Abstract
We herein describe the case of a 21-year-old woman with Stewart-Bluefarb syndrome presenting with recurrent ulcers on the right foot and multiple congenital arteriovenous malformations. The painful recurrent ulcers and brownish macules at the dorsum of the right foot had appeared at 13 years of age, and the size of the right foot gradually became larger than the left. She underwent conservative treatment and polyvinyl alcohol embolization but the ulcer was recurrent. Two macroscopic detectable feeding arteries to arteriovenous fistulas were ligated under Doppler sonography. At her 6 month follow-up, the chronic ulcer had begun to heal and pain had been alleviated.
Keywords: Acroangiodermatitis, Arteriovenous malformation, Recurrent foot ulcer, Stewart-Bluefarb syndrome
INTRODUCTION
Stewart-Bluefarb syndrome (SBS) is an acroangiodermatitis associated with arteriovenous malformation (AVM). This rare syndrome usually presents during the second decade of life and is characterized by cutaneous lesions. Due to the numerous small AVMs, surgical ligation or embolization is difficult to perform. Moreover, the treatment remains controversial, and most experts have recommended conservative treatment. However, several case reports presented patients who had been successfully treated by embolization. In the present report, we describe the case of a young woman with recurrent foot ulcers and AVM, who failed to improve with conservative treatment and embolization. Thereafter, she benefitted from surgical ligation of macroscopically detectable fistulas.
CASE REPORT
A 21-year-old wheelchair-bound woman presented to our outpatient clinic with chronic right foot ulcers along with progressive tenderness, swelling, and enlargement of the entire right foot. At 13 years of age, the patient had developed an ulcer with brownish macules, and the size of the right foot had gradually become larger than the left. The pain attributable to the ulcers was scored an 8 on the visual analogue scale, and the ulcers were mainly limited to the dorsum of the right foot, and usually bled spontaneously. She did not have any history of systemic disease, diabetes mellitus, thrombosis, or varicophlebitis or other diseases, including severe trauma of the leg. Conservative treatment including bed rest, foot elevation, compression bandage, and oral and topical antibiotics which had been recommended by previous clinicians.
On physical examination, the right leg was found to be longer than the left one. We observed nonblanching brownish to violaceous macules with a few nodules and ulcers on the right dorsum side of the foot, and marked varicosis on the right lower calf, ankle and foot. The temperature of the right foot was slightly elevated. Arterial pulses were strongly palpable on the dorsum of the right foot with bruit, thrills, and venous engorgement (Figure 1A).
Figure 1.
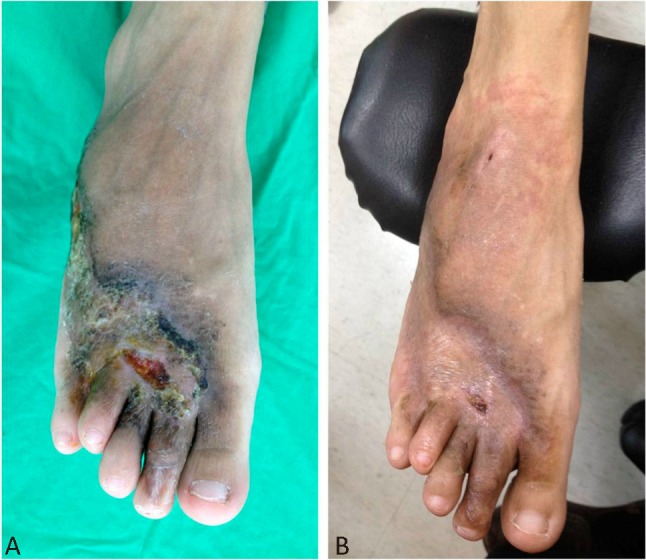
(A) The nonblanching brownish to violaceous macules with a few nodules and ulcers were on the right dorsum side of the foot. (B) At the 2-month follow-up, the macules improved and the chronic ulcer had started to heal.
The results of standard laboratory investigations were normal, and the enzyme-linked immunosorbent assay (ELISA) screening test for Human Immunodeficiency Virus (HIV) was negative. A wound culture indicated the growth of Staphylococcus aureus sensitive to oxacillin. A Doppler sonography showed 2 major AVM fistulas, with blood flowing into the feeding vessels at 45 and 23 ml/min. The magnetic resonance imaging revealed multiple AV communications between the dorsalis pedis artery and the recurrent foot ulcers resulting from arteriovenous malformations in the superficial venous system (Figure 2A). An arteriography confirmed the numerous arteriovenous communications, with early venous filling between the dorsalis pedis artery and dorsal venous arch (Figure 2B). The patient underwent embolization at the age of 20. The left lower extremity was catheterized through the femoral artery and polyvinyl alcohol was injected intermittently. Subsequently, she had a recurrence of the ulcer after 2 months.
Figure 2.
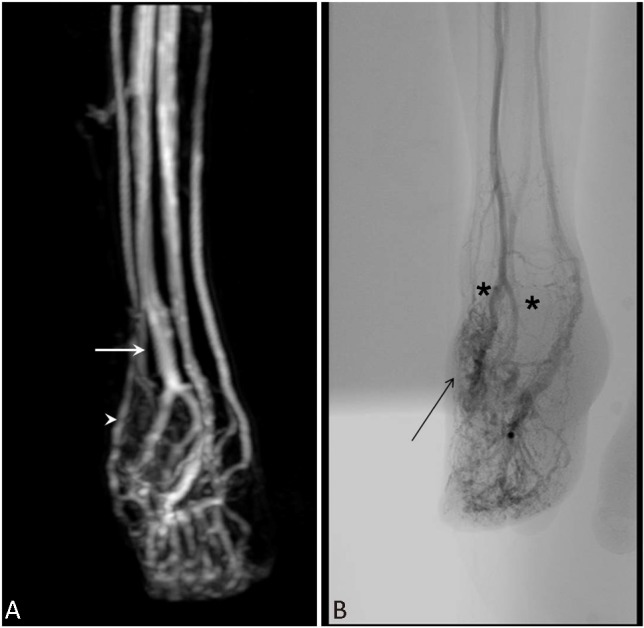
MRI revealed multiple arteriovenous communications between the dorsalis pedis artery (A, arrow) and superficial venous system (A, arrow head). Angiography showed extensive distribution of the arteriovenous malformation, with early engorged venous drainage, two major feeding arteries (B, astro) arising from the right pedis dorsalis artery, pedis plantaris artery, and its branches (B, arrow). MRI, magnetic resonance imaging.
Surgical ligation of the fistula between the AVMS was performed. The 2 major feeding vessels from the dorsalis pedis artery were identified by Doppler sonography and ligated with 6-0 Prolene sutures. The estimated blood loss was approximately 10 ml, with a total operation time of 47 min. She received oxacillin 2 gram intravenous every six hours for 7 days and shift to oral oxacillin for 2 weeks.
Following the operation, the bruit and thrill of the right foot immediately decreased. At the 2-month follow-up, the macules were found to be improved and the chronic ulcer started to heal. Right foot pain diminished from 8 to 3 on the visual analogue scale, and the patient was no longer wheel-chair bound (Figure 1B).
DISCUSSION
SBS is characterized by cutaneous lesions that usually present during the second decade of life. This skin lesion was first described by Bluefarb and Adams in 1967 in a patient with congenital AVMs.1,2 A reactive vascular proliferation following chronic venous insufficiency with capillary venous hypertension,3 SBS can manifest as brown macules, violaceous or purplish nodules, and plaques which become verrucous or ulcerative. The lesion mainly appears on the dorsum of the foot, particularly on the great toe and ankle. Laboratory assessment should be completed to distinguish this disease from systemic illnesses, and an ELISA screening test for HIV is necessary to exclude Kaposi’s sarcoma. Systemic, immuno-compromised, and infectious diseases should be ruled out through initial investigation.
Arteriovenous fistula may be clinically suspected during examination through palpable thrills, auscultation of a bruit, or detection of asymmetrical arterial pulses. The use of Doppler sonography is highly sensitive for the screening of AVMs. However, angiography is the gold standard tool for evaluating AVMs, with the most common sign of early venous filling, which is proportional to the length of the fistula.4 It can also reveal the extension and connection of the AVMs.
A literature review of the current treatment options for the vascular malformation of SBS was performed. Due to the presence of numerous small AVMs, invasive treatment was difficult. Therefore, conservative treatment with limb elevation, bed-rest, compression bandages, and topical steroids is recommended by some researchers.5-7 Successful embolization of the AVMs have been described (Table 1). Alioua et al.8 performed free-flow embolization using fragments of Ethibloc gelatin sponge. Klode et al.9 described an insufficient occlusion of the arteriovenous fistula after coil-embolization, with complete healing of the ulcer observed for a period of 10 years. Uterman et al.10 successfully used polyvinyl alcohol embolization for a single arteriovenous connection, and achieved long-term results. Behnia injected absolute alcohol to thrombosis and occlusion of AVMs, but the treatment induced sever systemic effects including alcohol intoxication, cherry-red urine and hyperthermia.11 Yamashita et al. developed a liquid material (Eudragit-E) for embolisation of AVMs,12 but the material was only used in animals. Others materials such as Gelfoam, acrylates, amino acids, avitene or silk was performed in the past.13
Table 1. Reported material for embolization of arteriovenous malformation.
| Author | Sex/age | Material for embolization | Follow-up period | Outcome |
| Alioua | M/33 | Ethibloc gelatin sponge | - | Healed |
| Klode | F/46 | coil | 10 years | Healed |
| Uterman | F/32 | Polyvinyl alcohol | 4 years | Healed |
| Behnia | M/17 | absolute alcohol | - | Failed |
| Yamashita | Animal | Eudragit-E | - | Healed |
F, female; M, male.
The ideal treatment for this disease is the resolution of the underlying vascular malformation. As this is not often possible due to the very distal location of the arteriovenous fistulas and the fact that surgical ligation only resolves macroscopically detectable fistulas; most experts suggest conservative treatment14 or embolization. Surgical ligation is indicated when there is a poor response to conservative treatment, functional impotence, refractory pain, recurrent infection, and/or bleeding.15,16 Wide excision of the multiple small fistulae with muscle flap reconstruction might be considered, if surgical ligation failed.
As we know, there was no reported successful treatment by surgical ligation before this case. This is likely in part because the AVMs have the following characteristics. First, the AVMs had only 2 major feeding vessels and these vessels could be macroscopically detectable by Doppler sonography. Second, these feeding vessels were superficial, which diminish the size of the surgical wound and decrease the time necessary for healing. The patient benefitted from ligation of the macroscopically detectable fistulas.
CONCLUSIONS
Although complete surgical ligation of the numerous small AVMs in SBS is challenging, ligation of macroscopically detectable fistulas can benefit patients with a poor response to conservative treatment and embolization.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Bluefarb SM, Adams LA. Arteriovenous malformation with angiodermatitis. Arch Dermatol. 1967;96:176–181. [PubMed] [Google Scholar]
- 2.Stewart WM. False Kaposi’s angiosarcoma caused by multiple arteriovenous fistulas. Bull Soc Fr Derm Syph. 1967;74:664–665. [PubMed] [Google Scholar]
- 3.Mali JW, Kuiper JP, Hamers AA. Acroangiodermatitis of the foot. Arch Dermatol. 1965;92:515–518. [PubMed] [Google Scholar]
- 4.Smiddy PF, Molloy MP, Flanagan N, Barnes L. Pseudo-Kaposi’s sarcoma: the association of arteriovenous malformations with skin lesions resembling Kaposi’s sarcoma. Australas Radiol. 2001;45:225–227. doi: 10.1046/j.1440-1673.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 5.Turk BG, Turk UO, Alioglu E, et al. Stewart-Bluefarb syndrome: a case report with angiographic findings. J Dermatol. 2009;36:415–418. doi: 10.1111/j.1346-8138.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel MI, Cuzzi T, Azeredo-Coutinho RB, et al. Acroangiodermatitis (pseudo-Kaposi sarcoma): a rarely-recognized condition. A case on the plantar aspect of the foot associated with chronic venous insufficiency. An Bras Dermatol. 2011;86:S13–S16. doi: 10.1590/s0365-05962011000700002. [DOI] [PubMed] [Google Scholar]
- 7.Mehta AA, Pereira RR, Nayak CS, Dhurat RS. Acroangiodermatitis of mali:a rare vascular phenomenon. Indian J Dermatol Venereol Leprol. 2010;76:553–556. doi: 10.4103/0378-6323.69090. [DOI] [PubMed] [Google Scholar]
- 8.Alioua Z, Lamsyah H, Sbai M, et al. Pseudo-Kaposi’s sarcoma secondary to superficial arteriovenous malformation: Stewart-Bluefarb syndrome. Ann Dermatol Venereol. 2008;135:44–47. doi: 10.1016/j.annder.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Klode J, Kröger K, Grabbe S, Dissemond J. Ulcers associated with arteriovenous fistula within a Stewart-Bluefarb syndrome: arterial and/or venous therapy? Vasa. 2007;36:134–137. doi: 10.1024/0301-1526.36.2.134. [DOI] [PubMed] [Google Scholar]
- 10.Utermann S, Kahle B, Petzold D. Successful longterm therapy of Stewart Bluefarb syndrome. Hautarzt. 2000;51:336–339. doi: 10.1007/s001050051128. [DOI] [PubMed] [Google Scholar]
- 11.Behnia R. Systemic effects of absolute alcohol embolization in a patient with a congenital arteriovenous malformation of the lower extremity. Anesth Analg. 1995;80:415–417. doi: 10.1097/00000539-199502000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita K, Taki W, Iwata H, Kikuchi H. A cationic polymer, Eudragit-E, as a new liquid embolic material for arteriovenous malformations. Neuroradiology. 1996;38:S151–S156. doi: 10.1007/BF02278144. [DOI] [PubMed] [Google Scholar]
- 13.Brenner S, Martínez de Morentin E. What’s new in pseudo-Kaposi’s sarcoma. J Eur Acad Dermatol Venereol. 2001;15:382–384. doi: 10.1046/j.1468-3083.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 14.Hueso L, Llombart B, Alfaro-Rubio A, et al. Stewart-Bluefarb syndrome. Actas Dermosifiliogr. 2007;98:545–548. doi: 10.1016/s0001-7310(07)70130-8. [DOI] [PubMed] [Google Scholar]
- 15.Marcoval J, Pagerols X, Iñiguez D, Peyri J. Acroangiodermatitis asociada a malformación arteriovenosa (síndrome de Bluefarb-Stewart) Actas Dermosifiliogr. 1992;83:143–145. [Google Scholar]
- 16.Punsoda G, Ribera M, Ferrándiz C. Acroangiodermatitis por fístula arterio-venosa. Pseudosarcoma de Kaposi tipo Bluefarb-Stewart. Actas Dermosifiliogr. 1991;82:337–340. [Google Scholar]


