Abstract
Background
The 6-minute walking test (6MWT) is a simple method used to evaluate exercise capacity in adults and children with cardiac diseases. Normal reference values in pediatric populations have been reported, but significant variations in the walking distance (6MWD) were noted among different studies. We aimed to provide and validate normal reference values of the 6MWD for healthy Taiwanese pediatric population between 7 and 17 years of age.
Methods
Healthy children and adolescents were recruited from 13 randomly selected schools in Kaohsiung City. From that recruitment effort, 762 participants (50.1% male) were included, and the 6MWT was conducted using standardized protocols. The main outcome measure utilized was the 6MWD, which was used to construct centile charts and Z score equations. Data from additional 64 healthy volunteers recruited from the National Taiwan University Children’s Hospital were used to validate these standards.
Results
There was an overall linear trend of increase in the 6MWD between 7 and 17 years of age (p < 0.001). Males covered significantly more distance than females after the age of 14 years, when the 6MWD essentially plateaued in female adolescents. Upon multivariate analysis, height was the most significant positive predictor of the 6MWD, while body mass index negatively correlated with the 6MWD. The height-based normal reference values of the 6MWD, derived from the 6MWT conducted in the school settings, were validated by a second cohort who received 6MWT inside the hospital.
Conclusions
Normal reference values of the 6MWD in healthy Taiwanese children and adolescents may serve as useful references for future clinical and research studies.
Keywords: Adolescents, Children, Six-minute walking test, Taiwan
INTRODUCTION
The 6-minute walking test (6MWT) is the distance a subject can walk at a constant and unhurried pace within 6 minutes. In adult patients with cardiorespiratory diseases, oxygen consumption during the 6MWT does not differ from the maximal oxygen consumption obtained in a standard cardiopulmonary exercise test.1 This makes the 6MWT a simple and less expensive method to evaluate exercise capacity.2 In adults, the walking distance covered in 6 minutes (6MWD) has been shown to be an independent predictor of morbidity and mortality in patients with heart failure,3 chronic respiratory diseases,4 and idiopathic pulmonary arterial hypertension.5 It can also be used to measure responses before and after treatment and to guide therapy.6
The use of 6MWT may be particular appealing for children and adolescents for whom performing standard cardiopulmonary exercise tests is sometimes problematic, requiring adequate body height, as well as a high degree of cooperation and motivation. Previous studies have demonstrated the usefulness of 6MWT in children and adolescents with congenital heart disease7 and obesity.8,9 Although reference values have been reported from several different countries,10-12 significant variations in these published values suggested that interpretation of the data obtained from individual local populations with reference to published norms must be viewed with caution. For example, racial difference may be one reason that could explain such variations.13,14 In Taiwan, normal reference values of the 6MWD have yet to be established for children and adolescents. Lack of these reference values might hinder the clinical usefulness of this test in our young cohort. Therefore, the purpose of this study was to establish and validate normal reference values of the 6MWD in Taiwanese healthy children and adolescents.
MATERIALS AND METHODS
Participants
Between April 2012 and June 2012, we prospectively studied healthy children and adolescents in Kaohsiung City between 7 and 17 years of age. They were recruited from randomly selected local elementary, junior high and senior high schools. All tests were conducted in the individual schools. Before recruitment, a questionnaire as well as informed consent was given to all eligible subjects as well as their parents. The questionnaire was used to identify children and adolescents with known cardiopulmonary illness, neuromuscular disease, long-term medication which might interfere with the test, or concurrent acute infection. Children and adolescents with negative disease and medication history, and written parental informed consent, constituted the target study population of our present study. All participants also willingly agreed to perform the 6MWT.
To validate the established normal reference values of the 6MWD which were obtained in a school setting, we recruited additional 64 healthy volunteers between 7 and 17 years of age from outpatient clinics of National Taiwan University Children’s Hospital for 6MWT. All of these subjects were referred for innocent heart murmur or nonspecific chest pain, and all had normal echocardiographic findings. The 6MWT was conducted inside the National Taiwan University Children’s Hospital after obtaining informed consent from both parents and participants.
6MWT
Before the start of the 6MWT, participants’ age, sex, height, weight, and body mass index were documented. According to the guidelines published by the American Thoracic Society,15 all 6MWTs were conducted using a marked hallway 30 m in length both at the participating schools and in the hospital. In a period of 6 minutes, the participants were asked to walk back and forth along this hallway as far as possible, at their own best pace but not to run or race. The tests were undertaken by each participant separately to prevent competition, and each participant had a personal instructor throughout the test. Standardized phases of encourage or announcement of time remaining, such as “You are doing well,” and “You have 3 minutes to go” were often given to the participants, although the frequency of such encouragement varied across studies from providing encouragement every 30 seconds to every 2 minutes. No comments were made with the intention of speeding up or slowing down the participant. The participants could adjust their speeds any time if they wanted. The participants were not aware of how long they had walked throughout the test. We used a wireless pulse oximeter (Nonin 4000, Nonin Medical, Plymouth, Minnesota, USA) attached to the participant’s wrist and a finger to monitor the oxygen saturation and heart rate continuously during the test. Data on heart rates and oxygen saturations before the start of the walk and after every minute during the test were recorded. At the end of test, the total distance walked within 6 minutes was measured.
Statistical analysis
Data are expressed as percentage, mean ± standard deviation, or median (25th-75th percentile), as appropriate. Comparisons between groups were made using unpaired Student’s t test or ANOVA. Potential predictors of the 6MWD were investigated by univariate analysis, followed by stepwise multivariate linear regression analysis. Multicollinearity between covariates was examined by calculating individual covariate variance inflation factor. Centile charts were constructed using the maximum penalized likelihood LMS method.10,16 The LMS method estimates the measurement centiles in terms of three height- and sex-specific cubic spline curves: the L curve (Box-Cox power to transform the data follow a normal distribution), M curve (median), and S curve (coefficient of variation). In brief, let Y(t) denote an independent positive datum (i.e., the 6MWD) at t height in centimeters, so that the distribution of Y(t) can be summarized by a normally distributed SD score (Z) as follows:
{[Y(t)/M(t)] - 1}/[L(t) ∙ S(t)]
Once the L(t), M(t), and S(t) have been estimated for each height t, the 100α centile at t height in cm can be derived from
C100α (t) = M(t)[1 + L(t)S(t) Zα]1/L(t)
where Zα is the α percentile of the normal distribution (e.g., for the 95th centile, α = 0.95 and Z = 1.65). The 1st, 3rd, 5th, 10th, 25th, 50th, 75th, 90th, 95th, 97th, and 99th centiles of the 6MWD at 5-cm height intervals were computed for boys and girls separately.
To obtain a 6MWD Z score for a given height, we used an exponential regression model as follows:
regression mean = ln(expected 6MWD) = β 1 + β 2 × ln(height)
where height was expressed in centimeters, and β1 and β2 were sex-specific. This model has been shown to offer good fit and yield Z scores evenly distributed around zero.17-19 Then Z score could be calculated from
Z score = ln(6MWD) – regression mean/√MSE
where 6MWD is the exact walking distance, and mean standard error (MSE) was sex-specific. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 15.0; SPSS Inc., Chicago, IL, USA) and R statistical software, version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).20 A p value < 0.05 defined statistical significance.
RESULTS
Participants from schools
There were 782 participants recruited from 6 elementary schools, 4 junior high schools, and 3 senior high schools, and all were of Asian ethnicity. Their heights were all within the normal age-appropriate ranges of Taiwanese children and adolescents (between 3rd and 97th percentile). However, we found that the case numbers of participants who are in the highest and lowest body height groups were limited, which might result in a deviation of the statistical assumption from the reality in these particular groups. Therefore, before the establishment of normal reference values for the 6MWD, we excluded subjects whose heights were ≤ 120 cm or > 180 cm for male participants (n = 9 and 3, respectively), and ≤ 110 cm or > 170 cm for female participants (n = 1 and 7, respectively). A total of 762 participants (50.1% male) were thereafter included in the following analysis.
Demographic and 6MWT data
Demographic and 6MWT data are shown in Table 1. All tests were completed uneventfully. The overall 6MWD was 513 ± 64 m for all participants. The mean oxygen saturation was 98% at baseline, and the lowest saturation was 94-95% during the test. During the test, the heart rate increased from a baseline of 103 ± 17 beats per minutes (bpm) reaching a peak rate of 142 ± 15 bpm. Pooling the data from both sexes together, there was no significant increase in 6MWD from one year to the next, but an overall trend of increase was noted between 7 and 17 years of age (p < 0.001). The mean 6MWD increased from 463 ± 62 m at 7 years to 527 ± 71 m at 12 years. The increment in 6MWD subsequently slowed, with the mean 6MWD of 542 ± 54 m at 15 years, and 545 ± 57 m at 17 years of age. Figure 1 shows the 6MWD in male and female participants in each age group. Between 7 and 14 years of age, the distance walked was similar when comparing male and female participants of the same age group. Between 14 and 17 years of age, however, male adolescents walked significantly longer than their female counterparts, while the 6MWD had essentially plateaued in female adolescents (Figure 1).
Table 1. Demographic data and 6-minute walking test results in different age groups (n = 762) .
| Age (year) | n | M/F | Height (cm) | Weight (kg) | BMI (kg/m2) | Resting SpO2 (%) | Lowest SpO2 (%) | Resting HR (bpm) | Peak HR (bpm) | 6MWD (m) |
| 7 | 67 | 32/35 | 124.6 ± 5.5 | 26.4 ± 5.8 | 16.9 ± 2.9 | 98 ± 1 | 95 ± 2 | 105 ± 11 | 148 ± 14 | 473 ± 62 |
| 8 | 77 | 38/39 | 131.6 ± 5.6 | 31.9 ± 6.9 | 18.3 ± 3.1 | 98 ± 1 | 95 ± 2 | 101 ± 17 | 147 ± 14 | 477 ± 68 |
| 9 | 76 | 40/36 | 135.8 ± 6.3 | 33.8 ± 9.1 | 18.1 ± 3.6 | 98 ± 1 | 95 ± 3 | 104 ± 15 | 150 ± 14 | 498 ± 57 |
| 10 | 80 | 41/39 | 141.7 ± 7.5 | 36.7 ± 8.8 | 18.1 ± 3.5 | 98 ± 1 | 95 ± 4 | 102 ± 15 | 147 ± 16 | 503 ± 57 |
| 11 | 88 | 41/47 | 147.2 ± 6.7 | 43.1 ± 9.7 | 19.8 ± 3.9 | 98 ± 1 | 95 ± 3 | 104 ± 17 | 143 ± 13 | 509 ± 65 |
| 12 | 85 | 45/40 | 153.7 ± 7.9 | 48.0 ± 10.3 | 20.2 ± 3.4 | 98 ± 1 | 95 ± 2 | 103 ± 16 | 139 ± 14 | 527 ± 71 |
| 13 | 58 | 26/32 | 156.6 ± 7.3 | 50.6 ± 9.4 | 20.6 ± 3.3 | 98 ± 1 | 95 ± 2 | 100 ± 18 | 136 ± 15 | 527 ± 52 |
| 14 | 65 | 31/34 | 161.2 ± 8.4 | 54.8 ± 13.5 | 21.0 ± 4.1 | 98 ± 2 | 94 ± 3 | 104 ± 19 | 138 ± 16 | 530 ± 48 |
| 15 | 62 | 36/26 | 165.1 ± 7.2 | 57.4 ± 12.2 | 21.0 ± 3.8 | 98 ± 2 | 95 ± 3 | 107 ± 18 | 138 ± 15 | 542 ± 54 |
| 16 | 52 | 27/25 | 165.6 ± 8.0 | 59.7 ± 12.1 | 21.6 ± 3.4 | 98 ± 1 | 94 ± 3 | 102 ± 21 | 135 ± 14 | 543 ± 61 |
| 17 | 52 | 25/27 | 164.9 ± 8.6 | 56.7 ± 12.8 | 20.7 ± 3.4 | 98 ± 2 | 94 ± 3 | 101 ± 16 | 135 ± 15 | 545 ± 57 |
| All | 762 | 382/380 | 148.5 ± 15.3 | 44.2 ± 14.7 | 19.5 ± 3.8 | 98 ± 1 | 95 ± 3 | 103 ± 17 | 142 ± 15 | 513 ± 64 |
BMI, body mass index; bpm, beats per minute; F, female; HR, heart rate; M, male; SpO2, oxygen saturation measured by pulse oximeter; 6MWD, 6-minute walking distance.
Figure 1.
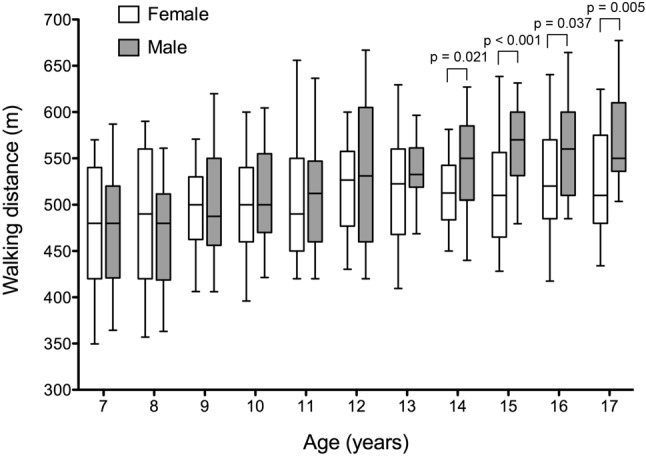
Six-minute walking distance in male and female participants in each age group. Data are shown as box-and-whiskers plots illustrating the median, 25th and 75th centile (box) and 5th and 95th centile (error bar). p values refer to the difference between male and female participants in each age group. p values not shown indicate no statistical difference.
Predictors of 6MWD
Results of univariate and multivariate linear regression between the 6MWD and relevant variables are shown in Table 2. Positive univariate correlates of 6MWD included male sex, age, height, weight, body mass index, and resting oxygen saturation. On stepwise multivariate analysis, male sex, height, peak heart rate, the difference between peak and resting heart rate, and resting oxygen saturation were positively related to 6MWD, while body mass index correlated negatively with 6MWD. None of the individual covariate variance inflation factors were greater than 2 (Table 2), indicating that multicollinearity is not likely to be a problem in this analysis. Because height correlated with the 6MWD most closely both in univariate and multivariate analysis, and is a routinely measured parameter in clinical settings, we chose height to construct normal centile charts and Z score equations of the 6MWD for Taiwanese children and adolescents.
Table 2. Factors associated with six-minute walking distance .
| Predictors | Univariate analysis | Multivariate analysis | |||
| β (95% CI) | p | β (95% CI) | p | VIF | |
| Male sex | 16.43 (7.33-25.54) | < 0.001 | 8.32 (0.15-16.5) | 0.05 | 1.03 |
| Age (years) | 7.74 (6.31-9.17) | < 0.001 | -- | -- | |
| Height (cm) | 1.70 (1.42-1.97) | < 0.001 | 2.16 (1.85-2.47) | < 0.001 | 1.41 |
| Weight (kg) | 1.34 (1.04-1.63) | < 0.001 | -- | -- | |
| BMI (kg/m2) | 1.89 (0.67-3.11) | 0.002 | -1.46 (-2.66--0.27) | 0.02 | 1.23 |
| Resting HR (bpm) | -0.19 (-0.47-0.08) | 0.17 | -- | -- | |
| Peak HR (bpm) | 0.09 (-0.21-0.39) | 0.56 | 0.52 (0.18-0.86) | 0.002 | 1.65 |
| Difference in HR (bpm) | 0.18 (-0.04-0.41) | 0.11 | 0.40 (0.15-0.65) | 0.002 | 1.57 |
| Resting SpO2 (%) | 5.93 (2.55-9.31) | 0.001 | 7.98 (4.95-11.00) | < 0.001 | 1.02 |
| Lowest SpO2 (%) | 0.86 (-0.77-2.50) | 0.30 | -- | -- | |
| Difference in SpO2 (%) | 0.61 (-1.19-2.40) | 0.51 | -- | -- |
Significant p values are given in bold.
BMI, body mass index; bpm, beats per minute; CI, confidence interval; HR, heart rate; SpO2, oxygen saturation measured by pulse oximeter; VIF, variance inflation factor.
Centile charts and Z score equations of the 6MWD
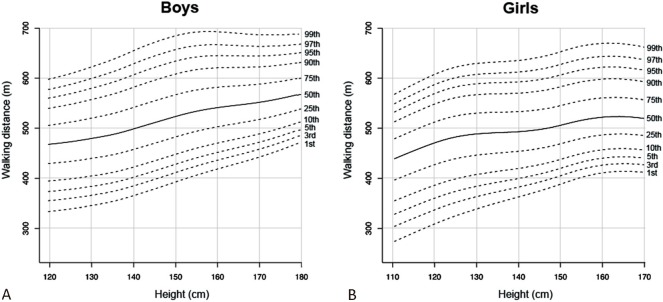
Figure 2A and B show the height-specific smoothed centile curves for the 6MWD for males (height > 120 cm and ≤ 180 cm) and females (height > 110 cm and ≤ 170 cm), respectively. The centile curves demonstrated that males generally could cover a greater 6MWD than their female counterparts for a given height. The 50th centile curve for females corresponded approximately to the 25th centile curves for males.
Figure 2.
Figure 2. Normal reference centile charts for 6-minute walking distance in boys (A) and girls (B).
Table 3 shows the estimated parameters in the regression model for calculating Z score for a given measurement of 6MWD. The Z score can be calculated from the following equation:
Table 3. Estimated parameters in regression models for Z score of 6-minute walking distance in boys and girls .
| β1 | β2 | MSE | |
| Boys (n = 382) | 3.5247 | 0.5443 | 0.0132 |
| Girls (n = 380) | 4.3204 | 0.3813 | 0.0138 |
MSE, mean standard error.
Z score = ln(6MWD) - β1 - β2 × ln(height)/√MSE
For example, to find the Z score corresponding to a 6MWD of 500 m for a boy with a height of 130 cm, first find the value of β1, β2, and MSE in Table 3 (3.5247, 0.5443, and 0.0132, respectively), and then put these values into the above equation:
Z score = ln(500) – 3.5247 – 0.5443 × ln(130)/√0.0132 = 0.352
Validation using 6MWT performed in the hospital
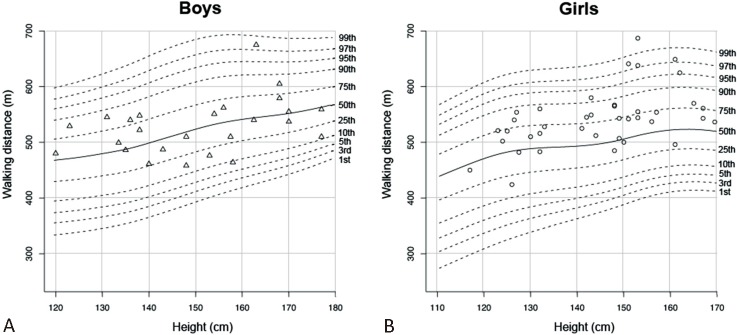
Data of the 64 participants whose 6MWT were performed inside the hospital were plotted on the normal centile charts of the 6MWD (Figure 3A and B). All measurements except one from a female participant were within the range between the 1st and 99th percentile curves. When converting these data into the scores using our constructed equations, the corresponding Z scores ranged between -1.22 and 1.89 for males (n = 25), and -0.98 and 2.50 for females (n = 39). These results validated the use of our centile charts and Z score equations for the tests conducted in the hospital setting.
Figure 3.
Walking distances for boys (A) and girls (B) recruited in the hospital plotted on the normal reference centile charts.
DISCUSSION
This study presented normal reference values of the 6MWD from a large cohort of Taiwanese children and adolescents between 7 and 17 years of age. We further validated the use of these normal reference values, which were obtained in the community setting, for tests that were conducted in the hospital setting. Both centile charts and Z score equations of the 6MWD from our present study could serve as useful references for future clinical practice and research application.
Including our present study, there are four reports from different countries that have published their own reference values pertaining to the 6MWD in healthy children and adolescents. Geiger and colleagues reported normal 6 MWD values in 528 Caucasian children from Austria, aged between 3 and 18 years of age.11 Based on their data, the median 6MWD was around 580 m in children aged between 6 to 8 years. The distance increased substantially to 728 m and 661 m in males and females aged over 16 years, respectively. Comparing our present study in an Asian cohort, their 6MWDs were considerably longer than ours (473 ± 62 m and 543 ± 61 m in 7 year old and 16 year old participants). Another report by Lammers et al. reported 6MWD normal values from 328 UK children of 4 to 11 years of age.12 Although 83% of their study subjects were Caucasians, interestingly, their data were quite similar to ours. They reported the value of 6MWD of 488 ± 35 m and 512 ± 41 m in 7 year old and 11 year old children, respectively, comparing to 473 ± 62 m and 509 ± 65 m in children with the same ages in our study cohort. Besides, reference values for the Asian pediatric population have been reported previously by a study from Hong Kong in Chinese children between 7 to 16 years of age.10 Li et al. reported an overall mean 6MWD of 664 m, which was significantly greater than our data. Based on these three previous reports10-12 and our present study, it seems that the results of 6MWT conducted in children varied significantly among different studies, whether the study populations were of similar ethnic background or not. Therefore, some other complicating factors might influence the 6MWT.
The variations in methodological details during the performance of 6MWT may be one crucial factor that affects the results of 6MWT. Although the American Thoracic Society has published a guideline to standardize 6MWT,15 several differences were still noted among various studies. First, the level of encouragement during the test varied. In Geiger’s study,11 they counted down the time left every minute, while investigators of the other 3 studies did not regularly inform the participants about any diminishing time element. Besides, a measuring wheel was used only in Geiger’s study. To avoid competition, the 6MWT should be performed separately or one by one. This methodological detail was not described in Li’s study.10 In addition, the track length varied from 20 to 50 m among these four studies.10-12 All these methodological variations may result in differences in the 6MWD of children and adolescents from different studies. A more strict standardization for the performance of 6MWT may reduce this difference.
A number of factors correlated with the 6MWD. Similar to most studies in healthy adults13,21-25 and the other three studies in children,10-12 our data in this pediatric cohort also showed that height was closely correlated with the 6MWD. Although we did not measure individual leg length, the correlation between height and the 6MWD might be attributed to the longer length of steps in taller individuals.11,22 Body weight, although a positive univariate predictor of the 6MWD, was not predictive of the 6MWD in multivariate analysis. This finding has also been noted previously.10,11 Since body weight and height were highly correlated with each other, the independent role of weight in predicting 6MWD may therefore become insignificant once the height was taken into the consideration. A similar situation was noted in terms of the effect of age on the 6MWD. Sex is another factor affecting the 6MWD. Male participants started to walk significantly longer than females after the age of 14 years, a difference that may persist through adulthood.22-25 Although the effect of sex on the 6MWD may be attributed to the difference in height, sex remains an independent predictor in the multivariate model after height was adjusted for. Such result may be explained by the higher muscle mass and greater strength in men after adolescence. Body mass index was initially a positive predictor of 6MWD in univariate analysis. However, it became an independent negative predictor after other relevant factors were adjusted for. As body mass index correlated with height (data not shown), the direct effect of increased body mass index on walking distance was likely through the influence of height. When body height was adjusted for in the multivariate model, the negative effect of body mass index on 6WMD indicated that subjects who are more overweight tended to walk shorter distances than those with the same height but lower body mass index. This relationship was not reported previously in the pediatric population. One possible explanation may be the difference of body mass index in the study population. In our present study cohort, the mean body mass index of all participants was 19.5 kg/m2, which was highest among all studies in healthy children and adolescents.10-12 A higher prevalence of obesity or overweight in the study population may unmask the influence of body mass index on the 6MWD. This finding not only highlights the potential threat of overweight or obesity on exercise capacity in contemporary Taiwanese pediatric cohort, but also emphasizes the need for reference values of the 6MWD that are specific for each population.
The sex-specific, height-based centile charts and Z score equations of the 6MWD not only serve as user-friendly tools in the clinical setting, but also provide standard references for future research. Among all anthropometric variables correlated with the 6MWD, height was the single most significant predictor in multivariate analysis. Furthermore, body height was easily measured, and would be routinely recorded before each pediatric clinic or laboratory evaluation. These features justify the use of height-based 6MWD reference values. The use of centile charts could provide an easy method to evaluate the level of exercise performance, and quickly identify an abnormal measurement. On the other hand, calculating a Z score could precisely quantify the exact functional capacity. This approach has been increasingly used in pediatric cardiology.18,19,26,27 Compared to other adjusting methods, there is no need to assume a constant variance across the range of body sizes when using Z scores. This advantage is particularly important within the pediatric population which covers a wide range of body sizes. Furthermore, a Z score, rather than a range of percentile derived from the centile charts, would be much more convenient for conducting statistical analysis. Of course, Z scores can also be converted to percentiles. However, the magnitude of an abnormality is much easier to appreciate with Z scores than with percentiles. This is another advantage of using Z scores for clinical and research purposes.
Although many studies have reported their own normal reference values of the 6MWD, only a few studies in adults have evaluated the validity of the reference equations which they proposed.21,23,28 As the reference values of the 6MWD provided by our present study were based on tests conducted in the schools, we prospectively evaluated additional individuals with the same ranges of age and height used in the development of our reference values, while their tests were performed inside the hospital. We demonstrated the reliability of centile curves and Z score equations of the 6MWD in this independent cohort. This result not only validated our reference values of the 6MWD, but also supported the use of these reference values in 6MWT conducted in the hospital settings, where most clinical and research studies were performed.
Our study had several limitations. We enrolled only subjects aged between 7 and 17 years, and body height between 120-180 cm for male and 110-170 cm for female. Therefore, caution should be taken when applying our centile curves and Z score equations to individuals whose age or height fall outside the ranges of our study cohort. Although we validated our normal reference values in another independent Taiwanese cohort, it remains unknown whether these values could be applied to populations from other countries or different ethnic backgrounds. Most of the data points from the female validation population appear to scatter above the median curve of normal centile charts. Although the explanation for this phenomenon is not clear, it is possible that the observed deviation from the median curve may be the result of random sampling since there are only 39 cases, and such a situation was not observed in the male counterpart. Besides, we performed only one test per participant. Therefore, the intra-observer and inter-observer variability could not be tested. Furthermore, as there might be a training effect on a repeated test,24,29 our reference values might systematically underestimate the 6MWD when the test was performed on a follow-up basis.
CONCLUSIONS
The 6MWT is a simple and practical means to assess exercise capacity in the pediatric population. This study provided data on normal reference values of the 6MWD in healthy Taiwanese children and adolescents. These standards may serve as useful references for future clinical and research studies.
Acknowledgments
This work was supported by a grant (CCFT1105) from the Cardiac Children’s Foundation, Taiwan. The authors would like to thank Ms. Chiu-Yi Hsu and Ms. Yi-An Shr for their efforts in the administration of 6MWT.
CONFLICT OF INTEREST
The authors have no conflicts to declare.
REFERENCES
- 1.Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;20:564–569. doi: 10.1183/09031936.02.02092001. [DOI] [PubMed] [Google Scholar]
- 2.Solway S, Brooks D, Lacasse Y, et al. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 3.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 4.Kessler R, Faller M, Fourgaut G, et al. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Oudiz RJ, Peacock A, et al. End points and clinical trial designs in pulmonary arterial hypertension:clinical and regulatory perspectives. J Am Coll Cardiol. 2004;43(12 Suppl S):48S–55S. doi: 10.1016/j.jacc.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Markevych I, Spiekerkoetter E, et al. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26:858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 7.Moalla W, Gauthier R, Maingourd Y, et al. Six-minute walking test to assess exercise tolerance and cardiorespiratory responses during training program in children with congenital heart disease. Int J Sports Med. 2005;26:756–762. doi: 10.1055/s-2004-830558. [DOI] [PubMed] [Google Scholar]
- 8.Calders P, Deforche B, Verschelde S, et al. Predictors of 6-minute walk test and 12-minute walk/run test in obese children and adolescents. Eur J Pediatr. 2008;167:563–568. doi: 10.1007/s00431-007-0553-5. [DOI] [PubMed] [Google Scholar]
- 9.Geiger R, Willeit J, Rummel M, et al. Six-minute walk distance in overweight children and adolescents:effects of a weight-reducing program. J Pediatr. 2011;158:447–451. doi: 10.1016/j.jpeds.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Li AM, Yin J, Au JT, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med. 2007;176:174–180. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- 11.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–399. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Lammers AE, Hislop AA, Flynn Y, et al. The 6-minute walk test:normal values for children of 4-11 years of age. Arch Dis Child. 2008;93:464–468. doi: 10.1136/adc.2007.123653. [DOI] [PubMed] [Google Scholar]
- 13.Poh H, Eastwood PR, Cecins NM, et al. Six-minute walk distance in healthy Singaporean adults cannot be predicted using reference equations derived from Caucasian populations. Respirology. 2006;11:211–216. doi: 10.1111/j.1440-1843.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao NA, Irfan M, Haque AS, et al. Six-minute walk test performance in healthy adult pakistani volunteers. J Coll Physicians Surg Pak. 2013;23:720–725. doi: 10.2013/JCPSP.720725. [DOI] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement:guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Green PJ. Smoothing reference centile curves:the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 17.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri L, Arling B, Friberg M, et al. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiogr. 2009;22:159–164. doi: 10.1016/j.echo.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Lin MT, Chang CH, Hsieh WC, et al. Coronary diameters in Taiwanese children younger than 6 years old:Z score regression equations derived from body surface area. Acta Cardiol Sin. 2014;30:266–273. [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes SM, Alexander ME, Graham DA, et al. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit Heart Dis. 2011;6:294–303. doi: 10.1111/j.1747-0803.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- 21.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–274. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 22.Camarri B, Eastwood PR, Cecins NM, et al. Six minute walk distance in healthy subjects aged 55-75 years. Respir Med. 2006;100:658–665. doi: 10.1016/j.rmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Ben Saad H, Prefaut C, Tabka Z, et al. 6-minute walk distance in healthy North Africans older than 40 years: influence of parity. Respir Med. 2009;103:74–84. doi: 10.1016/j.rmed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons WJ, Fruchter N, Sloan S, et al. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21:87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Chetta A, Zanini A, Pisi G, et al. Reference values for the 6-min walk test in healthy subjects 20-50 years old. Respir Med. 2006;100:1573–1578. doi: 10.1016/j.rmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Pettersen MD, Du W, Skeens ME, et al. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants,children,and adolescents:an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922–934. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Kampmann C, Wiethoff CM, Wenzel A, et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart. 2000;83:667–672. doi: 10.1136/heart.83.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alameri H, Al-Majed S, Al-Howaikan A. Six-min walk test in a healthy adult Arab population. Respir Med. 2009;103:1041–1046. doi: 10.1016/j.rmed.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc. 2003;35:169–174. doi: 10.1097/00005768-200301000-00025. [DOI] [PubMed] [Google Scholar]