Abstract
Background
Right ventricular dysfunction has been observed in uremic patients receiving percutaneous transluminal angioplasty (PTA). This prospective study focuses on the impact of tissue Doppler imaging echocardiographic parameters on assessing right ventricle function in uremic patients post PTA of dysfunctional hemodialysis access.
Methods
Sixty uremic patients were divided into two groups by angiographic findings: an occlusive group (26 patients) and a stenotic group (34 patients). All uremic patients underwent routine echocardiography with tissue Doppler imaging both before and immediately following PTA to assess the right ventricular (RV) function and pulmonary artery systolic pressure (PASP). The right ventricular (RV) myocardial performance index (MPI) was obtained during tissue Doppler imaging over the lateral tricuspid annulus. The M index was measured and defined as the peak early diastolic mitral inflow velocity divided by the RV MPI. The RV MPI, RV isovolumic relaxation time (IVRT) and M-index were used to evaluate RV function post-PTA.
Results
Immediately following PTA, PASP (31.6 ± 11.3 mmHg versus 42.6 ± 12.0 mmHg, p = 0.001), RV MPI (0.46 ± 0.08 versus 0.62 ± 0.13, p < 0.001) and IVRT (75.1 ± 12.9 versus 98.4 ± 27.7 ms, p < 0.001) increased significantly in the occlusive group. However, PASP and RV function did not change significantly in the stenotic group. In 42.3% patients from the occlusive group, the M-index fell below 112 and RV MPI rose above 0.55 post-PTA; this occurred in only 8.8% of the stenotic group.
Conclusions
This prospective study demonstrated that there was a higher incidence of RV dysfunction in uremic patients with elevated PASP with totally occluded hemodialysis access than those with stenotic access post-PTA.
Keywords: Myocardial performance index, Percutaneous transluminal angioplasty, Pulmonary hypertension, Tissue Doppler image, Uremic
INTRODUCTION
Several short-term or long-term complications may occur in uremic patients.1-6 Percutaneous transluminal angioplasty (PTA) is one of the most common methods used to manage aterio-venous graft or shunt failure in uremic patients.7 Right ventricular dysfunction related to pulmonary embolism has been observed in uremic patients receiving PTA of dysfunctional hemodialysis access as noted by pulmonary perfusion scintigraphy after the procedure.8-10 The advances in echocardiography have made it a useful tool for detecting right-ventricular (RV) function in specific patients.11,12 This prospective study was to evaluate the RV function immediately after PTA of dysfunctional hemodialysis access.
MATERIALS AND METHODS
Study population
Sixty-eight consecutive uremic patients with dysfunctional hemodialysis access, defined as an absence of thrill on physical examination or flow rate less than 250 ml/min on hemodialysis, underwent PTA at our cardiovascular center. Eight patients were excluded: three because of atrial fibrillation and another five due to failed procedure. Therefore, a total of sixty uremic patients participated in this study. The uremic patients were further divided into two groups according to angiographic findings. The occlusive group (26 patients, 14 of whom had grafts and 12 fistulae) had totally occluded hemodialysis access; the stenotic group (34 patients, 18 of whom had grafts and 16 fistulae) had partially occluded access. The Human Research Committee of our hospital approved the study protocol and written informed consent was obtained from all patients.
Percutaneous transluminal angioplasty procedure
PTA was performed with either 6- or 7-French vascular sheaths. Balloon catheters ranged from 4 to 9 mm in diameter and 20 to 40 mm in length, by angiographic findings. All uremic patients were pretreated with heparin sodium 5000 units intravenously. We used a 0.035″ hydrophilic guide wire (Radifocus, Terumo Corp., Japan) to cross the lesions with subsequent balloon angioplasty. All angiography was done with the contrast medium Omnipaque (350 mg I/ml, GE Health care, Ireland). PTA success was defined as complete reperfusion of the dysfunctional site, with contrast able to drain immediately into the central vein.
Measurement of thrombi
Angiography following the PTA procedures ascertained the presence of thrombi (Figure 1) in the occlusive group. The volume of a regular-shaped (cylindrical) thrombus was calculated by multiplying the cross-sectional area of the base by the length. The volume of a thrombus varying significantly in diameter was calculated segment by segment to avoid bias (Figure 2). The volume of a conical thrombus was calculated by multiplying the cross-sectional area of its base by its length and then dividing by three. Angiographic measurements were analyzed by two independent experienced observers blinded to the patients’ clinical and echocardiographic data.
Figure 1.
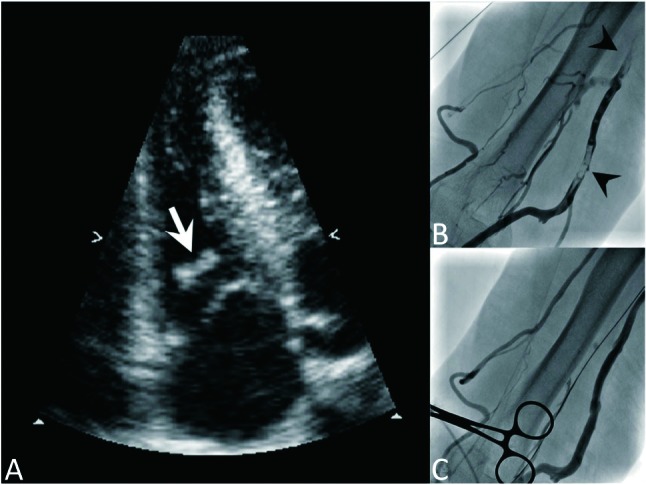
(A) Thrombus in RV papillary muscle after PTA. This is a 26-year-old male, whose hemodialysis access was total occluded with collateral vessels (B). A thrombus (white arrow) was found at the RV papillary muscle after PTA. The thrombus (arrow heads, B), had vanished on the angiogram following PTA (C). The collateral vessels became less prominent after restoration of venous flow. PTA, percutaneous transluminal angioplasty; RV, right ventricular.
Figure 2.
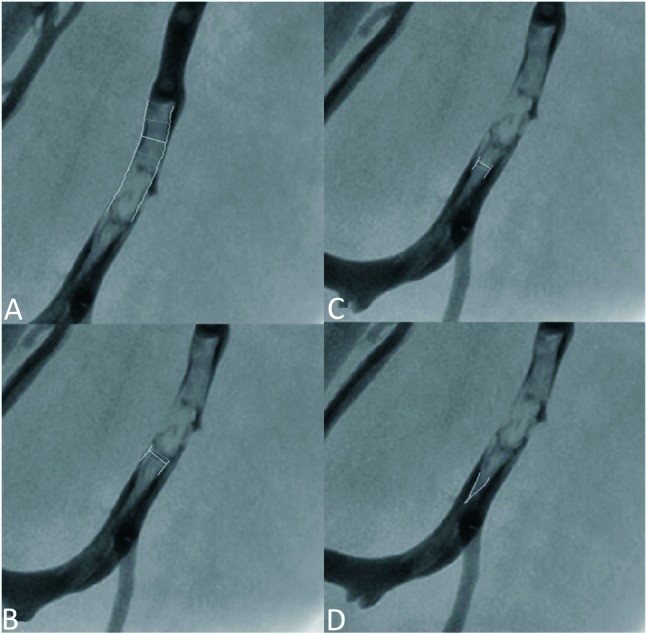
Measurement of volume of thrombi. Dislodged thrombi were defined as disappeared thrombi angiographically after procedure. Residual thrombus in situ was not taken into calculation. The cross section area and length of thrombus was measured by quantitative coronary analysis (QCA) method (total cardiology solution, TCS 2.02, Medcon, Israel). We assumed a cylindrical thrombus. The volume of thrombus was obtained by multiplying the cross-sectional area with the lesion length segment by segment to prevent measure bias (A-C). When the thrombus was conical (D), we ascertained its volume by multiplying the cross-sectional area of its base with its length and then dividing by 3.
Standard echocardiography examination
Echocardiography was performed with tissue Doppler imaging before and immediately after PTA, using a commercial echocardiography machine (SONOS 7500 imaging system, Philips Medical Systems, Andover, Massachusetts, USA) equipped with a S3 transducer. Standard apical 4, apical 5-chamber, and parasternal long-axis with short-axis view were used. Doppler images were obtained after expiration with patients holding their breath for 10 seconds. The mitral E velocity was acquired at apical four chamber view, between mitral leaflet tips during diastole. All data were recorded on VHS videotape and stored on magnetic optical discs. Two independent experienced observers blinded to the patients’ clinical data analyzed all echocardiography images, and all measurements followed recommendations of the American Society of Echocardiography. All echocardiography variables with tissue Doppler image parameters represent an average of 3 beats.13
Both Simpson’s method and the modified Simpson’s method were used to estimate left ventricular (LV) and right ventricular (RV) ejection fraction (EF).14 Using M-mode image, the tricuspid annular plane systolic excursion was measured in an apical 4-chamber view.15
Estimated pulmonary artery systolic pressure (PASP)
For PASP evaluation, a systemic search was undertaken to identify the most complete tricuspid regurgitant (TR) jet followed by continuous-wave Doppler acquisition of spectral envelopes of the greatest velocity and density, and calculated systolic transtricuspid pressure gradient with the modified Bernoulli equation: p = 4 × (V2). Right-atrial pressure was estimated using the inferior vena caval-respiratory index from long-axis subxiphoid views.16 Estimated PASP was calculated as the sum of the transtricuspid gradient and estimated right-atrial pressure.
Pulsed wave tissue Doppler imaging and calculation index
Pulse-wave tissue Doppler images were obtained using spectral pulse Doppler-signal filters, adjusting the Nyquist limit to 15 to 20 cm/s (approximately equal to myocardial velocities) with minimal optimal gain. The myocardial performance index was obtained from tissue Doppler with a 3-mm pulse-wave Doppler sample volume placed at the level of the lateral side of both tricuspid and mitral annuli, not TR jet and RV outflow tract flow. In patients with pulmonary hypertension, it may not be easy to gain satisfactory mitral signal. The mitral signal was not satisfactory in one of our patient. However, the patient was excluded later due to atrial fibrillation. The rest of our images showed satisfactory result. To minimize the incidence angle between Doppler beam and longitudinal wall motion, optimal apical views were taken. The pulse-wave tissue Doppler images were characterized by a myocardial systolic wave and two diastolic waves: early diastolic and atrial contraction. Isovolumic-contraction time, isovolumic-relaxation time (IVRT), and ejection time were measured, and myocardial performance index (MPI) was defined as the sum of isovolumic-contraction time and IVRT divided by ejection time.17-19 M-index was defined as mitral E velocity divided by RV MPI.
Statistical analysis
The analyses were performed using software (SPSS Inc., SPSS 18.0 for Windows, Chicago, Illinois, USA). Categorical data were presented as absolute values and percentages. Continuous variables were expressed as mean values ± standard deviation. Chi-squared tests were used for comparison of categorical data. Two-tailed student t-tests were performed for comparison of continuous variables. Simple linear-regression analysis was used to examine any relationship between two variables. A p-value of < 0.05 was considered statistically significant. For determination of interobserver variability, mean differences between the measurements of two observers were calculated; percentage variability was derived as the absolute difference between the two sets of measurements, divided by the mean of the two observations. Intraobserver variability was calculated using this method as well.
RESULTS
Basic characteristics of patients
The basic characteristics of the occlusive (n = 26) and stenotic (n = 34) groups are illustrated in Table 1. The baseline characteristics were similar in both groups. Echocardiography and tissue Doppler image parameters before and immediately after PTA in occlusive group are listed in Table 2. Those of the stenotic group are listed in Table 3.
Table 1. The basic characteristics of occlusive and stenotic group .
| Variable | Occlusive group (n = 26) | Stenotic group (n = 34) | p value |
| Age (yrs) | 61.7 ± 12.4 | 61.3 ± 10.0 | 0.92 |
| Men | 11 (42.3%) | 14 (41.2%) | 0.89 |
| Hypertension | 19 (73.1%) | 29 (85.3%) | 0.29 |
| Diabetes mellitus | 10 (38.5%) | 20 (58.8%) | 0.24 |
| Dyslipidemia | 4 (15.4%) | 6 (17.6%) | 0.74 |
| Smoking | 7 (26.9%) | 7 (20.6%) | 0.65 |
| Average PTA times in the past | 1.3 ± 1.5 | 2.9 ± 3.1 | 0.06 |
| Systolic blood pressure (mmHg) | 150 ± 14.9 | 148.0 ± 20.0 | 0.70 |
| Diastolic blood pressure (mmHg) | 76.3 ± 18.6 | 77.7 ± 13.6 | 0.78 |
| Hemodialysis duration (months) | 46.7 ± 43.9 | 68.5 ± 52.9 | 0.18 |
| Contrast volume used (ml) | 111.3 ± 63.1 | 98.6 ± 29.6 | 0.23 |
Values are means ± standard deviations, numbers of patients (percentages). PTA, percutaneous transluminal angioplasty.
Table 2. The echocardiographic and tissue Doppler parameters of occlusive group before and immediately after percutaneous transluminal angioplasty .
| Occlusive group (n = 26) | Before PTA | After PTA | p value |
| Systolic blood pressure (mmHg) | 157.7 ± 27.9 | 152.5 ± 20.5 | 0.21 |
| Diastolic blood pressure (mmHg) | 73.3 ± 15.6 | 70.1 ± 16.3 | 0.23 |
| Heart rare (beats per minutes) | 69.2 ± 15.2 | 77.5 ± 13.8 | 0.09 |
| 2-D Variable | |||
| RVEF (%) | 46.8 ± 4.9 | 44.7 ± 3.4 | 0.18 |
| LVEF (%) | 57.9 ± 8.3 | 57.7 ± 8.0 | 0.93 |
| M-mode variable | |||
| RV TAPSE (cm) | 2.3 ± 0.4 | 2.1 ± 0.3 | 0.08 |
| Doppler variable | |||
| RVOT TVI (m/s) | 19.8 ± 2.5 | 20.3 ± 2.8 | 0.40 |
| LVOT TVI (m/s) | 20.6 ± 3.0 | 20.1 ± 3.8 | 0.86 |
| Mitral E (cm/s) | 73.2 ± 25.0 | 66.8 ± 23.2 | 0.03 |
| Mitral A (cm/s) | 89.2 ± 19.5 | 87.0 ± 16.5 | 0.66 |
| Deceleration time (ms) | 189.9 ± 56.1 | 207.7 ± 63.8 | 0.63 |
| PASP (mmHg) | 31.6 ± 11.3 | 42.6 ± 12.0 | 0.001 |
| Calculation index | |||
| M-index (cm/s) | 156.3 ± 63.1 | 109.7 ± 58.8 | 0.02 |
| RV stroke volume (ml) | 59.5 ± 21.3 | 60.4 ± 19.3 | 0.80 |
| LV stroke volume (ml) | 60.3 ± 19.0 | 60.8 ± 20.3 | 0.92 |
| RV (lateral tricuspid annulus) | |||
| Sm (cm/s) | 11.8 ± 1.4 | 10.7 ± 2.5 | 0.16 |
| Em (cm/s) | 9.0 ± 2.8 | 8.3 ± 3.1 | 0.49 |
| Am (cm/s) | 13.8 ± 3.4 | 15.9 ± 4.6 | 0.57 |
| IVCT (ms) | 63.9 ± 10.3 | 67.9 ± 15.3 | 0.41 |
| IVRT (ms) | 75.1 ± 12.9 | 98.4 ± 27.7 | p < 0.001 |
| MPI | 0.46 ± 0.08 | 0.62 ± 0.13 | p < 0.001 |
Variables area means ± standard deviations.
Am, peak late diastolic myocardial velocity derived by pulsed-wave Doppler tissue; Em, peak early diastolic myocardial velocity derived by pulsed-wave Doppler tissue; IVCT, isovolumic contraction time derived from Doppler tissue imaging; IVRT, isovolumic relaxation time derived from Doppler tissue imaging; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; Mitral A, peak velocity of late diastolic mitral inflow; Mitral E, peak velocity of early diastolic mitral inflow; MPI, myocardial performance index, defined as the sum of isovolumetric contraction time and isovolumetric relaxation time divided by the ejection time obtained by Doppler tissue image; M-index, defined as Mitral E divided by RV MPI; PASP, pulmonary artery systolic pressure; RV, right ventricle; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; Sm, peak systolic myocardial velocity derived by pulsed-wave Doppler tissue; TAPSE, tricuspid annular plane systolic excursion; TVI, time velocity integral.
Table 3. The echocardiographic and tissue Doppler parameters of stenotic group before and immediately after percutaneous transluminal angioplasty .
| Stenotic group (n=34) | Before PTA | After PTA | p value |
| Systolic blood pressure (mmHg) | 149 ± 21.2 | 153 ± 23.0 | 0.46 |
| Diastolic blood pressure (mmHg) | 69.3 ± 13.4 | 72.4 ± 15.2 | 0.31 |
| Heart rate (beats per minutes) | 72.1 ± 12.5 | 73.2 ± 13.6 | 0.67 |
| 2-D variable | |||
| RVEF (%) | 44.1 ± 5.5 | 45.1 ± 5.5 | 0.47 |
| LVEF (%) | 56.9 ± 6.7 | 58.1 ± 6.4 | 0.52 |
| M-mode variable | |||
| RV TAPSE (cm) | 2.1 ± 0.5 | 2.2 ± 0.4 | 0.52 |
| Doppler variable | |||
| RVOT TVI (m/s) | 21.7 ± 3.6 | 21.5 ± 3.6 | 0.40 |
| LVOT TVI (m/s) | 21.2 ± 4.6 | 20.9 ± 5.0 | 0.41 |
| Mitral E (cm/s) | 66.9 ± 15.4 | 66.6 ± 16.1 | 0.77 |
| Mitral A (cm/s) | 94.9 ± 14.7 | 101.5 ± 22.6 | 0.21 |
| Deceleration time (ms) | 217.3 ± 70.6 | 201.5 ± 63.0 | 0.38 |
| PASP (mmHg) | 28.9 ± 9.6 | 30.6 ± 10.6 | 0.50 |
| Calculation Index | |||
| M-index (cm/s) | 149.5 ± 68.2 | 147.8 ± 71.0 | 0.90 |
| RV Stroke Volume (ml) | 63.3 ± 20.1 | 62.7 ± 24.0 | 0.65 |
| LV Stroke Volume (ml) | 63.1 ± 17.4 | 62.9 ± 18.7 | 0.75 |
| RV (lateral tricuspid annulus) | |||
| Sm (cm/s) | 12.7 ± 2.6 | 12.3 ± 2.6 | 0.80 |
| Em (cm/s) | 7.3 ± 2.5 | 7.6 ± 3.0 | 0.80 |
| Am (cm/s) | 15.3 ± 4.3 | 15.5 ± 4.1 | 0.56 |
| IVCT (ms) | 63.5 ± 17.0 | 67.3 ± 18.0 | 0.40 |
| IVRT (ms) | 77.1 ± 33.9 | 80.6 ± 37.0 | 0.69 |
| MPI | 0.50 ± 0.14 | 0.52 ± 0.16 | 0.59 |
Variables area means ± standard deviations. Abbreviations as in Table 2.
Volume of dislodged thrombi, change of PASP
The average dislodged thrombus in the occlusive group was 2.50 ± 2.30 ml, ranging from 0.41 to 8.14 ml. Immediately following PTA, PASP increased significantly in the occlusive group (31.6 ± 11.3 mmHg versus 42.6 ± 12.0 mmHg, p = 0.001). The change in PA pressure varied from 0.75 to 38.31 mmHg, with a mean value and standard deviation as 11.04 ± 7.8 mmHg. In the stenotic group, the post-PTA change in PASP was not significant (28.9 ± 9.6 mmHg versus 30.6 ± 10.6 mmHg, p = 0.5).
All PTA procedures were carried out smoothly, except for one patient from the occlusive group who exhibited chest tightness and dyspnea immediately after the procedure. This was a 72-year-old male with a past history of hypertension and type 2 diabetes and resultant diabetic nephropathy. There was no significant blood pressure change (pre-PTA, 128/76 mmHg; post-PTA 114/72 mmHg). However, the initial SpO2 was 98% with room air, dropping to 90% with nasal cannula 3 L/min after PTA. Follow-up ventilation-perfusion scintigraphy ascertained the presence of pulmonary emboli over bilateral lung field and symptoms improved three days later after treatment with heparin. We followed the patients’ clinical condition one month after procedure. The primary patency rate was 34.6% in the occlusive group and 41.2% in the stenotic group. The patient who suffered from pulmonary emboli after procedure was in stable condition during outpatient department follow-up until now. No patient exhibited cardiopulmonary distress at the one month follow-up.
Change of tissue Doppler image parameters and M-index
In the occlusive group, both RV MPI and RV IVRT increased significantly (0.46 ± 0.08 versus 0.62 ± 0.13, p < 0.001 and 75.1 ± 12.9 versus 98.4 ± 27.7 ms, p < 0.001, respectively, Table 2). The mitral E velocity (73.2 ± 25.0 versus 66.8 ± 23.2, p = 0.03) and M-index (156.3 ± 63.1 versus 109.7 ± 58.8, p = 0.02) decreased significantly. In 11 patients (42.3%) from the occlusive group, the M-index fell below 112 and RV MPI rose above 0.55 simultaneously post-PTA. Of these 11 patients, 6 were grafts and 5 were fistulae.
In the stenotic group, the RV MPI and RV IVRT did not change significantly post-PTA (0.50 ± 0.14 versus 0.52 ± 0.16, p = 0.59 and 77.1 ± 33.9 versus 80.6 ± 37.0 ms, p = 0.69, respectively, Table 3). The M-index also did not change significantly post-PTA (149.5 ± 68.2 versus 147.8 ± 71.0, p = 0.90). There were 3 patients (8.8%) from the stenotic group whose M-Index fell below 112 and RV MPI rose above 0.55 post-PTA. Of these 3 patients, 2 were grafts and 1 was fistula.
Reproducibility
Intraobserver differences of dislodged thrombi were 0.3 ± 3.2%. In the RV and left-ventricular ejection fractions, intraobserver differences were 3.8 ± 2.8% and 4.7 ± 3.4%, respectively. Intraobserver variability and differences, respectively, were 3.2 ± 2.1% and 0.3 ± 0.4% for myocardial systolic wave, 2.8 ± 2.0% and 0.2 ± 0.3% for myocardial early-diastolic wave, 3.9 ± 2.8% and 0.4 ± 0.4% for myocardial late-diastolic wave. Interobserver differences of dislodged thrombi were 0.3 ± 2.6%, and interobserver differences of RV EF and LV EF were 4.2 ± 3.1% and 5.3 ± 4.3%. Interobserver variability and differences, respectively, were 4.2 ± 3.5% and 0.5 ± 0.3% for isovolumic-contraction time, 4.9 ± 3.8% and 0.6 ± 0.5% for IVRT, and 4.6 ± 3.9% and 0.5 ± 0.4% for MPI.
DISCUSSION
Uremic patients receiving long-term hemodialysis often develop concurrent cardiovascular disease.20,21 Therefore, it is clinically important to identify uremic patients prone to developing post-PTA pulmonary hypertension or RV dysfunction in advance. No studies have focused on the post-PTA development of RV dysfunction in different patient populations. By assessing PASP and tissue Doppler parameters, we tried to identify patients prone to having RV dysfunction post-PTA. We chose echocardiography instead of multidetector computed tomography due to concerns about radiation dose and bedside availability. Echocardiography is also more suited to evaluate the RV dysfunction and PASP change.
In the occlusive group, the PASP increased immediately post-PTA; this could be due to increased venous return after successful PTA, or pulmonary emboli. However, after PTA, the time-velocity integral of both RV and LV outflow tracts, together with RV and LV stroke volume, did not differ between our groups (Table 2, Table 3). Mitral E velocity even decreased in the occlusive group after PTA (Table 2). These findings indicate that the venous return did not increase significantly. Parmley et al.22 once showed in dogs that pulmonary emboli prompted increases in PASP. To trace the cause of increase of PASP, we measured dislodged thrombi by angiography (Figure 2) and found that the increase of PASP correlated well with the volume of dislodged thrombi (r = 0.725, p < 0.001, Figure 3). This finding support the idea that the increase in PASP might related to pulmonary emboli.
Figure 3.
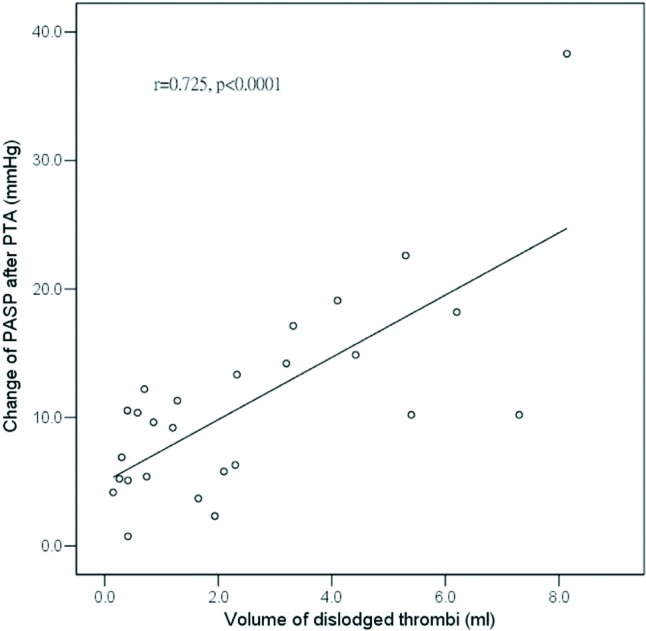
Relations among increases of PASP, and volume of dislodged thrombi. The coefficient of relation (r value) and p value were illustrated within the figure. PASP, pulmonary artery systolic pressure.
In the 11 patients, whose M-index fell below 112 and RV MPI rose above 0.55 simultaneously after procedure, the mean pre-procedure RV MPI was 0.46, ranging from 0.38 to 0.52. There were 3 patients whose RV MPI is greater than 0.5 (0.52 in two patients and the other is 0.51), which is near the cut-off value 0.55. Mean M-index before procedure was 156.3, ranging from 98.8 to 330.4. There was only one patient whose M-index was near cut-off value (125.5) before procedure. Furthermore, there are 3 patients (1 from the occlusive group, and 2 from the stenotic group) who had elevated PASP and high M-index, and low RV MPI value after procedure. This is seen in patients with low RV MPI value which may indicate impaired RV systolic function.
Clinical implications
In this prospective study, we found that uremic patients with totally occluded hemodialysis access are more likely to develop RV dysfunction post-PTA than patients with stenotic access. Furthermore, the increase of PASP correlated well with the volume of dislodged thrombi. Thus, for uremic patients whose hemodialysis accesses are totally occluded with the presentation of loss of thrill on physical examination, we should be more cautious to follow up patient’s condition status post-PTA.
Limitations
There are some limitations to our study. First, our patient population was small. Larger patient populations may disclose further correlations among these variables. Second, tissue Doppler imaging with isovolumic contraction time, IVRT, and MPI could be measured only in patients with sinus rhythm. This is an innate defect of tissue-Doppler imaging. Therefore, selection bias was possible. Third, we did not performed multidetector computed tomography or perfusion/ventilation scintigraphy routinely after procedure, thus, the diagnosis of pulmonary emboli was not sure.
CONCLUSION
This prospective study demonstrated that uremic patients with totally occluded hemodialysis access are more likely to develop RV dysfunction and elevation of PASP post-PTA, than uremic patients with stenotic access. The increase in PASP correlated well with the volume of dislodged thrombi.
Acknowledgments
Supported by the Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, Grant No. VGHKS 101-104, 101-043, 102-054, 102-008, and the National Science Council, Taiwan, NSC 101-2314-13-075B-008.
REFERENCES
- 1.Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy,heart failure,and chronic kidney disease:endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 2.Wu BT, Tsai-Pai MA, Hsu HY, et al. Effect of preload reduction by hemodialysis on left ventricular mechanical parameters by three-dimensional speckle tracking echocardiography. Acta Cardiol Sin. 2012;28:25–33. [Google Scholar]
- 3.Strempska B, Bilinska M, Weyde W, et al. The effect of high-tone external muscle stimulation on symptoms and electro-physio?logical parameters of uremic peripheral neuropathy. Clin Nephrol. 2013;79 Suppl 1:S24–S27. [PubMed] [Google Scholar]
- 4.Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376. doi: 10.1161/CIRCULATIONAHA.112.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreidy R, Stephan E, Salameh P, et al. Value of venous color flow duplex scan as initial screening test for geriatric inpatients with clinically suspected pulmonary embolism. Vasc Health Risk Manag. 2011;7:585–589. doi: 10.2147/VHRM.S23913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao SH, Lin SK, Huang WC, et al. Parameters derived from myocardial tissue Doppler imaging associated with major events in patients with uremia. Acta Cardiol Sin. 2007;23:254–262. [Google Scholar]
- 7.Lai CC, Mar GY, Fang HC, et al. A novel intragraft pressure-guided technique versus angiography-guided technique in salvage of thrombosed hemodialysis graft:a randomized controlled trial. Acta Cardiol Sin. 2012;28:324–331. [Google Scholar]
- 8.Smits HF, Van Rijk PP, Van Isselt JW, et al. Pulmonary embolism after thrombolysis of hemodialysis grafts. J Am Soc Nephrol. 1997;8:1458–1461. doi: 10.1681/ASN.V891458. [DOI] [PubMed] [Google Scholar]
- 9.Swan TL, Smyth SH, Ruffenach SJ, et al. Pulmonary embolism following hemodialysis access thrombolysis/thrombectomy. J Vasc Interv Radiol. 1995;6:683–686. doi: 10.1016/s1051-0443(95)71164-2. [DOI] [PubMed] [Google Scholar]
- 10.Kinney TB, Valji K, Rose SC, et al. Pulmonary embolism from pulse-spray pharmacomechanical thrombolysis of clotted hemodialysis grafts:urokinase versus heparinized saline. J Vasc Interv Radiol. 2000;11:1143–1152. doi: 10.1016/s1051-0443(07)61355-4. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao SH, Lee CY, Chang SM, et al. Pulmonary embolism and right heart function:insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:822–828. doi: 10.1016/j.echo.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao SH, Chang SM, Lee CY, et al. Usefulness of tissue Doppler parameters for identifying pulmonary embolism in patients with signs of pulmonary hypertension. Am J Cardiol. 2006;98:685–690. doi: 10.1016/j.amjcard.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Khoury DS, Thohan V, et al. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–1383. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 14.Miller D, Farah MG, Liner A, et al. The relation between quantitative right ventricular ejection fraction and indices of tricuspid annular motion and myocardial performance. J Am Soc Echocardiogr. 2004;17:443–447. doi: 10.1016/j.echo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Kaul S, Tei C, Hopkins JM, et al. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 16.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir K, Altunkeser BB, Icli A, et al. New parameters in identification of right ventricular myocardial infarction and proximal right coronary artery lesion. Chest. 2003;124:219–226. doi: 10.1378/chest.124.1.219. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz R, Celik S, Baykan M, et al. Pulsed wave tissue Doppler-derived myocardial performance index for the assessment of left ventricular thrombus formation risk after acute myocardial infarction. Am Heart J. 2004;148:1102–1108. doi: 10.1016/j.ahj.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir K, Altunkeser BB, Gok H, et al. Does the myocardial performance index affect pulmonary artery pressure in patients with mitral stenosis? A tissue Doppler imaging study. Echocardiography. 2003;20:249–256. doi: 10.1046/j.1540-8175.2003.03022.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Dev V, Kumar MV, et al. Left ventricular diastolic function in end-stage renal disease and the impact of hemodialysis. Am J Cardiol. 1993;71:1427–1430. doi: 10.1016/0002-9149(93)90604-b. [DOI] [PubMed] [Google Scholar]
- 21.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarctions among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 22.Parmley LF, Jr., North RL, Ott BS. Hemodynamic alternations of acute pulmonary thromboembolism. Circ Res. 1962;11:450–465. doi: 10.1161/01.res.11.3.450. [DOI] [PubMed] [Google Scholar]


