Abstract
Primary cardiac lymphoma is very rare, and the most common electrocardiographic finding in this condition is complete atrioventricular block. After electrolytic, metabolic, ischemic, infectious, and traumatic etiologies have been excluded, primary cardiac lymphoma should be consided as a possible cause of reversible atrioventricular block. Most patients with primary cardiac lymphoma are immunocompromised and have disease with a B-cell etiology. This is the first case report of a primary cardiac T-cell lymphoma with complete atrioventricular block and torsades de pointes in an immunocompetent patient who was successfully treated using chemotherapy.
Keywords: Atrioventricular block, T-cell lymphoma, Torsades de pointes
INTRODUCTION
Primary cardiac lymphoma (PCL) is an extremely rare and life-threatening malignancy.1 This condition is associated with a poor prognosis, as the tumor is clinically aggressive and is often detected late. Most PCLs in immunosuppressed adults are of B-cell origin.2 Herein, we report the first study of primary cardiac T-cell lymphoma with complete atrioventricular block (AVB) and torsades de pointes; the patient was successfully treated with a chemotherapy regimen. The diagnosis and management of this unusual tumor are reviewed.
CASE REPORT
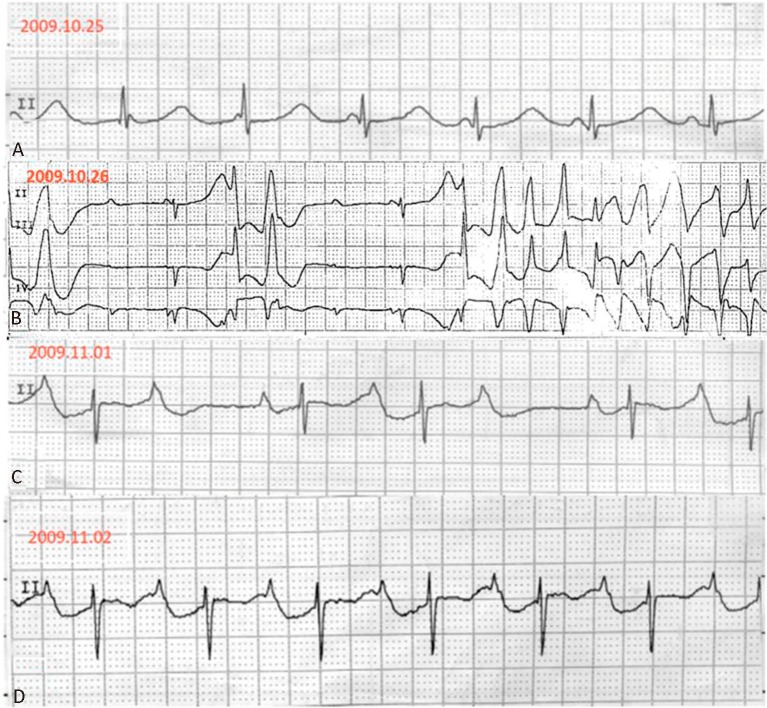
A 70-year-old immunocompetent woman without prior cardiovascular disease presented with a 1-month history of progressive shortness of breath. At the time of admission, her blood pressure was 102/56 mmHg, heart rate was 65 beats/min, and respiratory rate was 24 breaths/min. A physical examination revealed jugular vein distension, diminished breath sounds, dull sounds upon percussion, and mild edema of the lower extremities. Her physical cardiac assessment indicated distant heart sounds, a pericardial friction rub, and pulsus paradoxus. Chest radiography revealed a bilateral pleural effusion and an enlarged cardiac silhouette. Given that pericardial tamponade was strongly suspected, an emergency echocardiographic study was ordered. This revealed an ill-defined hyperechoic mass infiltrating the right ventricle, as well as a circumferential pericardial effusion. The patient felt more comfortable after pericardiocentesis, which produced 500 mL of serosanguineous exudate, and she was scheduled for computed tomography (CT) to confirm the location of the mass. However, during the night of her first day of hospitalization, an electrocardiogram indicated junctional rhythm with AV dissociation with a prolonged QT interval (QTc= 63 ms), then complete AVB, followed by polymorphic ventricular tachycardia (torsades de pointes) (Figure 1A, B). An electrolyte imbalance was not found (potassium level, 4.3 mmol/L; free calcium level, 1.12 mmol/L; and magnesium level, 1.36 mmol/L), and we had previously excluded any drug that could affect her QT interval or induce arrhythmia. Fortunately, the arrhythmia did not affect the patient’s hemodynamic status, and the ventricular tachycardia was terminated by the administration of lidocaine.
Figure 1.
Serial electrocardiographic strips upon patient admission. (A) Junctional rhythm with atrioventricular dissociation with prolonged QT interval. (B) Complete atrioventricular block followed by torsades de pointes before chemotherapy. (C) The complete atrioventricular block converted to 3:2 Wenckebach block on the sixth day of hospitalization. (D) To a first-degree atrioventricular block on the seventh day.
The next day, a CT scan demonstrated the presence of a low-density mass infiltrating the right and left atria, right ventricle, and atrioventricular septum, and extending to the periaortic space of the aortic root (Figure 2A, B). Aspiration of the pleural effusion was performed, and immunohistopathology showed a positive result for CD45RO, a T-lymphocyte surface marker (Figure 2C). The lymphoma staging workup was negative, which including an abdominal-pelvic CT scan, bone marrow biopsy, and human immunodeficiency virus (HIV) serology.
Figure 2.
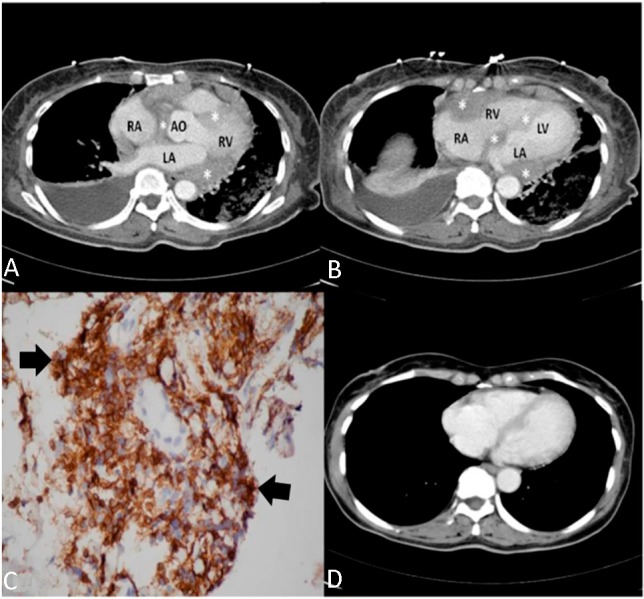
Primary cardiac T-cell lymphoma. (A and B) An axial contrast-enhanced computed tomography (CT) scan revealed a diffuse, low-density mass (asterisks) involving the right ventricle, right and left atria, and atrioventricular septum, as well as compressing the aortic root. (C) An immunohistochemical stain (arrow, with brown and tan color) was positive for CD45RO. (D) A follow-up CT scan demonstrates regression of the mass after sixth months. AO, aorta; LA, left atrium; LV, left ventricle; RA, right atrium, RV, right ventricle.
Chemotherapy, consisting of a cyclophosphamide/doxorubicin/vincristine/prednisolone (CHOP) regimen, was subsequently initiated. The electrocardiogram reverted to 3:2 Wenckebach block and then to first-degree AVB on the seventh day of hospitalization (Figure 1C, D); a normal sinus rhythm was regained eventually one month later. CT imaging showed that the tumor had vanished six months after the diagnosis (Figure 2D). The patient completed eight chemotherapy cycles and achieved complete remission, which was confirmed over a three-year follow-up period. The patient only had one admission because of neutropenic fever during follow-up.
DISCUSSION
A functional (pharmaceutical, metabolic, endocrine, or autonomic) or anatomical impairment in the myocardial conduction tissue can induce complete AVB. Anatomical impairment, which is characterized by fibrosis and sclerosis of the conduction system, is often the result of ischemic disease, congenital heart disease, infectious disease (Lyme disease), infiltrative disease, or trauma.3 The actual incidence of reversible complete AVB is unknown, but after the above factors have been excluded, the possibility of cardiac masses should be considered. Complete AVB is the most common electrocardiographic finding (19%) in patients with PCL,4 and most of them require temporary or permanent cardiac pacing.5 Reversible complete AVB associated with PCL is rare, however. To the best of our knowledge, this is the first case of a primary cardiac T-cell lymphoma with complete AVB and torsades de pointes that was successfully treated via chemotherapy where the patient experienced long-term survival. In view of the rapid regression of the tumor and stable hemodynamic status, we did not implant a pacemaker in this patient.
A clear definition of PCL is a non-Hodgkin’s lymphoma, confined only to the heart or pericardium, with no or minimal extracardiac involvement.5 The disorder is a rare malignancy, accounting for only 1% of primary cardiac tumors and 0.5% of extranodal lymphomas.2 Most PCLs occur in immunocompromised hosts, predominantly in men with HIV infections or transplant recipients,6 while data relating to immunocompetent persons are limited.4 We reviewed all 207 cases of PCL reported in the literature, including the estimates of Petrich et al.1 from 1949 to 2009 and other published reports up to 2012; only 6 cases were of T-cell origin.7-9 The present report is the only one that we reviewed that describes an immunocompetent patient with PCL of T-cell origin.
PCL progresses rapidly, and should be considered an oncologic emergency.2 There is no particular clinical presentation for PCL; therefore, diagnosis is often delayed. Transthoracic echocardiography is an important tool if there is suspicion of early-stage PCL. CT scans or magnetic resonance imaging are extremely valuable for both detection and staging.4 Cytology is diagnostic in only two-thirds of cases,5 but liquid cytology of the cardiac effusion or pleural effusion is very useful for rapid diagnosis, leading to a better prognosis. If cytology is not available, the diagnosis can be assisted by cardiac tissue biopsy.5 Our patient was promptly treated once the primary cardiac T-cell lymphoma was confirmed, based on the pleural effusion examination.
Currently, chemotherapy is the most effective treatment for PCL.4 Radiation therapy, alone, does not improve prognosis, and surgical excision is often difficult and incomplete.10 In the six patients reported with primary cardiac T-cell lymphoma,7-9 the diagnosis was not determined until autopsy in two patients; three received chemotherapy but died a short time later due to the rapid spread of the tumor or ventricular tachycardia; the sixth patient received palliative surgery with just a year follow-up. The literature suggests that T-cell PCL has a poorer response to chemotherapy than does B-cell-associated disease; however, in the present case, treatment with a CHOP regimen was successful. The present patient has the longest reported survival for patients reported with primary cardiac T-cell lymphoma.
To conclude, primary cardiac T-cell lymphoma can be treated successfully if detected early. Primary cardiac T-cell lymphoma could also manifest as AV nodal dysfunction (Mobiz type I AV block, or complete AVB) and polymorphic ventricular tachycardia, and rhythm disorder was reversed by early diagnosis and successful chemotherapy.
REFERENCES
- 1.Petrich A, Cho SI, Billett H. Primary cardiac lymphoma:an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117:581–589. doi: 10.1002/cncr.25444. [DOI] [PubMed] [Google Scholar]
- 2.Ceresoli GL, Ferreri AJ, Bucci E, et al. Primary cardiac lymphoma in immunocompetent patients:diagnostic and therapeutic management. Cancer. 1997;80:1497–1506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Crisel RK, Knight BP, Kim SS. Reversible, complete atrioventricular block caused by primary cardiac lymphoma in a nonimmunocompromised patient. J Cardiovasc Electrophysiol. 2012;23:1386–1389. doi: 10.1111/j.1540-8167.2012.02343.x. [DOI] [PubMed] [Google Scholar]
- 4.Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. 2011;149:358–363. doi: 10.1016/j.ijcard.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Yamabe H, Yamasaki H, et al. A case of reversible ventricular tachycardia and complete atrioventricular block associated with primary cardiac B-cell lymphoma. Pacing Clin Electrophysiol. 2009;32:816–819. doi: 10.1111/j.1540-8159.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen KW, Chang JH, Yeh SP, Lu CR. Primary cardiac B-cell lymphoma with atrioventricular block and paroxysmal ventricular tachycardia. J Cardiothorac Surg. 2012;7:70. doi: 10.1186/1749-8090-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Li R, Wu B, et al. Primary cardiac T cell lymphoma. J Card Surg. 2012;27:457–460. doi: 10.1111/j.1540-8191.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 8.Deepti AN, Noone ML, Mahadevan A, et al. Primary cardiac cytotoxic T-cell lymphoma presenting with neurological deficits:a case report. Cardiovasc Pathol. 2008;17:334–338. doi: 10.1016/j.carpath.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Patel J, Melly L, Sheppard MN. Primary cardiac lymphoma: B- and T-cell cases at a specialist UK centre. Ann Oncol. 2010;21:1041–1045. doi: 10.1093/annonc/mdp424. [DOI] [PubMed] [Google Scholar]
- 10.Frikha Z, Abid L, Abid D, et al. Cardiac tamponade and paroxysmal third-degree atrioventricular block revealing a primary cardiac non-Hodgkin large B-cell lymphoma of the right ventricle:a case report. J Med Case Rep. 2011;5:433. doi: 10.1186/1752-1947-5-433. [DOI] [PMC free article] [PubMed] [Google Scholar]