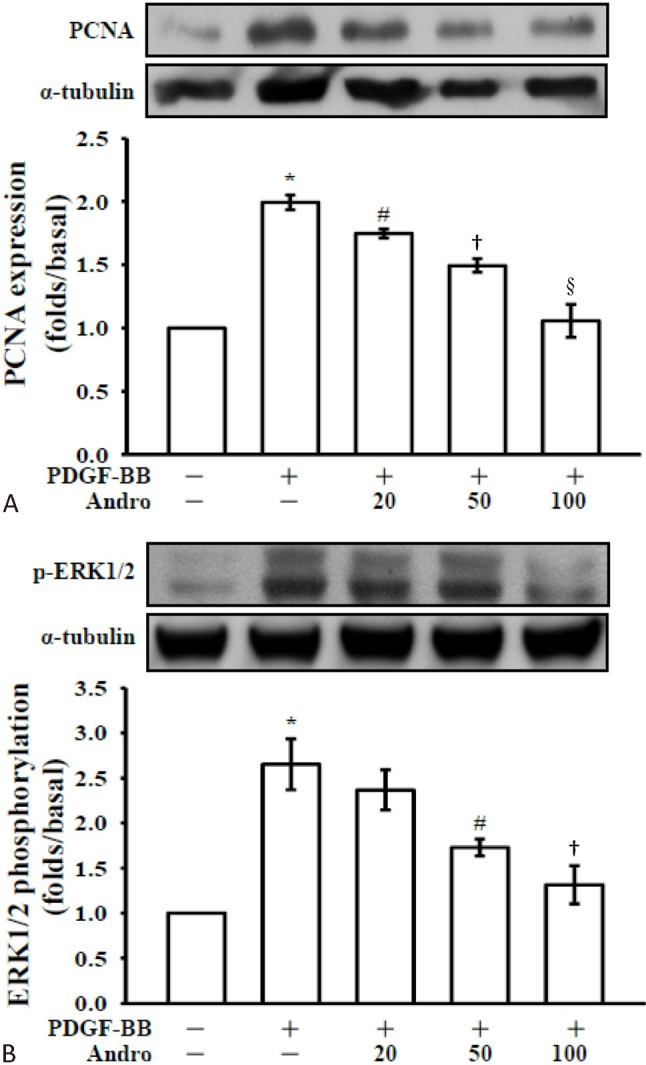
Effects of andrographolide on proliferating cell nuclear antigen (PCNA) and signal-regulated kinase (ERK)1/2 phosphorylation in platelet-derived growth factor-BB (PDGF-BB) stimulated vascular smooth muscle cell (VSMC)s. VSMCs (2 × 105 cells/dish) were treated with PBS (resting) or pretreated with a solvent control (0.1% DMSO) or andro (20, 50 and 100 μM), followed by the addition of PDGF-BB (10 ng/ml) to trigger (A) PCNA and (B) ERK1/2 phosphorylation. * p < 0.001, compared to the resting (PBS treatment) group; # p < 0.05; † p < 0.01 and § p < 0.001, compared to the PDGF-BB group. Data are presented as the means ± SEM (N = 5).
Andro inhibits p-ERK in PDGF-BB-induced VSMCs
Activation of PDGF signaling pathway is associated with PDGF-BB-stimulated cell proliferation of VSMCs. The level of ERK1/2, the early signals related to proliferation, was determined in PDGF-BB-stimulated VSMCs. PDGF-BB-induced ERK1/2 phosphorylation was inhibited by andro in a concentration-dependent manner (Figure 2B). These results indicated that andro may inhibit cell proliferation through ERK1/2 signaling pathway.
Effects of andro on LPS-induced iNOS and COX-2 expressions
Western blot analyses were performed to determine the effects of andro on LPS-stimulated COX-2 and iNOS expressions in CECs. CECs upon stimulation with LPS (10, 20 and 50 μg/ml) significantly increased the expressions of the pro-inflammatory enzymes, such as COX-2 (Figure 3B) and iNOS (Figure 3C) in a concentration-dependent manner. Moreover, expression of iNOS protein was significantly increased by stimulation with LPS and co-treatment with andro expressively inhibited iNOS expression in a concentration-dependent manner (Figure 4A). Under the same conditions, andro markedly decreased the expression of COX-2 (Figure 4B).
Figure 3.
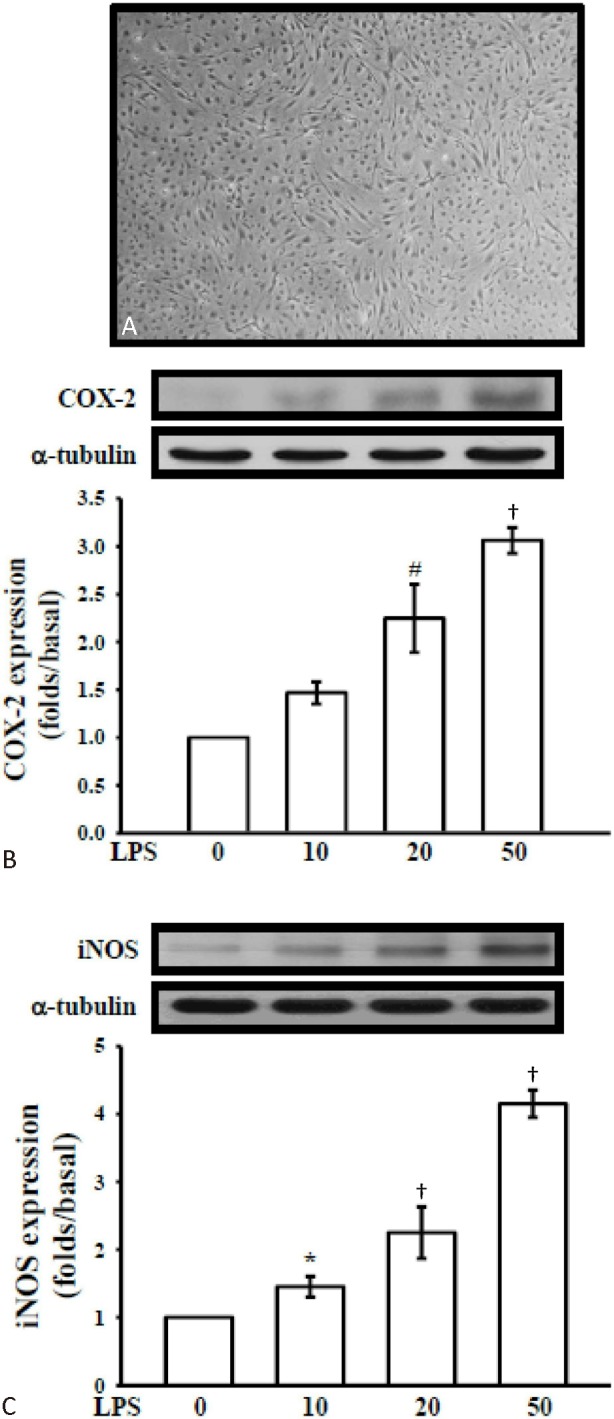
LPS-induced expressions of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in cerebral endothelial cell (CEC)s. (A) Morphology of primary cerebral endothelial cell. Cerebral endothelial cells (5 × 105 cells in 6-well plates) were treated with vehicle (0.5% DMSO) or various concentrations of lipopolysaccharide (LPS) (10, 20, and 50 μg/ml) for 24 hr. Cell lysates were obtained and analyzed for (B) COX-2 and (C) iNOS protein expression by Western blotting. α-tubulin normalized to the resting condition. Data are shown as the mean ± SEM of three independent experiments.* p < 0.05, # p < 0.01 and † p < 0.001, compared with the resting group.
Figure 4.
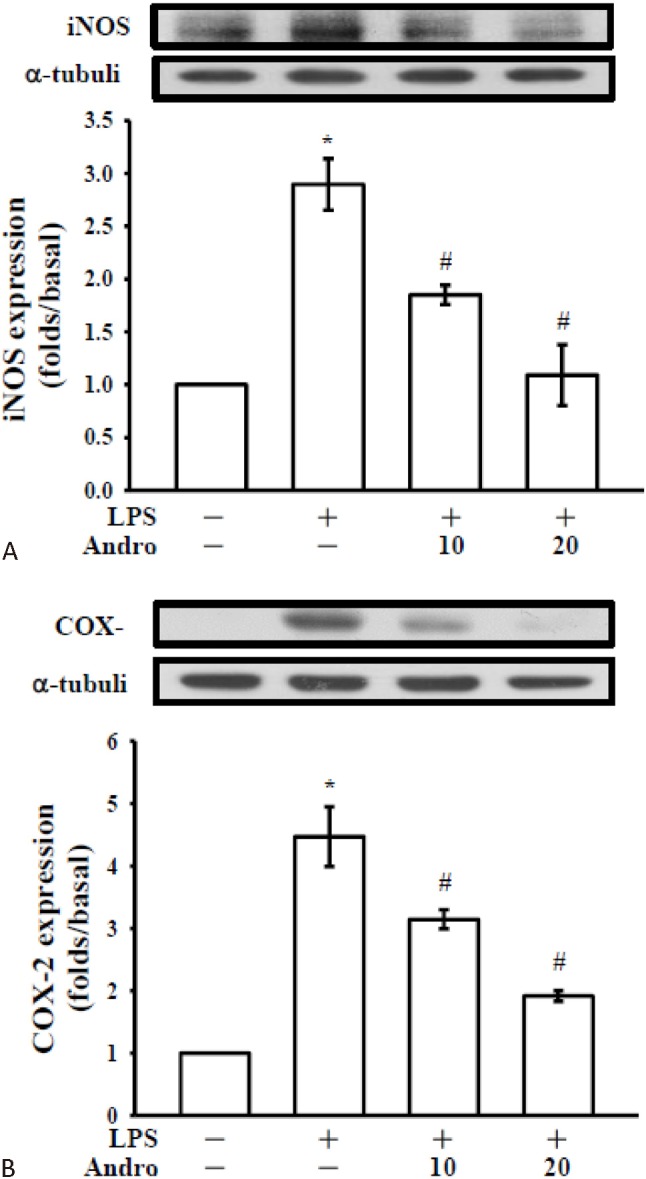
Effects of andrographolide on LPS-induced expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in cerebral endothelial cell (CEC)s. Cerebral endothelial (2 × 105) cells were pretreated with the vehicle (DMSO, 0. 1%, v/v) or andro (10 and 20 mM) for 3 hr and then stimulated by lipopolysaccharide (LPS) (50 μg/mL) for 24 h. Cell lysates were obtained and analyzed for (A) COX-2 and (B) iNOS protein expressions by Western blotting. a-tubulin is used as an internal control. Data are shown as the mean ± SEM of three independent experiments. * p < 0.001 compared with the resting group, # p < 0.001, compared with the vehicle group.
DISCUSSION
The abnormal growth of VSMCs is a prominent feature of vascular disease, including atherosclerosis and postangioplasty restenosis.22 Neointimal thickening is mainly due to VSMCs, which proliferate and migrate from the vascular media. Inhibition of VSMC proliferation and migration represents a potentially important therapeutic strategy for the treatment of cardiovascular diseases.23 Our previous study19 demonstrated that the therapeutic potential use of andrographolide in cardiovascular diseases is thought to be associated with the inhibition of LPS/IFN-γ-induced iNOS and MMP-9 expressions in rat VSMCs. It also demonstrated that andrographolide inhibited p65 Ser536 phosphorylation, reduced nuclear translocation of p65, and diminished p65 κB oligonucleotide binding in LPS/IFN-γ-stimulated rat VSMCs. In addition, (PP2A) contributed to these actions of andrographolide in rat VSMCs. A rat carotid injury model also demonstrated that andrographolide had beneficial effects in inhibiting balloon injury-induced neointimal formation and iNOS expression. In the present study, we investigated the inhibitory effect of andrographolide on PDGF-BB-induced proliferation, and its underlying mechanism in cultured VSMCs. This study also investigated the anti-inflammatory effect of andro via inhibiting LPS-induced COX2 and iNOS expressions in CEC cells. To our knowledge, this is first report shows that andro has potent effect on inhibiting PDGF-BB induced proliferation of VSMCs through suppressing ERK1/2 and PCNA expression and it also inhibited LPS-induced COX2 and iNOS expressions in CECs.
It is well-established that different stimuli are involved in the pathophysiology of cardiovascular diseases, which stimulate MAP kinase (MAPK) family members ERK1/2 and p38 and JNK in vascular cells.24 More importantly, these MAPK, particularly the ERK1/2 pathway, have an important role in VSMC proliferation.25 PDGF-BB activates the ERK1/2 pathway by triggering RAS-RAF activation, MEK1 phosphorylation, and ERK1 and ERK2 phosphorylations. Because PDGF-BB stimulation increases level of p44/p42 phosphorylation expressed in VSMC cells, it is assumed that ERK1/2 may modulate the reduced adhesion and increased proliferation of VSMCs. Moreover, ERK1/2 activation is required for its mitogenic signaling through a number of tyrosine kinase growth factor receptors, and the upregulation of PDGF-R expression is known to be associated with the development and progression of proliferative cardiovascular diseases such as hypertension26 and atherosclerosis.27 Therefore, an understanding of its inhibition on PDGFBB-stimulated VSMC proliferation is important in terms of developing methods of treating cardiovascular disease. Our results demonstrated that PDGF-BB induces activation of ERK1/2 and this activation was significantly suppressed by andro in a concentration-dependent manner. A work by Kim and Yun28 established that (NQ304), a newly synthesized 1,4-naphthoquinone derivative, significantly inhibited PDGF-BB-stimulated ERK1/2 activation in a concentration-dependent manner. One study also indicated that inhibition of MAPK/ ERK may inhibit VSMC proliferation and reduce neointima formation.29
VSMCs proliferation and PCNA expression are critical events in the development and progression of atherosclerosis. PCNA, a protein synthesized early in the G1 and S phases of the cell cycle, functions in cell progression, DNA replication and DNA repair. It is a very sensitive measure of cell proliferation including VSMC proliferation.30 In this study, PDGF-BB markedly promoted cell proliferation in proliferative VSMCs together with increased expression of PCNA. A study established that isorhynchophylline, an alkaloid from a traditional Chinese medicine, significantly blocked cell cycle progression in Ang II-treated VSMCs by decreasing the percentage of cells in S and G2/M phases and increasing cells in G0/G1 phase together with decreased expression of PCNA mRNA.31 Thus, our results indicated that andrographolide-mediated inhibition of PDGF-induced proliferation was associated with a remarkable reduction in ERK1/2 phosphorylation and decreased expression of PCNA in VSMCs.
The inflammation response is a complex reaction of the immune system that is regulated by many inflammatory mediators, such as NO, prostaglandins, and cytokines. Overproduction of iNOS-derived NO can have cytotoxic effects in pathological processes, especially in inflammatory and autoimmune disorders.32 COX-2-derived prostaglandin E2 (PGE2) is another important mediator that plays a regulatory role in a variety of physiological and pathological processes following an immune response and inflammation.33 It is well-known that the expression of iNOS and COX-2, the key enzymes for NO and PGE2 production, is up regulated in activated macrophages. In a variety of diseases, prostanoids produced by COX2 are responsible for the inflammatory processes by inducing vasodilation and disturbing the integrity of the vascular barrier.34 The iNOS is induced in cerebral blood vessels and inflammatory cells in response to cerebral ischemia.35 Nitric oxide produced excessively by iNOS contributes to oxidative injury, abnormal mitochondrial respiration, and DNA damage.36 In the present study, we showed that pre-treatment of CECs with LPS dose dependently increased COX-2 and iNOS expression. The reduction of COX2 and iNOS by andro (10-20 μM) may induce anti-inflammatory effects against LPS-induced toxicity, which may suppress COX-2-derived PGE2 as well as iNOS-derived NO production. In addition, although the present study was conducted in a cell culture environment, these findings should provide information concerning useful molecular mechanisms and the beneficial effects of andro in vascular diseases. In the future, further study will undoubtedly test whether these observations can be confirmed in in vivo experiments.
CONCLUSIONS
The results of the present study demonstrated that andrographolide, a novel NFκB inhibitor, at non-cytotoxic concentrations, inhibits PDGF-BB induced cell proliferation via suppressing ERK1/2 and PCNA expressions in VSMCs. Furthermore, andrographolide inhibits LPS-induced expression of iNOS and COX-2 proteins expression in mouse cerebral endothelial cells. Therefore, these results suggest that andrographolide can be used as a potential therapeutic compound for the prevention and treatment of vascular diseases.
Acknowledgments
The authors thank the National Science Council of Taiwan (NSC97-2320-B-038-016-MY3 and NSC100-2320-B-038-021-MY3), Cathay General Hospital (CGH-MR-10216), and National Taipei University of Technology-Taipei Medical University (NTUT-TMU-101-16) for the finance assistance provided.
CONFLICTS OF INTEREST STATEMENT
We declare that there are no conflicts of interest.
REFERENCES
- 1.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachinidis A, Locher R, Vetter W, et al. Different effects of platelet-derived growth factor isoforms on rat vascular smooth muscle cells. J Biol Chem. 1990;265:10238–10243. [PubMed] [Google Scholar]
- 3.Bornfeldt KE, Raines EW, Graves LM, et al. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 4.Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke. 2004;35:345–347. doi: 10.1161/01.STR.0000115161.10646.67. [DOI] [PubMed] [Google Scholar]
- 5.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31:641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 6.Coon JT, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections:a systematic review of safety and efficacy. Planta Med. 2004;70:293–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- 7.Bao Z, Guan S, Cheng C, et al. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am J Respir Crit Care Med. 2009;179:657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 8.Yang T, Shi HX, Wang ZT, Wang CH. Hypolipidemic effects of andrographolide and neoandrographolide in mice and rats. Phytother Res. 2013;27:618–623. doi: 10.1002/ptr.4771. [DOI] [PubMed] [Google Scholar]
- 9.Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol. 2000;129:1553–1560. doi: 10.1038/sj.bjp.0703191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HM, Pui-Hay But P. Pharmacology and Applications of Chinese Materia Medica. Vol. 2. Singapore: World Scientific; 1987. [Google Scholar]
- 11.Calabrese C, Berman SH, Babish JG, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–338. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Uttekar MM, Das T, Pawar RS, et al. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur J Med Chem. 2012;56:368–374. doi: 10.1016/j.ejmech.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Veeresham C, Swetha E, Rao AR, Asres K. In vitro and in vivo aldose reductase inhibitory activity of standardized extracts and the major constituent of Andrographis paniculata. Phytother Res. 2013;27:412–416. doi: 10.1002/ptr.4722. [DOI] [PubMed] [Google Scholar]
- 14.Lu WJ, Lee JJ, Chou DS, et al. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation:the pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J Mol Med (Berl) 2011;89:1261–1273. doi: 10.1007/s00109-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 15.Lu WJ, Lin KH, Hsu MJ, et al. Suppression of NF-kB signaling by andrographolide with a novel mechanism in human platelets:regulatory roles of the p38MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol. 2012;84:914–924. doi: 10.1016/j.bcp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh CY, Hsu MJ, Hsiao G, et al. Andrographolide enhances nuclear factor-kappaB subunit p65 Ser536 dephosphorylation through activation of protein phosphatase 2A in vascular smooth muscle cells. J Biol Chem. 2011;286:5942–5955. doi: 10.1074/jbc.M110.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lien LM, Su CC, Hsu WH, et al. Mechanisms of andrographolide-induced platelet apoptosis in human platelets: regulatory roles of the extrinsic apoptotic pathway. Phytother Res. 2013;27:1671–1677. doi: 10.1002/ptr.4911. [DOI] [PubMed] [Google Scholar]
- 18.Jayakumar T, Hsieh CY, Lee JJ, et al. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao G, Shen MY, Chang WC, et al. A novel antioxidant, octyl caffeate, suppression of LPS/IFN-gamma-induced inducible nitric oxide synthase gene expression in rat aortic smooth muscle cells. Biochem Pharmacol. 2003;65:1383–1392. doi: 10.1016/s0006-2952(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130:53–63. doi: 10.1016/s0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;62:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Inves. 1997;100:S87–S89. [PubMed] [Google Scholar]
- 24.Nguyen Dinh Cat A, Montezano AC, Burger D, et al. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 201;;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo AR, Koh SH, Cho GW, Kim SH. Inhibitory effects of cilostazol on proliferation of vascular smooth musclecells (VSMCs) through suppression of the ERK1/2 pathway. J Atheroscler Thromb. 2010;17:1009–1018. doi: 10.5551/jat.4309. [DOI] [PubMed] [Google Scholar]
- 26.Mulvany MJ. Structure and function of small arteries in hypertension. J Hypertens. 1990;8:S225–S232. [PubMed] [Google Scholar]
- 27.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 28.Kim TJ, Yun YP. Antiproliferative activity of NQ304, a synthetic 1,4-naphthoquinone, is mediated via the suppressions of the PI3K/Akt and ERK1/2 signaling pathways in PDGF-BB-stimulated vascular smooth muscle cells. Vascular Pharmcol. 2007;46:43–51. doi: 10.1016/j.vph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Gennaro G, Menard C, Michaud SE, et al. Inhibition of vascular smooth muscle cell proliferation and neointima formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation. 2004;110:3367–3371. doi: 10.1161/01.CIR.0000147773.86866.CD. [DOI] [PubMed] [Google Scholar]
- 30.Lavezzi AM, Ottaviani G, Matturri L. Biology of the smooth muscle cells in human atherosclerosis. APMIS. 2005;113:112–121. doi: 10.1111/j.1600-0463.2005.apm1130204.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Sun AS, Yu LM, et al. Effects of isorhynchophylline on angiotensin II-induced proliferation in rat vascular smooth muscle cells. J Pharm Pharmacol. 2008;60:1673–1678. doi: 10.1211/jpp/60.12.0014. [DOI] [PubMed] [Google Scholar]
- 32.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 33.Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16:181–206. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Gong C, Ennis SR, Hoff JT, Keep RF. Inducible cyclooxygenase-2 expression after experimental intracerebral hemorrhage. Brain Res. 2001;901:38–46. doi: 10.1016/s0006-8993(01)02186-2. [DOI] [PubMed] [Google Scholar]
- 35.Forster C, Clark HB, Ross ME, Iadecola C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol. 1999;97:215–220. doi: 10.1007/s004010050977. [DOI] [PubMed] [Google Scholar]
- 36.Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]


