Abstract
Background
Uric acid (UA) is an independent risk factor for the development of coronary heart disease. Serum UA levels have been correlated with all major forms of death from cardiovascular disease, including acute, subacute, and chronic forms of coronary artery disease (CAD), heart failure, and stroke. However, its value in acute ST-segment elevation myocardial infarction (STEMI) remains unclear. The aim of this study was to evaluate the prognostic value of UA in patients with STEMI undergoing primary percutaneous coronary intervention (PCI).
Methods
We prospectively enrolled 434 consecutive Turkish STEMI patients (mean age 55.4 ± 12.4 years, 341 male, 93 female) undergoing primary PCI. The study population was divided into tertiles based on admission UA values. The high UA group (n = 143) was defined as a value in the third tertile (> 5.7 mg/dl), and the low UA group (n = 291) included those patients with a value in the lower two tertiles (≤ 5.7 mg/dl). Clinical characteristics, in-hospital and six-month outcomes of primary PCI were analyzed.
Results
Compared to the low UA group, only Killip class > 1 at admission was more prevalent in the high UA group (3.4% vs. 17.5%, p < 0.001, respectively). Higher in-hospital cardiovascular mortality and six-month all-cause mortality rates were observed in the high UA group than in the lower group (12.6% vs. 1.7%, respectively, p < 0.001) and (19.6% vs. 4.1%, respectively, p < 0.001). In Cox multivariate analysis; a high admission UA value (> 5.7 mg/dl) was found to be a powerful independent predictor of six-month all-cause mortality (hazard ratio: 5.57, 95% confidence interval: 1.903-16.3, p = 0.002).
Conclusions
These results suggest that a high level of UA on admission was associated with increased in-hospital cardiovascular mortality, and six-month all-cause mortality in Turkish patients with STEMI undergoing primary PCI.
Keywords: Primary angioplasty, ST elevation myocardial infarction, Uric acid
INTRODUCTION
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality. Conseguently, risk stratification is a very important issue in the prevention and management of CAD.1-3 Uric acid (UA) is the end product of purine catabolism in humans and is readily tested in routine clinical practice, as well as being an independent risk factor for the development of CAD.4-8 Serum UA levels have been correlated with all major forms of death from cardiovascular disease, including acute, subacute, and chronic forms of CAD, heart failure, and stroke.9 Although an association between elevated UA levels and early adverse outcomes in acute myocardial infarction (MI) has been documented,9 the relationship between UA levels and mid-term outcomes in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) has not been fully evaluated. In this prospective study, we hypothesized that elevated UA levels would be associated with in-hospital and long-term adverse outcomes after primary PCI for STEMI.
METHODS
Patient population
In this prospective observational study, we included 464 consecutive patients with acute STEMI presenting at the Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Center, Training and Research Hospital, between December 2010 and May 2012. The study inclusion criteria were as follows: electrocardiography (ECG) revealing STEMI, which was defined as > 30 minutes of continuous typical chest pain and ST-segment elevation ≥ 2 mm in two contiguous electrocardiography leads within 12 hours of symptom onset, or for up to 18 hours if there was evidence of continuing ischemia or hemodynamic instability. We excluded 30 patients from our analysis because they had no indication of PCI (n = 15), were not suitable for PCI (n = 10), or had missing or unavailable data about uric acid level upon admission (n = 5). Therefore, the final study population consisted of 434 patients. The population was divided into tertiles based on admission UA values. A high UA group (n = 143) was defined as a value in the third tertile (> 5.7 mg/dl), and a low UA group (n = 291) was set as a value in the lower two tertiles (≤ 5.7 mg/dl).
All primary PCI procedures were performed in a single high-volume tertiary care center (> 3000 PCI/year) by expert operators who carry out an average of > 75 PCI/year. The study protocol was approved by the Ethics Committee of the hospital.
Analysis of patient data
Upon admission, the patients’ medical histories and a special questionnaire on lifestyle and risk factors were taken. Reperfusion time and door-to-balloon time were also recorded. Complete blood counts and other serum values were determined on admission before catheterization procedures. A 12-lead ECG was recorded in each patient just after hospital admission, and the myocardial infarction type was also obtained from patient ECGs. At 24 to 72 hours after revascularization, a transthoracic echocardiographic study was performed using a Vivid S5 probe 3S-RS (GE Healthcare) with a 1.7/3.4 MHz phased-array transducer, and the left ventricular ejection fraction (LVEF) was calculated using the biplane Simpson’s method.10 The glomerular filtration rate (GFR) was estimated by the simplified Modification of Diet in Renal Disease (MDRD) equation.11
Analysis of ST-segment resolution
Measurements were taken during the first ECG, which was obtained immediately before angioplasty, and also in the second, 60 minutes after first balloon inflation. ST-segment elevation in mm was measured 20 milliseconds after the J point. The sum of ST-segment elevations were measured in leads I, aVL, and V1 through V6 for anterior infarctions, and in leads II, III, aVF, V5, and V6 for inferior infarctions. The difference between 2 measurements was accepted as resolution of the sum of ST-segment elevation and expressed as Σ:STR. According to classification of Schroder et al.,12,13 patients with Σ:STR ≥ 50% were accepted as no-reflow phenomenon (-), and patients with Σ:STR < 50% were accepted as no-reflow phenomenon (+).
Coronary angiography, primary angioplasty, and stenting
All patients received a chewable 300 mg aspirin and clopidogrel (600 mg loading dosage) before coronary angiography. Angiographic data of the patients were evaluated from catheter laboratory records. Emergency coronary angiography and angioplasty were performed using the percutaneous femoral approach. A nonionic, low-osmolality contrast media was used in all patients. The artery that was presumed to be unobstructed was injected first. Blood flow in the infarct-related artery (IRA) was graded according to the Thrombolysis in Myocardial Infarction (TIMI) classification.14 Heparin (100 IU/kg) was administered when the coronary anatomy was first defined.
After visualizing the left and right coronary arteries, 2.5 μg of nitrate was selectively injected into the IRA to rule out a possible coronary spasm. An angiographic evaluation was made by visual assessment. Primary angioplasty (including balloon angioplasty and/or stent implantation) was performed only for IRA according to lesion type. For each procedure, interventional success at the acute phase was defined as reducing to < 30% of obstruction and stenosis of the IRA with TIMI 3 flow just after primary angioplasty. After angioplasty, all patients were admitted to the coronary care unit, where 100 mg aspirin and 75 mg clopidogrel were continued in all patients. The use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the operator.
Definition
Reperfusion time was measured as the time from symptom onset until coronary reperfusion was obtained with balloon inflation. The door-to-balloon time was defined as the time between hospital admission and balloon inflation. Patients were evaluated according to the Killip clinical examination classification.15 Advanced heart failure was defined as New York Heart Association (NYHA) classification ≥ 3. Anemia was set as a baseline hemoglobin concentration < 13 mg/dl in males and < 12 mg/dl in females. Renal failure was defined as a GFR < 60 ml/min/1.73 m2, which was estimated by the modification of diet in renal disease (MDRD) equation.11 Patients with diabetes mellitus (DM) were determined to be those with documented DM currently using either oral hypoglycemic agents or insulin treatment at admission. Cardiovascular mortality was defined as unexplained sudden death, death due to acute STEMI, heart failure, or arrhythmia. We set the repeat target vessel revascularization (TVR) as the need for PCI or coronary surgery because of restenosis or reocclusion of the IRA. Reinfarction was described as an elevation of serum creatinine kinase-MB (CK-MB) enzyme levels at least two times the upper limit of normal and ST-segment re-elevations.
Follow-up
Follow-up data were obtained from hospital records or by interviewing patients (directly or by telephone), their families, or their personal physicians. Major adverse cardiac events (MACE) were defined as cardiovascular mortality, reinfarction, or repeat TVR (percutaneous or surgical).
Statistical analysis
Quantitative variables were expressed as mean value ± SD, and qualitative variables were shown as a percentage (%). The comparison of parametric values between the two groups was performed using a two-tailed Student’s t test. Categorical variables were compared by the likelihood-ratio χ2 test or the Fisher’s exact test. The cumulative survival curve for six-month all-cause mortality was constructed using the Kaplan-Meier method, with differences assessed with log-rank tests. Pearson analysis was used for correlation. A backward stepwise Cox multivariate analysis, which included variables with p < 0.1, was performed to identify independent predictors of six-month all-cause mortality. Age, female gender, DM, hypertension, TIMI score, uric acid > 5.7 mg/dl, Killip class > 1, anemia upon admission, three-vessel disease, unsuccessful procedure, LVEF < 40%, tirofiban usage, no-reflow and GFR < 60 ml/min/1.73 m2 were entered into the model. A p-value < 0.05 was considered statistically significant. All statistical studies were carried out with the Statistical Package for the Social Sciences (SPSS) software program (version 15.0, SPSS, Chicago, Illinois, USA).
RESULTS
Baseline characteristics
The baseline characteristics are listed in Table 1. The baseline uric acid level of the study population was 5.3 ± 1.6 mg/dl (range: 1 to 14.6). Also, the upper tertile of UA values were 6 mg/dl in men, and 5.2 mg/dl in women. The mean UA values were 5.47 ± 1.54 mg/dl in men, and 5.07 ± 1.3 mg/dl in women (p = 0.04). Compared to the low UA group, only Killip class > 1 at admission was more prevalent in the high UA group. Age, anterior MI, female gender, MI history, PCI history, hypertension (HT), DM, and current smoker were not statistically different between the two groups.
Table 1. Baseline characteristics of study patients .
| Variable | Uric acid ≤ 5.7 (n = 291) | Uric acid > 5.7 (n = 143) | p value |
| Age, years (SD) | 54.8 ± 11.6 | 56.8 ± 13.9 | 0.12 |
| Female gender, n (%) | 70 (24.1) | 23 (16.1) | 0.06 |
| DM, n (%) | 61 (21) | 28 (19.6) | 0.78 |
| Hypertension, n (%) | 97 (33.3) | 54 (37.8) | 0.34 |
| By-pass history, n (%) | 5 (1.7) | 4 (2.8) | 0.45 |
| PCI history, n (%) | 37 (12.7) | 20 (14) | 0.69 |
| MI history, n (%) | 42 (14.4) | 26 (18.2) | 0.30 |
| Current smoker, n (%) | 223 (76.6) | 98 (68.5) | 0.08 |
| Anterior MI, n (%) | 127 (43.6) | 72 (50.3) | 0.23 |
| Killip class > 1, n (%) | 10 (3.4) | 25 (17.5) | < 0.001 |
| Reperfusion time, min (SD) | 249.8 ± 140 | 253.3 ± 152.8 | 0.82 |
| Door-to-balloon time, min (SD) | 42.9 ± 14.5 | 42.9 ± 17.3 | 0.97 |
DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Laboratory findings
Table 2 lists the patients’ laboratory data. Higher baseline blood creatinine and lower baseline GFR were observed in the higher more so than in the lower UA group (1.13 ± 0.52 vs. 0.87 ± 0.39, p < 0.001, and 77.1 ± 26.7 vs. 95.4 ± 26.1, p < 0.001, respectively). There was a negative linear correlation with GFR and UA value (r: 0.38, p < 0.001). Peak CK-MB; was also higher in the high UA group (176.9 ± 145.4 vs. 130.4 ± 131.7, p = 0.001). Baseline glucose and anemia at admission, total-cholesterol and triglyceride levels were not statistically different between the two groups.
Table 2. Laboratory findings of patients .
| Variable | Uric acid ≤ 5.7 (n = 291) | Uric acid > 5.7 (n = 143) | p value |
| Baseline creatinine, mg/dl (SD) | 0.87 ± 0.39 | 1.13 ± 0.52 | < 0.001 |
| Baseline glucose, mg/dl (SD) | 164 ± 71 | 178.4 ± 105.1 | 0.1 |
| Anemia at admission, n (%) | 35 (12) | 22 (15.4) | 0.33 |
| Baseline hemoglobin, g/dl (SD) | 14.7 ± 7.4 | 14.5 ± 7.2 | 0.67 |
| Baseline GFR, ml/min/1.73m2 (SD) | 95.4 ± 26.1 | 77.1 ± 26.7 | < 0.001 |
| Renal failure, n (%) | 7 (2.4) | 23 (16.1) | < 0.001 |
| Peak CK-MB, IU/L (SD) | 130.4 ± 131.7 | 176.9 ± 145.4 | 0.001 |
| Total-cholesterol, mg/dl (SD) | 197.4 ± 47 | 196.4 ± 47 | 0.84 |
| LDL-cholesterol, mg/dl (SD) | 132 ± 37.1 | 132.9 ± 36.6 | 0.82 |
| HDL-cholesterol, mg/dl (SD) | 42 ± 10.2 | 40.2 ± 10.9 | 0.10 |
| Triglyceride, mg/dl (SD) | 125.2 ± 81.4 | 128.1 ± 80.2 | 0.81 |
CK-MB, creatinine kinase-MB; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Angiographic and procedural characteristics
Angiographic and procedural characteristics are depicted in Table 3. Culprit lesions were similar in the two groups. Compared to the low UA group, only unsuccessful procedure was more prevalent in the high UA group (10.5% vs. 4.5%, p = 0.02). Stent type was not statistically different between the two groups (p = 0.63). Stent usage, stent length, stent diameter, number of diseased vessels, and tirofiban usage were not statistically different between the two groups.
Table 3. Angiographic and procedural characteristics of patients .
| Variable | Uric acid ≤ 5.7 (n = 291) | Uric acid > 5.7 (n = 143) | p value |
| Culprit lesion | |||
| LMCA, n (%) | 0 (0) | 0 (0) | |
| LAD, n (%) | 126 (43.3) | 73 (51) | |
| CX, n (%) | 37 (12.7) | 14 (9.8) | |
| RCA, n (%) | 122 (41.9) | 56 (39.2) | |
| Others, n (%) | 3 (1) | 2 (1.4) | |
| No. of diseased vessels | 0.07 | ||
| 1 | 126 (43.3) | 67 (46.9) | |
| 2 | 101 (34.7) | 36 (25.2) | |
| 3 | 62 (21.3) | 42 (29.4) | |
| Unsuccessful procedure, n (%) | 13 (4.5) | 15 (10.5) | 0.02 |
| Stent type | 0.63 | ||
| BMS | 249 (97.6) | 123 (97.6) | |
| SES | 6 (2.4) | 3 (2.4) | |
| Stent usage, n (%) | 255 (87.6) | 126 (88.1) | 0.79 |
| Stent length, mm (SD) | 23.3 ± 9.3 | 23.6 ± 10.5 | 0.8 |
| Stent diameter, mm (SD) | 3.3 ± 1.7 | 3.4 ± 1.9 | 0.63 |
| Tirofiban usage, n (%) | 96 (32) | 50 (35) | 0.77 |
BMS, bare metal stent; CX, circumflex coronary artery; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; RCA, right coronary artery; SD, standard deviation; SES, sirolimus-eluting stent.
In-hospital outcomes
Table 4 presents the in-hospital outcomes after primary PCI. The high UA group had a significantly higher incidence of in-hospital cardiovascular mortality than the low UA group (12.6% vs. 1.7%, respectively, p < 0.001). MACE, advanced heart failure, cardiopulmonary resuscitation, inotropic agent usage, cardiogenic shock, intra-aortic balloon pump usage, atrial fibrillation, transient pace, gastrointestinal bleeding, ventilator necessity, no-reflow phenomenon, and lower LVEF occurred more frequently in the high UA group.
Table 4. In-hospital cardiac events of all study patients .
| Variable | Uric acid ≤ 5.7 (n = 291) | Uric acid > 5.7 (n = 143) | p value |
| Cardiovascular mortality, n (%) | 5 (1.7) | 18 (12.6) | < 0.001 |
| Reinfarction, n (%) | 18 (6.2) | 7 (4.9) | 0.6 |
| Target-vessel revascularization, n (%) | 18 (6.2) | 7 (4.9) | 0.6 |
| MACE, n (%) | 22 (7.6) | 24 (16.8) | 0.003 |
| Stroke, n (%) | 1 (0.3) | 2 (1.4) | 0.22 |
| Cardiopulmonary resuscitation, n (%) | 6 (2.1) | 22 (15.4) | < 0.001 |
| Dialysis, n (%) | 1 (0.3) | 2 (1.4) | 0.22 |
| Advanced heart failure, n (%) | 13 (4.7) | 30 (21) | < 0.001 |
| Cardiogenic shock, n (%) | 7 (2.4) | 20 (14) | < 0.001 |
| Inotropic agent usage,n (%) | 13 (4.5) | 23 (16.1) | < 0.001 |
| IABP usage, n (%) | 5 (1.76) | 12 (8.4) | 0.001 |
| Atrial fibrillation, n (%) | 04 (1.4) | 7 (4.9) | 0.03 |
| Transient pace, n (%) | 5 (1.7) | 9 (6.3) | 0.01 |
| Gastrointestinal bleeding, n (%) | 0 (0) | 2 (1.4) | 0.04 |
| Blood transfusion | 1 (0.3) | 2 (1.4) | 0.22 |
| Ventilator necessity | 5 (1.7) | 16 (11.2) | < 0.001 |
| No-reflow phenomenon | 45 (15.5) | 48 (33.6) | < 0.001 |
| LVEF, % (SD) | 49.2 ± 9.2 | 45 ± 11.1 | < 0.001 |
IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events (cardiovascular mortality, reinfarction, target-vessel revascularization).
Six-month outcomes
Six-month outcomes are depicted in Table 5. The high UA group had significantly higher incidence of all-cause mortality and cardiovascular mortality than the low UA group (19.6% vs. 4.1%, respectively, p < 0.001, and 17.5% vs. 4.1% respectively, p < 0.001). Three patients died because of non-cardiac reason (cerebral hemorrhage, lung cancer and pulmonary embolism). The Kaplan-Meier survival plot for six-month all-cause mortality is presented in Figure 1. Non-cardiac mortality, advanced heart failure, fatal reinfarction, stroke and MACE were also more frequently observed in the high UA group. Independent predictors of six-month all-cause mortality were determined by a backward stepwise Cox multivariate analysis. These predictors of all-cause mortality are depicted in Table 6. Age, uric acid > 5.7 mg/dl, three vessel disease, unsuccessful procedure, and LVEF < 40 were found to be independent predictors of six-month all-cause mortality. In a receiver operating characteristic (ROC) curve analysis, a UA value of 5.7 was identified as an effective cut-point in the STEMI of six-month all cause mortality [area under curve = 0.73, 95% confidence interval (CI) 0.64 to 0.81, p < 0.001]. A UA of > 5.7 yielded a sensitivity of 70% and a specificity of 71% for six-month all-cause mortality (Figure 2).
Table 5. Six-month events of all study patients .
| Variable | Uric acid ≤ 5.7 (n = 291) | Uric acid > 5.7 (n = 143) | p value |
| All-cause mortality, n (%) | 12 (4.1) | 28 (19.6) | < 0.001 |
| Cardiovascular mortality, n (%) | 12 (4.1) | 25 (17.5) | < 0.001 |
| Non-cardiac mortality, n (%) | 0 | 3 (2.1) | 0.01 |
| Fatal reinfarction, n (%) | 8 (2.7) | 14 (9.8) | 0.002 |
| Target-vessel revascularization, n (%) | 21 (7.2) | 16 (11.2) | 0.16 |
| Stroke, n (%) | 1 (0.3) | 4 (2.8) | 0.02 |
| Advanced heart failure, n (%) | 46 (15.8) | 47 (32.9) | < 0.001 |
| Cardiac-hospitalization, n (%) | 53 (18.2) | 37 (25.9) | 0.06 |
| MACE, n (%) | 32 (11) | 38 (26.6) | < 0.001 |
MACE, major adverse cardiac events (cardiovascular mortality, reinfarction, target-vessel revascularization).
Figure 1.
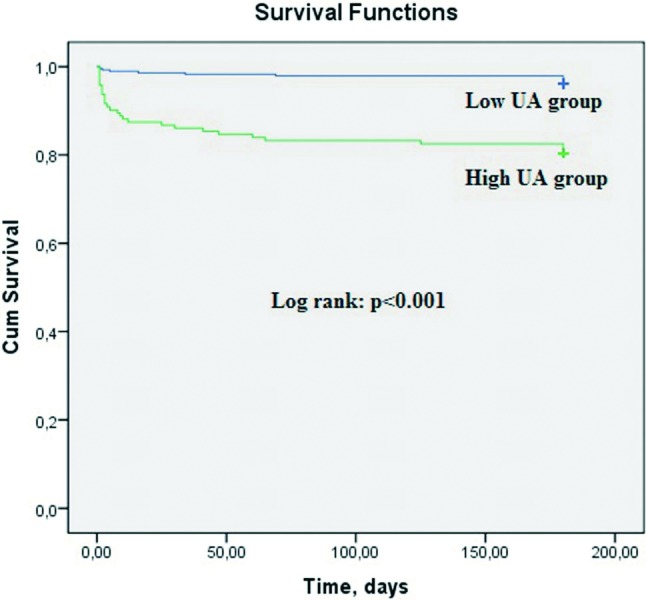
The Kaplan-Meier survival plot for six-month all-cause mortality according to UA groups.
Table 6. Effects of multiple variables on the six-month all-cause mortality in univariate and multivariate Cox analyses .
| Univariate HR | 95% CI | p value | Multivariate HR | 95% CI | p value | |
| Age | 1.084 | 1.057-1.112 | < 0.001 | 1.071 | 1.034-1.110 | lt; 0.001 |
| Female gender | 2.2 | 1.138-4.211 | 0.02 | |||
| DM | 3.18 | 1.657-6.087 | 0.001 | |||
| Hypertension | 1.94 | 1.028-3.668 | 0.04 | |||
| TIMI-score | 1.06 | 1.042-1.079 | < 0.001 | |||
| Killip class > 1 | 11.238 | 5.948-21.233 | < 0.001 | |||
| Anemia at admission | 2.444 | 1.187-5.032 | 0.02 | |||
| Uric acid > 5.7 | 5.7 | 2.835-11.442 | < 0.001 | 5.570 | 1.903-16.3 | 0.002 |
| Three-vessel disease | 2.656 | 1.411-5.003 | 0.002 | 3.613 | 1.407-9.280 | 0.008 |
| Unsuccessful procedure | 8.86 | 4.475-17.536 | < 0.001 | 3.82 | 1.254-11.642 | 0.02 |
| LVEF < 40% | 16.894 | 7.486-38.125 | < 0.001 | 11.956 | 4.332-32.995 | < 0.001 |
| Tirofiban usage | 1.874 | 0.936-3.752 | 0.08 | |||
| GFR < 60 ml/min/1.73m2 | 6.144 | 2.759-13.684 | < 0.001 | |||
| No-reflow phenomenon | 9.424 | 4.77-18.6 | < 0.001 |
DM, diabetes mellitus; GFR, glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIMI, Thrombolysis in Myocardial Infarction.
Figure 2.
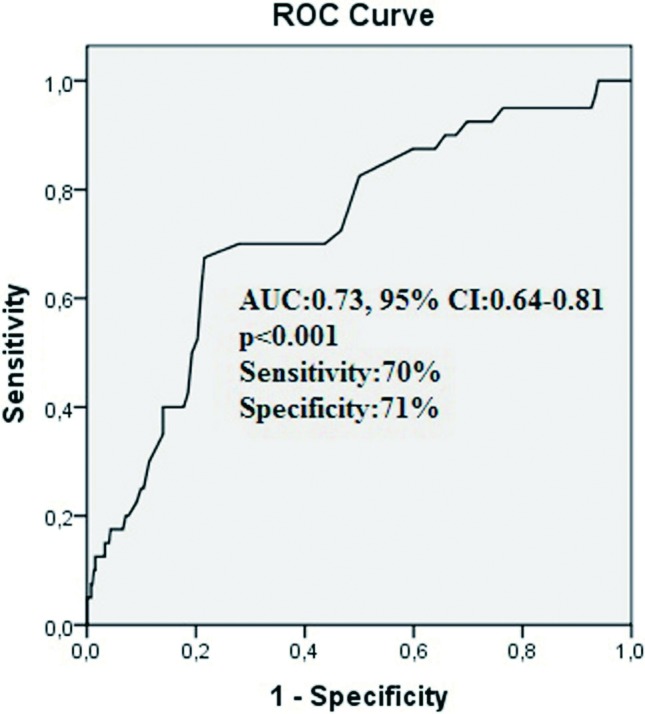
The receiver-operating characteristic (ROC) curve with regard to all-cause mortality at six-months for high uric acid (UA) with area under curve of 0.73 (95% CI, 0.64 to 0.81).
DISCUSSION
The main findings of the present single-center study are as follows: 1) patients in the high UA group had a higher prevalence of unsuccessful procedures, lower LVEF, lower GFR, higher peak CK-MB, no-reflow phenomenon, atrial fibrillation, gastrointestinal bleeding, and advanced Killip class; 2) high UA levels were associated with a remarkable increase in in-hospital cardiovascular mortality, six-month cardiovascular mortality, and six-month all-cause mortality; and 3) after adjustment for potential confounders, a high level of UA was one of the independent predictors of six-month all-cause mortality.
Some epidemiological studies have established a relationship between UA levels and cardiovascular diseases, and also with UA levels and adverse outcomes and mortality.4-6
Kojima et al.16 reported that mortality in patients whose serum UA levels were in the highest quartile was significantly higher than in those whose UA levels were in the lowest quartile. Lazzeri et al.9 demonstrated that a high UA level was an independent predictor of in-hospital mortality in patients with STEMI who underwent primary PCI. Contrary to this study, in another study with a larger patient population with STEMI, Lazzeri et al.17 revealed that the UA level predicted the occurrence of complications in the intensive care unit, but not early mortality. Akpek et al.18 reported that there was a significant relationship between baseline UA levels and impaired coronary flow and in-hospital MACEs in 289 patients. Duran et al.19 also demonstrated that a high level of serum UA is associated with impaired development of coronary collateral vessels in patients with acute coronary syndrome. In another recent study, Kowalczyk et al.20 demonstrated that elevated levels of UA predicted short-term and long-term mortality, regardless of the degree of renal dysfunction in patients with acute myocardial infarction and impaired renal function treated with PCI. In our study, an elevated UA level was significantly correlated with higher creatinine values and lower GFR values.
In this large prospective study of 434 patients, we found that an elevated UA level on admission was independently associated with the no-reflow phenomenon after primary PCI. As a result, both short-term and mid-term adverse outcomes were significantly increased.
In another study, Huczek et al.21 reported that the mean platelet volume at the time of admission was associated with impaired reperfusion and six-month mortality in primary PCI patients. The present study suggests that the serum UA level at admission is also an additional biomarker associated with in-hospital adverse outcomes and cardiovascular mortality. In a retrospective study, Kaya et al.22 recently reported that elevated UA levels were associated with in-hospital and long-term adverse outcomes after primary PCI for STEMI. In addition, Ndrepepa et al.23 reported that UA level was associated with mortality across the whole spectrum of patients with acute coronary syndrome (ACS) who underwent PCI. In our prospective study, we found similar results, with increased in-hospital mortality, adverse outcomes, and increased mid-term all-cause mortality in patients with STEMI undergoing primary PCI.
Coronary artery disease is an atherosclerotic process,24 and mechanisms underlying this process mainly involve oxidative stress, inflammation, and endothelial dysfunction. Research has shown that a high UA level is strongly associated with atherosclerosis acting through inflammation,25 oxidative stress,26 and endothelial dysfunction.27,28
UA is an end product of the enzyme activity of xanthine oxidase during purine catabolism,29 and the UA level is, in part, related to the activity of xanthine oxidase and may be associated with the level of oxidative stress. During the production of UA and the corresponding activity of xanthine oxidase, oxygen free radicals are generated.30 The generation of oxygen-free radicals is one of the underlying causes of impaired coronary flow and the no-reflow phenomenon after primary PCI.31 The coronary flow is regulated according to the demands of the cardiovascular system by endothelial-derived mediators.32
We know that the major vasodilator endothelial-derived mediator is nitric oxide and that this plays a pivotal role in coronary blood flow.33 Hyperuricemia adversely affects the production of nitric oxide in vascular endothelial cells.34 Therefore, elevated levels of UA not only contribute to oxygen free radical generation, but also to a decrease in the amount of nitric oxide in circulation. This, in turn, inhibits vasodilatation and increases the prevalence of the no-reflow phenomenon. Schröder et al.12,13 showed that early ST-segment resolution in acute ST-elevation myocardial infarction was associated with good myocardial perfusion and that this method was a simple and strong predictor of the no-reflow phenomenon and of adverse outcomes and prognosis. It is notable that the no-reflow phenomenon and lower LVEF were seen more frequently in patients with elevated levels of UA in the present study.
Study limitations
This study was a single-center study. It was nonrandomized and thus subject to selection bias. However, we were careful to include consecutive patients. We did not evaluate the high-sensitivity C-reactive protein, the B-type natriuretic peptide, other pro-inflammatory cytokines, or markers of oxidative stress. Despite adjusting for multiple risk factors, it is possible that residual confounding conditions and medications may have been present. Due to the very limited number of patients receiving allopurinol therapy, the impact of this drug on the prognosis could not be assessed. We used ΣSTR, which is an indirect method for detecting microvascular function. Quantitative myocardial contrast echocardiography can give more information about microvascular circulation. Lastly, the study population was composed exclusively of Turkish people, which may limit its generalizability to other racial groups.
CONCLUSION
Our study showed that patients with high levels of UA had poorer clinical outcomes and higher mortality than patients with low levels of UA, both while they were in the hospital and during the subsequent six-month period. In summary, UA is a powerful prognostic factor in patients undergoing primary PCI for STEMI. In light of the present study, we suggest that the UA level is a simple and easily measurable biomarker that can be used in risk stratification in patients with STEMI, and that STEMI patients with high UA levels should be monitored closely to determine in-hospital adverse outcomes and long-term prognosis. Although the clinical significance of the treatment of patients after developing STEMI is not known, the relationship between UA levels and primary PCI outcomes is still relevant. We think that further studies are needed to determine optimal treatment of STEMI patients, and that close monitoring of hyperuricemia is needed to prevent no-reflow and adverse outcomes in STEMI patients with increased UA levels.
REFERENCES
- 1.Fox KA, Cokkinos DV, Deckers J, et al. The ENACT study:a pan-European survey of acute coronary syndromes. Eur Heart J. 2000;21:1440–1449. doi: 10.1053/euhj.2000.2185. [DOI] [PubMed] [Google Scholar]
- 2.Fox KA, Goodman SG, Anderson FA , Jr., et al. From guidelines to clinical practice:the impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes. The Global Registry of Acute Coronary Events (GRACE). Eur H. 2003;24:1414–1424. doi: 10.1016/s0195-668x(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 3.Kolansky DM. Acute coronary syndromes:morbidity,mortality,and pharmacoeconomic burden. Am J Manag . 2009;15:S36–S41. [PubMed] [Google Scholar]
- 4.Bickel C, Rupprecht HJ, Blankenberg S, et al. Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am J Cardiol. 2002; 89:12–17. doi: 10.1016/s0002-9149(01)02155-5. [DOI] [PubMed] [Google Scholar]
- 5.Bos MJ, Koudstaal PJ, Hofman A, et al. Uric acid is a risk factor for myocardial infarction and stroke:the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 6.Niskanen LK, Laaksonen DE, Nyyssönen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men:a prospective cohort study. Arch Intern Med. 2004; 164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DS, Williamson DF, Gunter EW , et al. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-Up Study. Am J Epidemiol . 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 8.Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death:the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lazzeri C, Valente S, Chiostri M, et al. Uric acid in the acute phase of ST elevation myocardial infarction submitted to primary PCI:its prognostic role and relation with inflammatory markers:a single center experience. Int J Cardiol. 2010;138:206–209. doi: 10.1016/j.ijcard.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function measured and estimated glomerular filtration rate . N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 12.Schröder R, Dissmann R, Bruggemann T, et al. Extent of early ST segment elevation resolution:a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24:384–391. doi: 10.1016/0735-1097(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 13.Schröder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110:506–510. doi: 10.1161/01.CIR.0000147778.05979.E6. [DOI] [PubMed] [Google Scholar]
- 14.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. . Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 15.Killip T, Kimball JT. reatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 16.Kojima S, Sakamoto T, Ishihara M, et al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study) Am J Cardiol. 2005;96:489–495. doi: 10.1016/j.amjcard.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Lazzeri C, Valente S, Chiostri M, et al. Uric acid in the early risk stratification of ST-elevation myocardial infarction. Intern Emerg Med. 2012;7:33–39. doi: 10.1007/s11739-011-0515-9. [DOI] [PubMed] [Google Scholar]
- 18.Akpek M, Kaya MG, Uyarel H, et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis. 2011;219:334–341. doi: 10.1016/j.atherosclerosis.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Duran M, Ornek E, Murat SN, et al. High levels of serum uric acid impair development of coronary collaterals in patients with acute coronary syndrome. Angiology. 2012;63:472–475. doi: 10.1177/0003319711422433. [DOI] [PubMed] [Google Scholar]
- 20.Kowalczyk J, Francuz P, Swoboda R, et al. Prognostic significance of hyperuricemia in patients with different types of renal dysfunction and acute myocardial infarction treated with percutaneous coronary intervention. Nephron Clin Pract. 2010;116:c114. doi: 10.1159/000314660. [DOI] [PubMed] [Google Scholar]
- 21.Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and longterm mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 22.Kaya MG, Uyarel H, Akpek M, et al. Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;109:486–491. doi: 10.1016/j.amjcard.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Ndrepepa G, Braun S, Haase HU, et al. Prognostic value of uric acid in patients with acute coronary syndromes. Am J Cardiol. 2012;109:1260–1265. doi: 10.1016/j.amjcard.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient:a call for new definitions and risk assessment strategies:part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 25.Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart J. 2006;27:1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174:288–291. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Hisatome I, Tomikura Y, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol. 2005;96:1576–1578. doi: 10.1016/j.amjcard.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 28.Erdogan D, Gullu H, Caliskan M, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59:1276–1282. doi: 10.1111/j.1742-1241.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 29.Caymaz O. Chronic heart failure and uric acid. Turk J C. 2006;9:110–114. [Google Scholar]
- 30.Saugstad OD. Role of xanthine oxidase and its inhibitor in hypoxia:reoxygenation injury. Pediatrics. 1996;98:103–107. [PubMed] [Google Scholar]
- 31.Romano M, Buffoli F, Tomasi L , et al. The no-reflow phenomenon in acute myocardial infarction after primary angioplasty:incidence,predictive factors,and long-term outcomes. J Cardiovasc Med (Hagerstown) 2008;9:59–63. doi: 10.2459/JCM.0b013e328028fe4e. [DOI] [PubMed] [Google Scholar]
- 32.Pohl U, Busse R. Endothelium-dependent modulation of vascular tone and platelet function. Eur Heart J. 1990;11:35–42. doi: 10.1093/eurheartj/11.suppl_b.35. [DOI] [PubMed] [Google Scholar]
- 33.Parent R, Paré R, Lavallée M. Contribution of nitric oxide to dilation of resistance coronary vessels in conscious dogs. Am J Physiol. 1992;262:10–16. doi: 10.1152/ajpheart.1992.262.1.H10. [DOI] [PubMed] [Google Scholar]
- 34.Farquharson CA, Butler R, Hill A , et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]


