Abstract
There have been numerous studies focusing on the assessment of left ventricular mechanical dyssynchrony. These studies are diverse in their purposes, which include more effectively predicting the response to cardiac resynchronization therapy, improving the guidance of the left ventricular lead position, and better prediction of outcome in patients with heart failure. This article reviews the current assessment methods, clinical applications and limitations of left ventricular dyssynchrony indices derived from echocardiography, magnetic resonance imaging and radionuclide imaging in patients with heart failure.
Keywords: Cardiac resynchronization therapy, Dyssynchrony, Echocardiography, Heart failure
INTRODUCTION
A normal left ventricle contracts synchronously with little more than 40 ms variation at the onset of electrical activation of the left ventricle, with very similar low variation in the timing of mechanical activation throughout the wall. Mechanical dyssynchrony refers to the abnormal prolongation of the timing of contraction or relaxation between the atrium and ventricle (atrioventricular dyssynchrony), between the right ventricle and left ventricle (interventricular dyssynchrony), or between different left ventricular (LV) segments (intraventricular or LV dyssynchrony).1 LV dyssynchrony is frequently observed in patients with heart failure (HF).2 It is often induced by electrical conduction delay in some regions of the left ventricle, which in turn leads to discoordinate LV contraction and reduced cardiac efficiency. The prevalence of LV dyssynchrony depends on the methodology of measurement and characteristics of patients, e.g. the QRS duration, QRS morphology, severity of LV dysfunction, loading condition, and HF etiology.2-4 Numerous studies have suggested that assessment of LV dyssynchrony is useful for the exploration of disease mechanism, selection of treatment, stratification of risk, and prediction of treatment response especially in HF patients who are eligible for cardiac resynchronization therapy (CRT).4-10
ASSESSMENT OF LV DYSSYNCHRONY
Echocardiography
Echocardiographic assessment of LV dyssynchrony has been extensively used because it is noninvasive, widely available, and has no known risk or side effect. Most of the prior assessment modalities used tissue Doppler imaging (TDI, Figure 1A).5,8,11,12 However, more recent studies have employed the use of speckle-tracking echocardiography (STE, Figure 1B) and 3-dimensional (3D) echocardiography.6,13,14 Despite the major advantages of high temporal resolution, wide availability, and ability to assess regional timing of contraction or relaxation, the major limitations of TDI in properly assessing LV dyssynchrony include angle dependency, measurement variability, and an inability to distinguish active contraction from passive motion (Table 1). A multicenter study of CRT, known as the PROSPECT (predictors of response to CRT) study, has shown that 12 echocardiographic (conventional and TDI) dyssynchrony indices did not have enough predictive power to replace routine selection criteria (QRS duration) for CRT.15 The limitations of conventional and TDI echocardiography have led to a search for newer echocardiographic approaches or non-echocardiographic imaging techniques to optimize decision making for CRT.
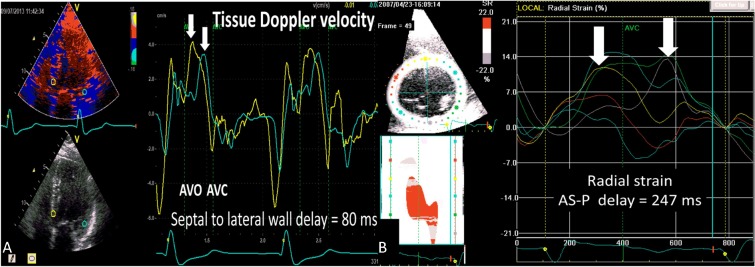
Figure 1.
(A) An example of significant dyssynchrony (> 65 ms) with septal to lateral wall delay of 80 ms by color-coded tissue Doppler velocity. (B) An example of significant dyssynchrony (> 130 ms) with anteroseptal to posterior wall delay of 247 ms by speckle tracking radial strain.
Table 1. Comparison of echocardiographic assessment methods to quantify left ventricular dyssynchrony.
| Method | Dyssynchrony indices | Advantages | Limitations |
| M-mode | Septal to posterior wall motion delay. | Wide availability. Simple method. No specialized software required. | Inability to differentiate passive motion from active contraction. Myocardial infarction or scar involving septum or posterior wall. Measurement variability. |
| Tissue Doppler velocity | Septal-to-lateral wall delay. Maximal delay of time-to-peak velocity. Yu index. | Wide availability. High temporal resolution. | Doppler angle dependency. Inability to differentiate passive motion from active contraction. Measurement variability. |
| Two-dimensional speckle tracking strain | Anteroseptal to posterior delay. SD of time to peak strain. Radial discoordination index. Strain delay index. Septal rebound stretch. | Angle independency. Ability to differentiate passive motion from active contraction. High signal to noise ratio. | Through plane motion. Relatively low frame rate. Analysis affected by image quality. Measurement variability. |
| Three-dimensional echocardiography | Systolic dyssynchrony index. | Angle independency. Simultaneous assessments of volumes and systolic function. | Low temporal resolution. Low spatial resolution. |
| Three-dimensional speckle tracking strain | SD of time-to-peak area strain. SD of time-to-peak minimal endocardial surface area. Area-tracking based strain dyssynchrony index. | Automated analysis. Ability to quantify area strain. Simultaneous assessments of volumes, systolic function, regional strain and torsion. | Low temporal resolution. Limited clinical experience. Need for careful image acquisition. Specialized software required. |
| Cardiac magnetic resonance imaging | Circumferential uniformity ratio estimate. | High spatial resolution. High reproducibility. Ability to quantify scar. | Time-consuming image acquisition. Relatively limited clinical experience. Incompatibility with implanted pacemaker. Specialized software required. |
| Radionuclide imaging | Phase SD. Histogram bandwidth. | Automated analysis. High reproducibility. Ability to assess scar location. | Low temporal and spatial resolution. Limited clinical experience. Limited availability of analysis software. |
SD, standard deviation.
The use of two-dimensional (2D) STE is an established approach to assess LV dyssynchrony. Two-dimensional STE utilizes 2D gray scale images to identify discrete speckle patterns within myocardium, and then tracks the motion of these speckles from frame to frame to quantify myocardial deformation.6,16 Myocardial deformation by 2D STE can provide 3 directions of strain data. Among the 3 deformation patterns, most of the literature favors the use of the radial strain for prediction of response to CRT.13,16,17 A possible explanation may be that radial thickening mirrors circumferential and longitudinal shortening. The measure of LV dyssynchrony by radial strain provides more information in a single assessment than longitudinal strain and circumferential strain could provide alone. Mid-ventricular radial strain with an anteroseptal to posterior wall delay (AS-P delay) ≥ 130 ms is the most commonly-used STE-derived dyssynchrony index.6,16 Similar to the approach used for TDI-derived dyssynchrony indices, the standard deviation (SD) or maximal difference of time to peak strain over multiple segments was attempted, but did not prove superior to the simpler AS-P delay index.16 Compared to the TDI velocity-based indices, 2D STE-derived indices have several advantages, including the ability to differentiate active motion from passive tethering, angle independency, and a higher signal to noise ratio.6 The main limitations of 2D STE are through plane motion and analysis affected by image quality, region of interest, and frame rate (Table 1).
Most of the aforementioned dyssynchrony indices focus on the time delay of mechanical events. The alternative approaches use echocardiography to assess the presence, severity, and/or distribution of discoordinate LV contraction caused by asynchronous electrical activation. The easiest and most available method to assess dyssynchrony-related LV discoordination is to recognize characteristic LV wall motion abnormalities associated with left bundle branch block (LBBB).18-20 Septal flash (Figure 2) and apical transverse motion (Figure 3) are the two recognized LV wall motion features associated with LBBB.18-20 Compared to the qualitative analysis of the two wall motion features, the development of 2D STE enables the quantification of discoordinated LV contraction due to asynchronous electrical activation. The potential dyssynchrony indices derived from 2D STE include the strain delay index, systolic rebound stretch, and radial discoordination index (Figure 4).17,21-28 The strain delay index calculates the sum of the differences between the absolute peak longitudinal strain and the end-systolic strain across 16 LV segments;21,22,29 the systolic rebound stretch quantifies the absolute longitudinal stretching that occurs after the initial myocardial shortening.23-26 Both indices quantify the inefficient deformation in absolute terms, and may represent the gain in myocardial shortening that would occur by resynchronization of the left ventricle. The radial discoordination index expresses the ratio of inefficient deformation to myocardial thickening during the ejection phase.17,27,28 All of the three indices incorporate the information of temporal delay and strain amplitude into one parameter, thereby obviating some of the problems inherent in the time delay indices. Although these new measures are promising, several limitations regarding the mechanisms of measurements still exist. First, these measures are technically challenging and have not been implemented in commercially available analysis software (Table 1). Second, methodologies selectively assessing counteracting strain in the systolic phase are susceptible to errors in the definition of time period and variability in strain amplitude measurement. Finally, clinical experiences with these indices are sparse and further studies regarding the feasibility and reproducibility of results are required.
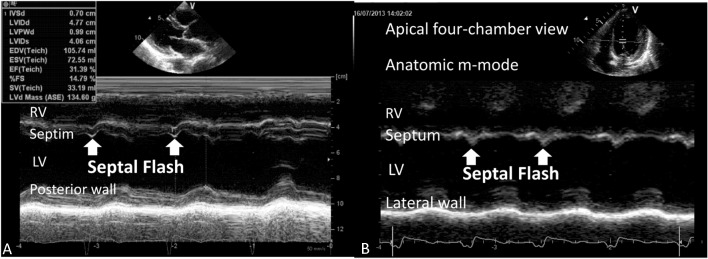
Figure 2.
(A) An example of septal flash which represents early septal systolic thickening and thinning in a patient with heart failure and left bundle branch block.19 (B) Septal flash can also be observed using anatomic M-mode from the apical 4-chamber view.
Figure 3.
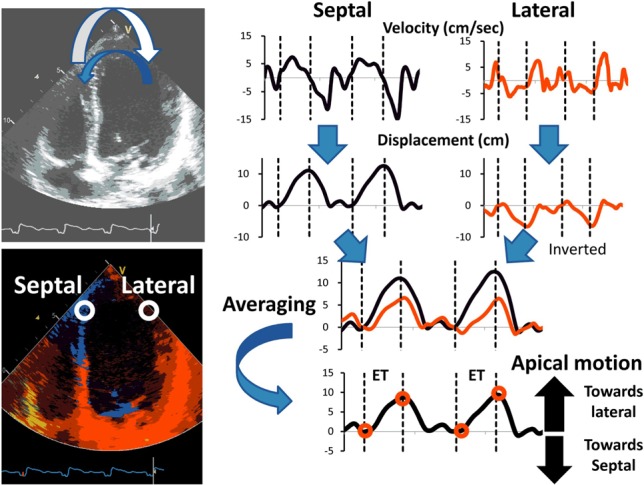
Apical transverse motion is a characteristic apical wall motion abnormality of left bundle branch block with early septal motion during the isovolumic contraction time followed by inverted lateral motion during the ejection phase.20 For calculation of apical transverse motion, velocity traces are integrated to displacement traces. Traces of septal and inversed latercal displacement are averaged to obtain the apical transverse motion during the ejection phase. ET, ejection time.
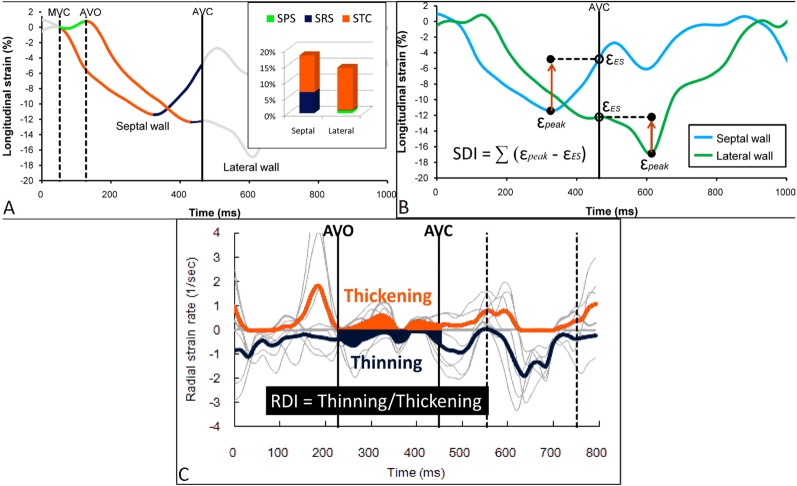
Figure 4.
Measurements of systolic rebound stretch (SRS),26 strain delay index (SDI)21 and radial discoordination index (RDI).28 (A) Within the systolic phase, the strain curve is classified as systolic total shortening (STC, red), systolic pre-stretch (SPS, green) and SRS (blue). SRS is the cumulative amount of systolic stretching after early systolic shortening. (B) SDI is the sum of differences between the peak strain (εpeak) and the end-systolic strain (εES) across 16 myocardial segments. (C) Radial discoordination index is calculated as a ratio of the average myocardial thinning to the average myocardial thickening during the ejection phase.
The assessment of LV dyssynchrony has gradually shifted towards a 3D approach, providing more comprehensive information about LV mechanics as a whole. Three-dimensional echocardiography with regional volumetric analysis is the initial 3D technique to assess LV dyssynchrony.14 The systolic dyssynchrony index (SDI), being the main 3D volumetric dyssynchrony parameter, is defined as the SD of the time to minimum volume of 16 or 17 LV segments, expressed as a percentage of R-R duration.14 The advantages of SDI are 1) angle-independency, 2) ability to assess the whole left ventricle, and 3) simultaneous assessments of LV volumes and systolic function. The main limitations of SDI are low temporal and spatial resolution, and conflicting data on reproducibility and cutoff values (Table 1).
The recent development of 3D STE offers a promising tool for the assessment of LV dyssynchrony based on the analysis of myocardial deformation instead of LV volume. The advantage of 3D STE is that it can provide a quantitative assessment of global and regional LV mechanics, including LV volumes, ejection fraction, global and regional 3D strain, area deformation, torsion, and mechanical dyssynchrony in one rapid and highly automated analysis.30 There are several 3D STE-based parameters for quantifying LV dyssynchrony. These measures include SD of time-to-peak regional systolic strain, SD of time to minimum endocardial surface area, maximal opposing wall delay in time-to-peak radial strain, and area tracking-based strain dyssynchrony index.31 The limitations of 3D STE are the relatively low temporal resolution (20-30 volumes/second), the need for careful image acquisition of a full-volume dataset, and the requirement of specific analysis software (Table 1). In a recent study, 3D STE enabled the derivation of an index of LV global performance that incorporate LV dyssynchrony (SDI), 3D strain, and torsion for the sensitive detection of subtle LV dysfunction in childhood cancer survivors.32 This study demonstrates the potential of any robust index which combines various aspects of global LV mechanics.32
Magnetic resonance imaging (MRI)
Cardiac MRI is a potential alternative to echocardiography for the assessment of LV dyssynchrony. Cardiac MRI has the advantages of high spatial resolution, highly reproducible wall motion tracking and the capability to assess LV scar, volumes, systolic function, velocity, strain, and torsion. Compared to echocardiography, the image acquisition of cardiac MRI is less dependent on the operator. There are 3 major MRI techniques for the assessment of LV dyssynchrony: myocardial tagging, tissue phase velocity mapping, and displacement encoding with stimulated-echo (DENSE) techniques.33-35 The myocardial tagging technique produces a grid-like pattern on the myocardial images and is used to quantify regional myocardial strain or distortion. The tagging technique has the advantage over 2D STE in its ability to measure circumferential strain directly across several cross-sections.34 Tissue phase velocity mapping uses pixel-wise velocity mapping for the assessment of regional velocities in all directions with high spatial resolution covering the whole left ventricle.35 Use of the DENSE technique encodes myocardial displacement directly into the phase of the MRI signal, provides rapid analysis, high spatial resolution, and accurate measures of the circumferential, longitudinal, and radial strain, without the need for tag detection.33 The dyssynchrony indices derived from cardiac MRI are based on the assessment of regional strain heterogeneity by Fourier transform (FT),33,34 or direct quantification of temporal delay of regional contraction.33 The circumferential uniformity ratio estimate (CURE) index is the main FT-based measure for MRI dyssynchrony.33,34 In a recent canine study using myocardial tagging and cine DENSE techniques, automated calculation of the CURE index has shown its superiority over time-to-peak parameters in the prediction of clinical improvement following CRT.33
The 3D nature of cardiac MRI data, which allows for a multi-directional analysis of strain for assessment of LV dyssynchrony, and the ability to assess myocardial viability by late gadolinium enhancement greatly enhance the power of cardiac MRI (Table 1). The limitation of MRI dyssynchrony is that these MRI parameters were investigated based on a small sample size. Clinical experiences with cardiac MRI for assessing LV dyssynchrony are few. To apply these techniques for effective clinical application, we still need to define cutoff values from the derived indices, which have yet to be established.
Radionuclide imaging
Phase analysis of gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has been used for the assessment of LV dyssynchrony by radionuclide imaging.36 Phase analysis is based on the partial volume effect, which indicates that LV regional maximal counts in SPECT MPI images are proportional to the regional wall thickness. Phase analysis approximates the variation of regional maximal counts over the cardiac cycle with the first Fourier harmonic function to measure the onset of mechanical contraction.36 The phase SD and histogram bandwidth of onset of mechanical contraction can be calculated and are the commonly used indices of LV dyssynchrony by radionuclide imaging. Compared to other imaging modalities, phase analysis of SPECT MPI has several advantages such as automated calculation, better reproducibility, and the ability to simultaneously assess myocardial scar location and severity for optimizing CRT (Table 1). The main limitations are the relatively low temporal and spatial resolution when compared with echocardiography and the limited availability of dedicated software for phase analysis, and the fact that clinical applications are limited to a few single center experiences and relatively small sample sizes.
Diastolic dyssynchrony
Systolic dyssynchrony has been extensively studied in patients with HF after the introduction of CRT. It has been shown that systolic dyssynchrony is a relatively common finding in HF patients with reduced ejection fraction (EF) and has better prognostic value than the QRS duration.3,11 The measurement of diastolic dyssynchrony is similar to that of systolic dyssynchrony, in which the parameters are used to calculate the temporal dispersion of myocardial relaxation, as referred to the SD or maximal difference among a certain amount of myocardial segments (Figure 5).4 The systolic and diastolic dyssynchrony indices are significantly correlated, but different in patients with diastolic HF or in patients with end-stage renal disease, indicating they are physiologically related but measure different LV mechanics.4 Echocardiographic TDI velocity is the major modality for the assessment of diastolic dyssynchrony due to its high temporal resolution and obvious signal of early diastole in most patients.4,7 Yu et al. reported that the presence of isolated systolic dyssynchrony, isolated diastolic dyssynchrony, and combined systolic and diastolic dyssynchrony were observed in 25%, 22%, and 14% of 92 patients with diastolic HF, respectively.7 They also reported 102 patients with acute coronary syndrome and demonstrated that these patients complicated by acute HF had significantly increased diastolic dyssynchrony.37 Diastolic dyssynchrony is closely linked to diastolic dysfunction grade and noninvasive estimates of LV filling pressure, suggesting that diastolic dyssynchrony may be a contributing factor for diastolic HF. Wang et al. reported that the presence of systolic and diastolic dyssynchrony was observed in 33% and 58% of 60 hypertensive patients with diastolic HF, respectively.4 Compared to patients without systolic dyssynchrony, those with systolic dyssynchrony had a lower EF, reduced mid-wall fractional shortening, and lower stroke work. On the other hand, diastolic dyssynchrony is associated with a worse degree of diastolic dysfunction as determined by the mean pulmonary wedge pressure and time constant of LV relaxation when compared with those patients without diastolic dyssynchrony. Besides, patients with a higher LV mass have a more advanced degree of diastolic dyssynchrony, while the LV systolic function is preserved. The strong association between diastolic dyssynchrony and LV mass raises the possibility that LV hypertrophy and myocardial interstitial fibrosis may be the causative factors of diastolic dyssynchrony.4 Medical therapies in patients with diastolic HF decrease systemic blood pressure, resulting in a significant improvement of diastolic dyssynchrony, which in turn is linked to the improvement of LV stiffness and reduction of filling pressure.4 These findings suggest that diastolic dyssynchrony may reflect disease severity and treatment response in patients with diastolic HF.
Figure 5.
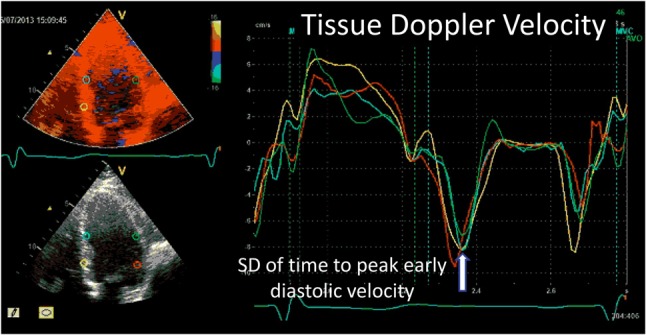
An example of measurement of diastolic dyssynchrony. Diastolic dyssynchrony is evaluated by calculating the standard deviation (SD) or maximal difference of time to peak early diastolic tissue velocity (arrow).
The recent development in phase analysis of gated SPECT MPI to approximate the variation of myocardial wall thickness in diastole enables the assessment of diastolic dyssynchrony by calculating the onset of mechanical relaxation using radionuclide imaging.38 It has been shown that patients with LV diastolic dysfunction were not necessarily afflicted with LV diastolic dyssynchrony, indicating that the indices of diastolic dyssynchrony characterized independent mechanisms of LV diastolic function. Unlike patients with systolic HF, the presence of systolic and diastolic dyssynchrony in patients with diastolic HF may occur as a result of underlying myocardial disease rather than electromechanical delay.
Dynamic dyssynchrony
It has been shown that LV dyssynchrony is not a stable phenomenon. Changes in loading condition, myocardial ischemia, exercise, or drug therapy may significantly alter the presence and degree of LV dyssynchrony.4 Exercise and dobutamine stress echocardiography are often used to unmask LV dyssynchrony. In a study of 81 patients with LV hypertrophy, both systolic and diastolic dyssynchrony during stress were more prevalent and more severe in HF patients with preserved EF than in those without HF, despite a similar severity of resting dyssynchrony in both groups.39 The dynamic increase may contribute to exercise-induced changes in mitral regurgitation, limitation of stroke volume increase during exercise, or symptom of exertional dyspnea. These findings contribute to a better understanding of the pathophysiology of HF with preserved EF, and add further insights into treatment strategies. As LV dyssynchrony could be absent at rest but provoked by stress, evaluation of dynamic dyssynchrony by means of a stress test may help identify high-risk patients. Finally, whether therapies targeting regression of LV hypertrophy would reverse dynamic dyssynchrony and lead to improvement of symptoms and myocardial function reserve is unknown and warrants further study.
CLINICAL APPLICATIONS
Prediction of response and outcome in CRT patients
Although the current guidelines recommend CRT implantation in HF patients with LV EF ≤ 35%, and a QRS duration ≥ 120 ms,40 it remains an ongoing challenge that non-response to CRT is regularly observed in about one-third of patients.15 Given that correction of LV mechanical dyssynchrony has been suggested to be one of the major mechanisms for CRT, its detection should be of clinical importance in identifying patients who will ultimately benefit from CRT. Several single-center studies have shown that LV dyssynchrony evaluated by TDI velocity identifies the CRT responders. However, the value of TDI dyssynchrony in the prediction of CRT response was not supported by the PROSPECT study.15 The PROSPECT study showed that the ability of the 12 conventional and TDI dyssynchrony indices to predict CRT response was suboptimal. The results suggested that measures of LV mechanical dyssynchrony derived from conventional and TDI had limited incremental value in patient selection for CRT.15 A number of studies with newer echocardiographic techniques were conducted in the post-PROSPECT era to test the ability of other potential indices in predicting response to CRT. In 90 patients with CRT, Soliman et al. found that 3D echocardiography-derived SDI > 10% could predict LV reverse remodeling.41 Using 2D STE radial strain, Delgado et al. have shown that the AS-P delay ≥ 130 ms can predict LV reverse remodeling with both sensitivity and specificity more than 80%.16 The Speckle TrAcking and Resynchronization (STAR) study demonstrated that radial and transverse dyssynchrony by 2D STE can predict CRT response and long-term outcome in 132 patients.13 However, in the recent Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) study, HF patients with narrow QRS and LV dyssynchrony by TDI velocity or 2D STE strain did not benefit from CRT.42 Even with the new assessment method of LV dyssychrony (speckle-tracking strain), the study reinforces the notion that QRS width remains the primary determinant of response to CRT.
Measurements of the temporal delay of mechanical events have inherent limitations. Compared to a normal heart, the failing heart with conduction delay is usually characterized by multi-phasic motion or deformation especially at the septum, which renders the definition of onset or peak mechanical events more difficult and increases the measurement variability.24 In addition, numerous studies suggest that patients with LV dyssynchrony may not respond to CRT due to scarred myocardium and lack of residual contractile reserve that can be recruited by CRT.43,44 To overcome these limitations, newer indices derived from echocardiography and non-echocardiographic techniques also consider time delay and myocardial contractility. The strain delay index, systolic rebound stretch, CURE, internal stretch fraction, or radial discoordination index are the potential indices for improved prediction of CRT response by taking the phase and strain amplitude into account rather than by simply quantifying the time delay.17,21,23,26,27,29 Several studies have shown the superiority of these new indices over temporal dyssynchrony in the prediction of LV reverse remodeling and long-term outcome after CRT.17,21-23,25-27,29 It is now known that the presence of LV dyssynchrony is one of the factors which helps determine response to CRT or long-term outcome. A multi-parameter approach considering all known predictors of CRT response, such as ischemic etiology, QRS duration, LBBB morphology, scar burden, and LV dyssynchrony may help us better identify patients who will benefit from CRT.19
Guiding LV lead placement in CRT
It has been hypothesized that CRT will be most effective when the LV lead is placed in the scar-free and latest-activated myocardium, which is supported by the important results of Targeted left ventricular lead placement to guide CRT (TARGET) and Speckle Tracking Assisted Resynchronization Therapy for Electrode Region (STARTER) trials.45,46 Both studies are prospective, double-blind, randomized trials. In the TARGET group, the LV lead was positioned at the latest site of peak contraction with an amplitude > 10% by 2D STE radial strain to signify freedom from scar. The result indicated that TARGET patients had higher rates of clinical response and LV reverse remodeling, as well as a lower rate of combined death and HF-related hospitalization, as compared to the control groups after 6-month follow up. The STARTER trial investigated if the incremental benefit to CRT would be gained by echocardiography-guided transverse LV lead placement. The result indicated that the echocardiography guided group had a significantly more favorable event-free survival over a 1.8-year follow-up period. Both studies have suggested that assessment of LV dyssynchrony could be used to guide the LV lead position and improve the response rate and outcome in CRT patients. In a pilot study of 7 patients with HF and conduction delay, activation imaging with 3D STE has been validated against the invasive 3D electrical voltage mapping systems.47 Good correlations with invasive electrical activation time have been found for basal (r2, 0.56-0.71), mid (r2, 0.53-0.71), and apical (r2, 0.66-0.89) segments. The technique could be used clinically for visualizing the wavefront propagation of mechanical activation similar to the invasive electrical mapping systems.
Outcome prediction in patients with HF
Several studies reported the importance of LV dyssynchrony as the prognostic implication in various heart diseases.8-10,48,49 It should be noted that the presence of LV dyssynchrony at baseline is associated with a better outcome if patients have received CRT. By contrast, the presence of LV dyssynchrony is a poor-outcome predictor in HF patients with narrow QRS duration.48 Bader et al. reported a total of 104 patients with LV EF < 45% followed for one year. Fifty-five percent of the patients had narrow QRS duration (< 120 ms).8 These results indicated that LV dyssynchrony was the most important predictor of HF hospitalization. Cho et al. investigated a total of 106 patients with LV EF < 35% and QRS duration < 120 ms for a mean follow-up of 17 months.48 They concluded that the presence of LV dyssynchrony by TDI velocity was associated with a significant increase in total mortality and adverse clinical events including HF hospitalization or cardiac transplantation. They also found that in 167 admitted HF patients with LV EF < 35%, LV dyssynchrony (opposite wall delay ≥ 65 ms by TDI velocity) or electrical dyssynchrony (QRS duration ≥ 120 ms) was associated with a poor outcome. Those patients with both electrical dyssynchrony and mechanical dyssynchrony had a greater risk of adverse cardiac events when compared with those with normal QRS duration or those without mechanical dyssynchrony.
LV dyssynchrony can be used for outcome prediction after cardiovascular surgery. Penicka et al. investigated 215 consecutive HF patients with ischemic cardiomyopathy undergoing coronary artery bypass graft (CABG).10 The degree of LV dyssynchrony was evaluated by TDI velocity before and 1 month after CABG. The presence of pre-CABG dyssynchrony ≥ 119 ms had the highest predictive accuracy for in-hospital mortality. Using the Cox regression analysis, post-CABG dyssynchrony ≥ 72 ms and ≥ 5 viable segments were identified as independent predictors of clinical events, while QRS duration did not predict cardiac events during the follow up period. Recent studies indicated that LV dyssynchrony by 2D STE longitudinal strain predicted LV arrhythmias in patients with dilated, ischemic, or arrhythmogenic right ventricular cardiomyopathy independent of LV EF or QRS duration.49,50 These studies have all suggested that LV dyssynchrony compares more favorably than the QRS duration for providing important prognostic value in various heart diseases.
CONCLUSIONS
Numerous studies have shown that assessment of LV dyssynchrony is useful for predicting response to CRT, guiding LV lead position and predicting outcome in patients with HF. Despite the complexity of the disease, and the fact that its therapy makes accurate prediction unlikely, assessment of LV dyssynchrony has continued to evolve with new concepts and approaches which will provide additional insight into the disease process and help us to optimize treatment in patients with HF.
REFERENCES
- 1.Gorcsan J, 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the american society of echocardiography dyssynchrony writing group endorsed by the heart rhythm society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Yu CM, Lin H, Zhang Q, et al. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal qrs duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyazaki C, Powell BD, Bruce CJ, et al. Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation. 2008;117:2617–2625. doi: 10.1161/CIRCULATIONAHA.107.733675. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Kurrelmeyer KM, Torre-Amione G, et al. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Suffoletto MS, Dohi K, Cannesson M, et al. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 7.Yu CM, Zhang Q, Yip GW, et al. Diastolic and systolic asynchrony in patients with diastolic heart failure:a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. doi: 10.1016/j.jacc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004;43:248–256. doi: 10.1016/j.jacc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Antoni ML, Boden H, Hoogslag GE, et al. Prevalence of dyssynchrony and relation with long-term outcome in patients after acute myocardial infarction. Am J Cardiol. 2011;108:1689–1696. doi: 10.1016/j.amjcard.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Penicka M, Bartunek J, Lang O, et al. Severe left ventricular dyssynchrony is associated with poor prognosis in patients with moderate systolic heart failure undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2007;50:1315–1323. doi: 10.1016/j.jacc.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 11.Cho GY, Kim HK, Kim YJ, et al. Electrical and mechanical dyssynchrony for prediction of cardiac events in patients with systolic heart failure. Heart. 2010;96:1029–1032. doi: 10.1136/hrt.2009.167585. [DOI] [PubMed] [Google Scholar]
- 12.Wo HT, Chang PC, Chen TH, et al. Cardiac resynchronization therapy in patients with and without atrial fibrillation. Acta Cardiol Sin. 2011;27:46–51. [Google Scholar]
- 13.Tanaka H, Nesser HJ, Buck T, et al. Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the speckle tracking and resynchronization (STAR) study. Eur Heart J. 2010;31:1690–1700. doi: 10.1093/eurheartj/ehq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapetanakis S, Kearney MT, Siva A, et al. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112:992–1000. doi: 10.1161/CIRCULATIONAHA.104.474445. [DOI] [PubMed] [Google Scholar]
- 15.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to crt (prospect) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 16.Delgado V, Ypenburg C, van Bommel RJ, et al. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–1952. doi: 10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Wang CL, Powell BD, Redfield MM, et al. Left ventricular discoordination index measured by speckle tracking strain rate imaging predicts reverse remodelling and survival after cardiac resynchronization therapy. Eur J Heart Fail. 2012;14:517–525. doi: 10.1093/eurjhf/hfs025. [DOI] [PubMed] [Google Scholar]
- 18.Parsai C, Baltabaeva A, Anderson L, et al. Low-dose dobutamine stress echo to quantify the degree of remodelling after cardiac resynchronization therapy. Eur Heart J. 2009;30:950–958. doi: 10.1093/eurheartj/ehp050. [DOI] [PubMed] [Google Scholar]
- 19.Parsai C, Bijnens B, Sutherland GR, et al. Toward understanding response to cardiac resynchronization therapy: left ventricular dyssynchrony is only one of multiple mechanisms. Eur Heart J. 2009;30:940–949. doi: 10.1093/eurheartj/ehn481. [DOI] [PubMed] [Google Scholar]
- 20.Voigt JU, Schneider TM, Korder S, et al. Apical transverse motion as surrogate parameter to determine regional left ventricular function inhomogeneities: a new, integrative approach to left ventricular asynchrony assessment. Eur Heart J. 2009;30:959–968. doi: 10.1093/eurheartj/ehp062. [DOI] [PubMed] [Google Scholar]
- 21.Lim P, Buakhamsri A, Popovic ZB, et al. Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation. 2008;118:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.750190. [DOI] [PubMed] [Google Scholar]
- 22.Lim P, Donal E, Lafitte S, et al. Multicentre study using strain delay index for predicting response to cardiac resynchronization therapy (MUSIC study) Eur J Heart Fail. 2011;13:984–991. doi: 10.1093/eurjhf/hfr073. [DOI] [PubMed] [Google Scholar]
- 23.Chan YH, Wu LS, Kuo CT, et al. Incremental value of inefficient deformation indices for predicting response to cardiac resynchronization therapy. J Am Soc Echocardiogr. 2013;26:307–315. doi: 10.1016/j.echo.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Lumens J, Leenders GE, Cramer MJ, et al. Mechanistic evaluation of echocardiographic dyssynchrony indices: Patient data combined with multiscale computer simulations. Circ Cardiovasc Imaging. 2012;5:491–499. doi: 10.1161/CIRCIMAGING.112.973446. [DOI] [PubMed] [Google Scholar]
- 25.Leenders GE, De Boeck BW, Teske AJ, et al. Septal rebound stretch is a strong predictor of outcome after cardiac resynchronization therapy. J Card Fail. 2012;18:404–412. doi: 10.1016/j.cardfail.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 26.De Boeck BW, Teske AJ, Meine M, et al. Septal rebound stretch reflects the functional substrate to cardiac resynchronization therapy and predicts volumetric and neurohormonal response. Eur J Heart Fail. 2009;11:863–871. doi: 10.1093/eurjhf/hfp107. [DOI] [PubMed] [Google Scholar]
- 27.Chan YH, Kuo CT, Yeh YH, et al. Incremental value of radial discoordination index for the prediction of response to cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging. 2013;14:213–222. doi: 10.1093/ehjci/jes112. [DOI] [PubMed] [Google Scholar]
- 28.Wang CL, Wu CT, Yeh YH, et al. Recoordination rather than resynchronization predicts reverse remodeling after cardiac resynchronization therapy. J Am Soc Echocardiogr. 2010;23:611–620. doi: 10.1016/j.echo.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Kydd AC, Khan FZ, O'Halloran D, et al. Radial strain delay based on segmental timing and strain amplitude predicts left ventricular reverse remodeling and survival after cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2013;6:177–184. doi: 10.1161/CIRCIMAGING.112.000191. [DOI] [PubMed] [Google Scholar]
- 30.Thebault C, Donal E, Bernard A, et al. Real-time three-dimensional speckle tracking echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Eur J Echocardiogr. 2011;12:26–32. doi: 10.1093/ejechocard/jeq095. [DOI] [PubMed] [Google Scholar]
- 31.Tatsumi K, Tanaka H, Matsumoto K, et al. Mechanical left ventricular dyssynchrony in heart failure patients with narrow qrs duration as assessed by three-dimensional speckle area tracking strain. Am J Cardiol. 2011;108:867–872. doi: 10.1016/j.amjcard.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Yu HK, Yu W, Cheuk DK, et al. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr. 2013;26:846–852. doi: 10.1016/j.echo.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Budge LP, Helms AS, Salerno M, et al. Mr cine dense dyssynchrony parameters for the evaluation of heart failure: comparison with myocardial tissue tagging. JACC Cardiovasc Imaging. 2012;5:789–797. doi: 10.1016/j.jcmg.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation. 2005;111:2760–2767. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delfino JG, Bhasin M, Cole R, et al. Comparison of myocardial velocities obtained with magnetic resonance phase velocity mapping and tissue doppler imaging in normal subjects and patients with left ventricular dyssynchrony. J Magn Reson Imaging. 2006;24:304–311. doi: 10.1002/jmri.20641. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Garcia EV, Bax JJ, et al. Spect myocardial perfusion imaging for the assessment of left ventricular mechanical dyssynchrony. J Nucl Cardiol. 2011;18:685–694. doi: 10.1007/s12350-011-9392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AP, Zhang Q, Yip G, et al. Lv mechanical dyssynchrony in heart failure with preserved ejection fraction complicating acute coronary syndrome. JACC Cardiovasc Imaging. 2011;4:348–357. doi: 10.1016/j.jcmg.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Kalogeropoulos AP, Verdes L, et al. Left-ventricular systolic and diastolic dyssynchrony as assessed by multi-harmonic phase analysis of gated spect myocardial perfusion imaging in patients with end-stage renal disease and normal lvef. J Nucl Cardiol. 2011;18:299–308. doi: 10.1007/s12350-010-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AP, Song JK, Yip GW, et al. Importance of dynamic dyssynchrony in the occurrence of hypertensive heart failure with normal ejection fraction. Eur Heart J. 2010;31:2642–2649. doi: 10.1093/eurheartj/ehq248. [DOI] [PubMed] [Google Scholar]
- 40.McMurray JJ, Adamopoulos S, Anker SD, et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 41.Soliman OI, Geleijnse ML, Theuns DA, et al. Usefulness of left ventricular systolic dyssynchrony by real-time three-dimensional echocardiography to predict long-term response to cardiac resynchronization therapy. Am J Cardiol. 2009;103:1586–1591. doi: 10.1016/j.amjcard.2009.01.372. [DOI] [PubMed] [Google Scholar]
- 42.Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013 in press doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 43.Wong JA, Yee R, Stirrat J, et al. Influence of pacing site characteristics on response to cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2013;6:542–550. doi: 10.1161/CIRCIMAGING.111.000146. [DOI] [PubMed] [Google Scholar]
- 44.Ypenburg C, Roes SD, Bleeker GB, et al. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol. 2007;99:657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 45.Khan FZ, Virdee MS, Palmer CR, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the target study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 46.Saba S, Marek J, Schwartzman D, et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circ Heart Fail. 2013;6:427–434. doi: 10.1161/CIRCHEARTFAILURE.112.000078. [DOI] [PubMed] [Google Scholar]
- 47.Seo Y, Yamasaki H, Kawamura R, et al. Left ventricular activation imaging by 3-dimensional speckle-tracking echocardiography. Circ J. 2013 doi: 10.1253/circj.cj-13-0380. [DOI] [PubMed] [Google Scholar]
- 48.Cho GY, Song JK, Park WJ, et al. Mechanical dyssynchrony assessed by tissue doppler imaging is a powerful predictor of mortality in congestive heart failure with normal qrs duration. J Am Coll Cardiol. 2005;46:2237–2243. doi: 10.1016/j.jacc.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 49.Haugaa KH, Goebel B, Dahlslett T, et al. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr. 2012;25:667–673. doi: 10.1016/j.echo.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Haugaa KH, Smedsrud MK, Steen T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging. 2010;3:247–256. doi: 10.1016/j.jcmg.2009.11.012. [DOI] [PubMed] [Google Scholar]