Abstract
Wnt/β-catenin is an important signaling pathways involved in the tumorgenesis, progression and maintenance of cancer stem cells (CSCs). In the present study, the role of Wnt/β-catenin signaling in CSC-mediated tumorigenesis and invasion in liver CSCs was investigated. A small population of cancer stem-like side population (SP) cells (3.6%) from liver cancer samples were identified. The cells were highly resistant to drug treatment due to the enhanced expression of drug efflux pumps, such as ABC subfamily G member 2, multidrug resistance protein 1 and ATP-binding cassette subfamily B member 5. Furthermore, using TOPflash and reverse transcription-quantitative polymerase chain reaction analysis, Wnt/β-catenin signaling and the transcriptional regulation of Wnt/β-catenin target genes including dickkopf Wnt signaling pathway inhibitor 1, axis inhibition protein 2 and cyclin D1 were observed to be markedly upregulated in liver cancer SP cells. As a consequence, SP cells possessed infinite cell proliferation potential and the ability to generating tumor spheres. In addition, upon reducing Wnt/β-catenin signaling, the rates of proliferation, tumor sphere formation and tumor invasion of SP cells were markedly reduced. Therefore, these data suggest that Wnt/β-catenin signaling is a potential therapeutic target to reduce CSC-mediated tumorigenicity and invasion in liver cancer.
Keywords: Wnt/β-catenin, drug resistance, anticancer drugs, cancer stem cells, cyclin D1
Introduction
Numerous previous studies have demonstrated that cancer stem cells (CSCs) are a key obstacle in providing effective treatment and improving survival rate in patients with cancer (1–3). CSCs are a small population within the heterogeneous tumor that escape conventional therapies and are responsible for minimal residual disease (4). Previous studies involving solid tumors have reported that CSCs are potentiators of differentiation and drug resistance, and are highly self-renewal due to the expression of stem cell surface proteins, including CD133, CD44, octamer-binding transcription factor 4 (Oct-4) and Nestin, which contribute to metastasis and tumor invasion (5–8). Therefore, improved characterization of CSCs and the elucidation of additional signaling factors involved in CSC-mediated therapy failure and tumor recurrence are crucial for providing effective cancer treatment.
Hepatocellular carcinoma (HCC) is one of the most common types of cancer, and is associated with a high mortality rate. HCC is frequently diagnosed at the stage of metastases to the lungs, adrenal and lymph nodes, and therefore the overall survival rate of patients is poor following treatment (1,9,10). Previous studies in liver cancer reported that the upregulation of stemness and anti-apoptotic genes, the enhanced expression of dysadherin and the overproduction of chemokines o serve crucial roles in therapy failure, cancer metastases and invasion (2,3,11,12). In addition, point mutations in the p53 gene and the inactivation of Bcl-2 leads to the upregulation of anti-apoptotic pathways in CSCs (13). Furthermore, previous studies in breast, liver and colon cancer (5,6,14) have suggested that the Wnt/β-catenin pathway is involved in the regulation of CSC survival maintenance. Therefore, the current study investigated the Wnt/β-catenin pathway and Wnt/β-catenin targeted gene expression in CSCs from liver cancer samples. In addition, the Wnt/β-catenin pathway was investigated in cancer stem-like side-population (SP) liver cancer cells for its involvement in proliferation, tumorigenicity and invasiveness.
Materials and methods
Sample collection and cell culture
HCC samples were obtained from patients during surgery according to the ethical principle approved by the Department of Hepatopancreatobiliary Surgery, Second Affiliated Hospital, School of Medicine, Zhejiang University (Hangzhou, China). In total, samples were obtained from 30 patients (15 male and 15 female; age, 49–57) undergoing surgery at the Department of Hepatopancreatobiliary Surgery. The collected tumor samples were minced to fine fragments and cultured in 1 ml fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA). For the subsequent culturing, Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) with 10% FBS, supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) was used. Cultures were maintained in T-75 flasks at 37°C in a humidified 5% CO2 and 95% air atmosphere. Once cells were 90% confluent, they were removed from the culture flask using Trypsin-ethylenediaminetetraacetic acid (EDTA EDTA, Sigma-Aldrich) [0.25% 53 mM (EDTA)]. Cells were then washed and suspended in DMEM with 10% FBS and centrifuged at 4,000 × g for 6 min. Cells were resuspended in DMEM with 10% FBS, and cells were counted using a Bulker-Turk hemocytometer (Sunlead Glass Corp., Saitama, Japan).
Fluorescence-activated cell sorting (FACS) analysis
Cells were cultured in DMEM with 10% FBS, supplemented with antibiotics and maintained in T-75 flasks at 37°C in a humidified 5% CO2 and 95% air atmosphere. Once cells were 90% confluent, they were removed from the culture flask using Trypsin-EDTA (0.25% 53 mM EDTA), washed and suspended in DMEM with 10% FBS. A cell count was taken using hemocytometer. The study groups were as follows: Control, cells labeled with Hoechst 33342 dye (Sigma-Aldrich) alone (n=9); and drug-treated cells, treated with verapamil (Sigma-Aldrich) and Hoechst 33342 dye (n=9). Cells were counted using a hemocytometer, and ~106 cells/ml in DMEM with 10% FBS were labeled with Hoechst 33342-bis-benzimide (5 μl/ml) either with dye alone or in combination with drug [ABC transporter inhibitor verapamil (50 μmol/l)]. Further, cells were counter stained with 2 μg/ml propidium iodide (Sigma-Aldrich). The cells were sorted using a FACS Aria II flow cytometer (BD Biosciences, Franklin Lakes, CA, USA) and the sorted cells were cultured and maintained in DMEM/F-12 supplemented with 10% FBS. The Hoechst 33342 emission was first split by using a 610 nm dichroic short-pass filter, and the red and the blue emissions were collected through 670/30 and 450/65 nm band pass filters, respectively.
Cell resistance assay
In total ~1×103 cells/well were cultured in 96-well plates and treated with the chemotherapeutic drugs at the following concentrations: 10 μ/ml 5-fluorouracil (5-FU), 250 mM gemcitabine, 100 mM oxaliplatin, 30 ng/ml paclitaxel, 5 mg/ml cisplatin, 10 mg/ml etoposide and 2 μg/ml oxaliplatin (all from Sigma-Aldrich). The mean value of the optical density (OD) 450 obtained was represented as a graph. Cell resistance in each group was calculated using the following formula: Cell resistance rate (%) = (experimental group OD450 value / control group OD450 value) × 100. The values presented in the graph are the average of three independent experiments. OD values were determined using a spectrophotometer (Multiskan FC Microplate Photometer, Thermo Scientific, Inc.)
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted, and 2 μg RNA was reverse transcribed into cDNA using a Reverse Transcriptase kit (Fermentas; Thermo Fisher Scientific, Inc.). RT-qPCR analysis was subsequently performed on an iCycler IQ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), using IQ Supermix with SYBR-Green (Bio-Rad Laboratories, Inc.). The sequences of the human specific primers used were as follows (5′-3′): CD133, forward TCT TGA CCG ACT GAG AC and reverse ACT TGA TGG ATG CAC CAA GCA C); cyclin D1 (CCND1), forward TGA TGC TGG GCA CTT CAT CTG and reverse TCC AAT CAT CCC GAA TGA GAG TC); Oct-4, forward ATC CTG GGG GTT CTA TTT GG and reverse CTC CAG GTT GCC TCT CAC TC); ATP-binding cassette (ABC) subfamily B member 5 (ABCB5), forward CAC AAG TTG GAC TGA AAG GA and reverse ACC ACT AGG CAT GTC CTT CC); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward GCA CCG TCA AGG CTG AGA AC and reverse TGG TGA AGA CGC CAG TGG A); ABC subfamily G member 2 (ABCG2), forward TCA ATC AAA GTG CTT CTT TTT TATG and reverse TTG TGG AAG AAT CAC GTG GC); multidrug resistance protein 1 (MDR1), forward ACA GGA AGA GAT TGT GAG GG and reverse TAT CCA GAG CTG ACG TGG CT); axis inhibition protein 2 (AXIN2), forward CTG GCT TTG GTG AAC TGT TG and reverse AGT TGC TCA CAG CCA AGA CA); and dickkopf Wnt signaling pathway inhibitor 1 (DKK1), forward AGC ACC TTG GAT GGG TAT TC and reverse CAC AAT CCT GAG GCA CAG TC (15,16). The PCR cycling conditions were as follows: 58°C for 1 min and 96°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 58°C for 30 sec and extension at 72°C for 60 sec. A final extension step was conducted at 72°C for 10 min. GAPDH was used as a housekeeping gene (primers: Forward AATCCCATCACCATCTTCCA and reverse TGGACTCCACGACGTACTCA). The values presented in the quantification graph are the average values of three independent experiments. The 2−ΔΔCq method was used for the quantification of the RT-qPCR results (17).
Luciferase assay
A total of ~106 cells were seeded into 12-well plates and were transfected with 100 ng TOPflash or FOPflash (Upstate Cell Signaling Solutions, Billerica, MA, USA) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), A total of 20 ng p-cytomegalovirus-Renilla luciferase reporter Promega Corporation (Madison, WI, USA) was cotransfected as an internal control. Cell lysates were collected at 24 h post-transfection and the luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega Corporation) according to the manufacturer's instructions.
RNA interference
A small interfering RNA (siRNA) sequence specific to β-catenin (GeneBank accession no. CTNNB1, NM001904), was purchased from Dharmacon (GE Life Sciences, Lafayette, CO, USA). siRNA transfection with a final concentration of 200 nm was conducted using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. A scramble siRNA sequence (5′-TTCTCCGAACGTGTCACGT-3′) was used as a control (Gima Biol Engineering Inc., Shanghai, China). The transfected siRNA cells were analyzed following 48 h of transfection.
Invasion assay
The cellular invasiveness of SP and non-SP cells was investigated using 6-well Matrigel invasion chambers (BD Biosciences). Cells were seeded in DMEM at a density of 2×105/insert. Outer wells were filled with DMEM containing 5% FBS as a chemoattractant and incubated at 37°C for 48 h. Subsequently, the non-invading cells were washed by swabbing the top layer of the Matrigel with a Q-tip. The membrane containing the invading cells was stained with hematoxylin for 3 min, washed and mounted on slides. The entire membrane containing the invading cells was counted using a CX31 light microscope (Olympus, Tokyo, Japan) at 40× objective. The values presented in the graph are the mean value of three independent experiments.
Western blotting
Cell extracts were harvested from the SP and non-SP cells using RIPA buffer (Sigma-Aldrich) containing protease inhibitor cocktail (Roche Diagnostics Deutschland GmbH, Mannheim, Germany) and protein concentration was determined using a Bradford assay (Sigma-Aldrich) (18). Protein lysates (40 μg) from each sample were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Sigma-Aldrich). Separated proteins were transferred to a polyvinylidene difluoride membrane (Sigma-Aldrich). The membranes were treated with the primary antibodies against β-catenin (1:2,400; cat. no. ab6302; Abcam, Shanghai, China) and GAPDH (0.7 μg/ml; cat. no. ab37168, Abcam). Subsequently, the membranes were incubated in the secondary antibodies horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG with alkaline phosphatase markers; cat. no. ab97048, Abcam). The protein was detected using chemiluminescence reagents (Amersham Biosciences). Blots were scanned using a Bio-Rad GS-710 densitometer (Bio-Rad Laboratories, Inc.).
In vitro proliferation activity
The sorted SP and non-SP cells were seeded in a 96-well plate at 2×106 cells/well and then cultured in a CO2 incubator. Each group was analyzed in triplicate. Cell proliferation was measured daily for 7 days. Each well was supplemented with Cell Counting kit-8 solution (10 μl; Dojindo Laboratories, Kumamoto, Japan) and incubated in a CO2 incubator for 2–3 h. The OD was determined at 450 nm. These data were used to calculate cell growth graphs based on the mean value of OD450 and the standard deviation values for each well.
Differentiation assay
Following 16–18 days of cell sorting, cells were cultured in normal RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.). Subsequently, differentiation ability of two subpopulations was determined using an Olympus CX31 microscope.
Immunofluorescence staining
The sorted SP and non-SP cells were fixed onto glass slides in ice-cold 4% formaldehyde (4°C, 10 min), and blocked with blocked with 1% bovine serum albumin (Sigma-Aldrich) for 30 min. Slides were incubated with mouse monoclonal anti-CD133 (1:200; cat. no. ab5558; Abcam), anti-Oct-4 (1:100; cat. no. ab59545; Abcam) and anti-ABCG2 (1:100; cat. no. ab3380; Abcam) for 1 h. Following washing the slides with phosphate-buffered saline, slides were incubated with fluorescein isothiocyante-conjugated chicken anti-mouse IgG (1:200; cat. no. ab112455; Abcam) overnight in dark room. Nuclei were counterstained with 4,6-diamidino-2-phenylindole and viewed using a DMI 4000 B fluorescence microscope (Leica, Wetzlar, Germany). All images were processed using Adobe Photoshop, version CS6 (Adobe System, Inc., San Jose, CA, USA).
Sphere formation assay
The sorted SP cells and non-SP cells were plated at a density of 1,000 cells/ml and suspended in tumor sphere medium consisting of serum-free 1:1 mixture of Ham's F-12/DMEM, N2 supplement, 10 ng/ml human recombinant basic fibroblast growth factor (Sigma-Aldrich) and 10 ng/ml epidermal growth factor(Sigma-Aldrich), and subsequently cultured in ultra-low attachment plates for ~2 weeks. Sorted SP and non-SP cells were seeded at a low density of 20 cells/l and the number of generated spheres (>100 μM) was counted following 7 days of culture. The values presented in the graph are the mean values of three independent experiments.
Tumor cell implantation
NOD SCID mice (n=20; age, 6 weeks) were purchased from the Hubei Provincial Center of Disease Control and Prevention (Wuhan, China). They were kept in individually ventilated cages with access to food and water ad libitum. Mice were kept in a 14/10 h light/dark cycle at 25°C and 55–60%.humidity as approved by Institutional Ethical Committee of the School of Medicine (Zhejiang University, Hangzhou, China). FACS sorted SP and non-SP cells (4×105 cells) were mixed with Matrigel and administered to NOD/SCID mice by subcutaneous injection. The subsequent steps were conducted as described previously (19). At 21 days following implantation, mice were sacrificed by cervical dislocation and tumors were harvested and observed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are represented as the mean ± standard deviation. Statistical differences between the experimental groups were analyzed by Student's t-test, one-way analysis of variance and with post-hoc test where needed. P<0.05 was considered to indicate a statistically significant difference.
Results
Existence of CSCs in liver cancer
The liver cancer samples were assessed for the presence of cancer stem-like SP cells using a FACS-based Hoeschst 33342 dye exclusion method. This identified a small population of SP cells (3.6%; Fig. 1A, gated region) whose presence was significantly reduced to 0.6% (Fig. 1B, gated region) following treatment with verapamil. The function of ABC transporter proteins may be efficiently blocked by verapamil and therefore the SP cell population was significantly reduced.
Figure 1.
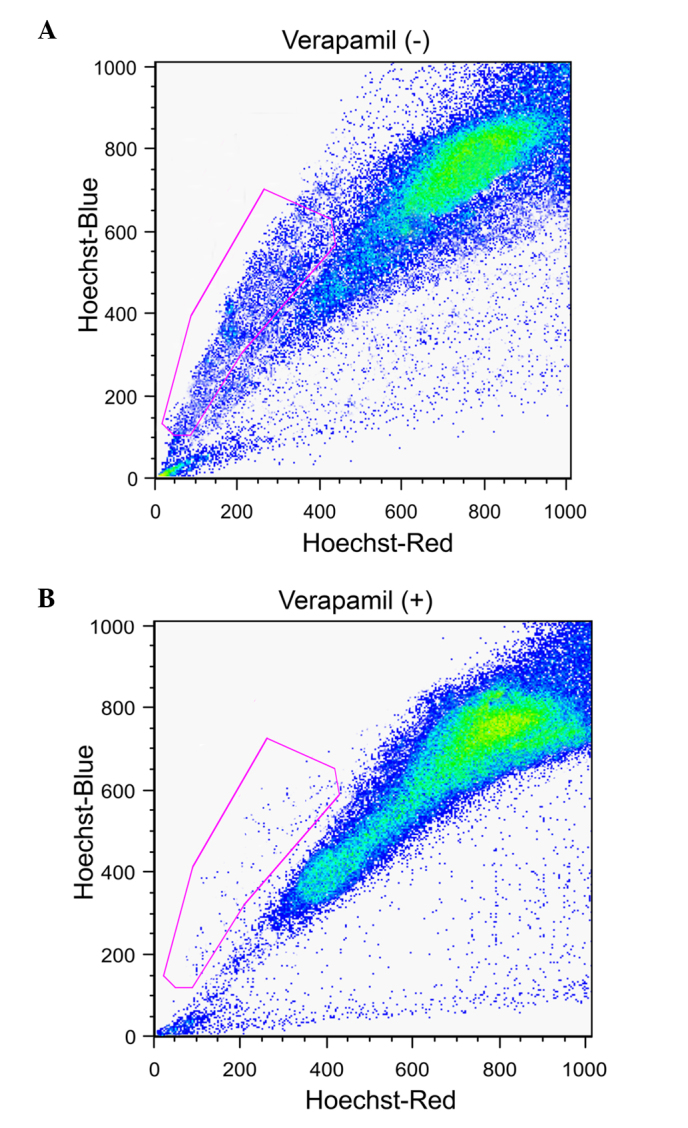
Analysis of cancer stem-like SP cells in liver cancer samples. (A) Cells were stained using Hoechst 33342 dye and analyzed using flow cytometry. Outlined is a gated population of SP cells (3.6%) from the main population of non-SP cells. (B) The prevalence of SP cells was reduced to 0.3% following treatment with verapamil. SP, side population.
Phenotypic characterization of liver cancer SP cells
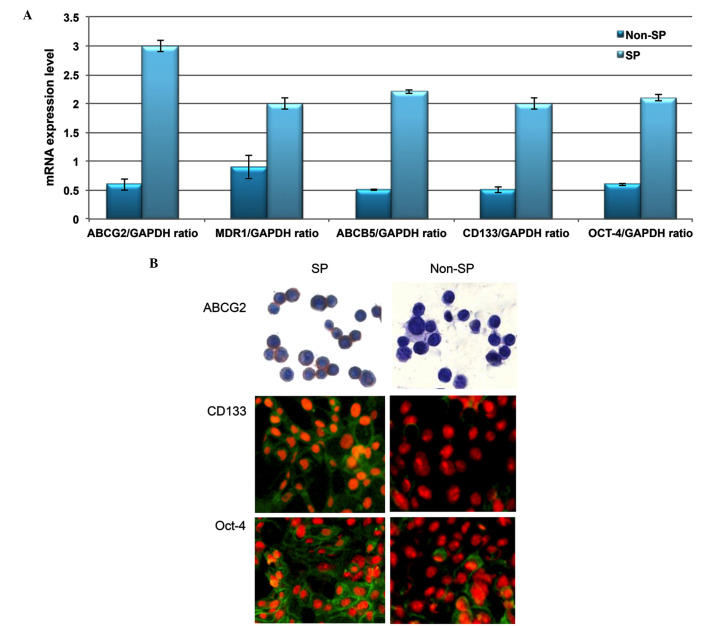
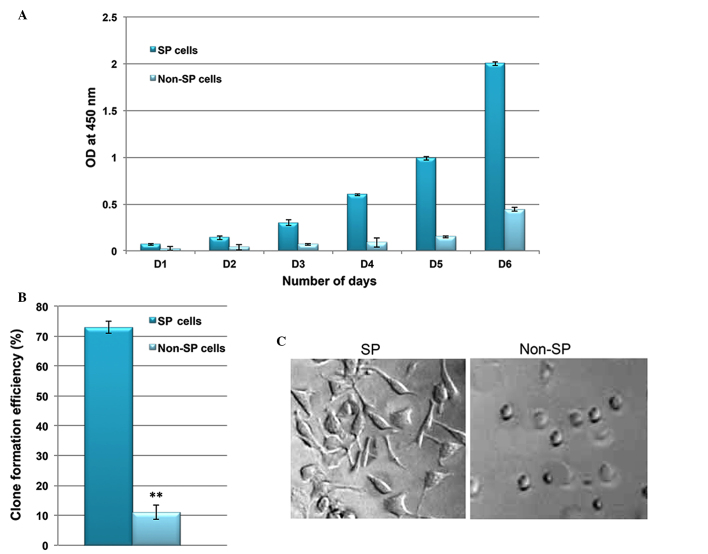
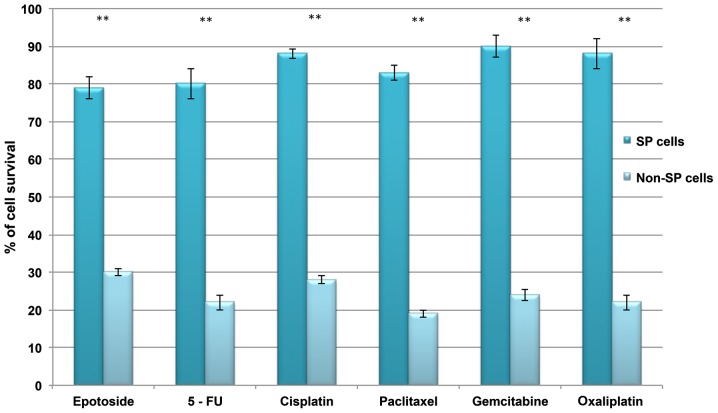
Using RT-qPCR analysis, the transcriptional regulation of drug efflux genes (ABCG2, MDR1 and ABCB5) and stem cell surface genes (CD133 and Oct-4) was investigated. Increased levels of these genes were observed in SP cells compared with non-SP cells (Fig. 2A). In addition, the SP cells exhibited enhanced staining intensity for ABCG2, CD133 and Oct-4 (Fig. 2B). Furthermore, the in vitro proliferation and clone formation efficiency assays indicated that liver cancer SP cells exhibit an enhanced rate of proliferation, with a high potential for generating tumor spheres compared with non-SP cells (Fig. 3A and B). Additionally, SP cells were observed to lose their normal morphological appearance following 5–7 days in culture, SP cells began to form filamentous structures, whereas the non-SP cells did not form these structures (Fig. 3C). The SP cells are able to resist DNA targeting drugs, including 5-FU, gemcitabine, oxaliplatin, paclitaxel, cisplatin, etoposide and oxaliplatin, as indicated by the increased cell survival rate in SP cells (Fig. 4). Together, these data suggest that the presence of a small proportion of SP cells in liver cancer which possess stem cell features may be responsible for chemotherapeutic failure and tumor recurrence.
Figure 2.
Liver cancer SP cells possess stem cell-like properties. (A) Reverse transcription-quantitative polymerase chain reaction analysis indicated the increased mRNA expression of ABC transporter genes and stemness genes in SP cells. Quantification of the data from three independent experiments. GAPDH is used as a housekeeping gene. (B) Representative images of staining in SP and non-SP cells. Magnification, ×100. Values are presented as the mean ± standard deviation. **P<0.01. SP, side-population; ABC, ATP-binding cassette; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ABCG2, ABC subfamily G member 2; MDR1, multidrug resistance protein 1; ABCB5, ABC subfamily B member 5; Oct-4, octamer-binding transcription factor 4.
Figure 3.
SP cells are highly proliferative and have a high potential for differentiation. (A) In vitro proliferation assay indicating that the rate of proliferation of SP cells is significantly greater compared with non-SP cells. (B) Clone formation efficiency of SP cells indicating that SP cells rapidly generate more tumor spheres than non-SP cells. (C) The morphology of SP cells alters following 7 days of culturing, forming filament-like structures. Magnification, ×100. Values are presented as the mean ± standard deviation. **P<0.01.
Figure 4.
Liver cancer SP cells resist chemotherapy. Comparison of cell survival rate between SP and non-SP cells following treatment with DNA targeting drugs 5-FU, gemcitabine, oxaliplatin, paclitaxel, cisplatin, etoposide and oxaliplatin. SP, side-population; 5-FU, 5-fluorouracil. **P<0.01 vs. SP cells.
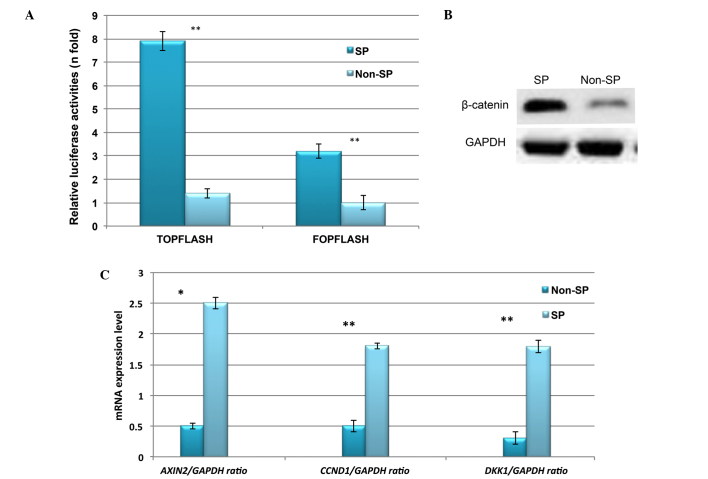
Constitutive expression of Wnt/β-catenin signaling in liver cancer SP cells
Abnormal activation of β-catenin and its downstream signaling targets, such as cyclin D1, have been demonstrated to be involved in the enhanced proliferation and self-renewal of CSCs (15,16). Therefore, the expression profile of Wnt/β-catenin in liver cancer SP cells was investigated. Using TOPflash and FOPflash luciferase reporter assays, the transcriptional regulation of Wnt/β-catenin was observed to be highly upregulated in liver cancer SP cells (Fig. 5A). In addition, the protein expression of β-catenin was increased in SP cells (Fig. 5B). Furthermore, RT-qPCR indicated significantly increased relative mRNA expression of Wnt/β-catenin target genes including CCND1, DKK1 and AXIN2 (Fig. 5C). Together, these results suggest that the abnormal activation of Wnt/β-catenin signaling in SP cells may serve a role in the SP cell phenotype.
Figure 5.
Constitutive expression of Wnt/β-catenin signaling in liver cancer SP cells. (A) TOPflash and FOPflash assay indicating that β-catenin is markedly upregulated in liver cancer SP cells. (B) Western blot image indicating the increased β-catenin protein expression in SP cells. (C) Reverse transcription-quantitative polymerase chain reaction analysis indicating the increased mRNA expression of Wnt/β-catenin target genes in SP cells. SP, side-population; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; AXIN2, axis inhibition protein 2; CCND1, cyclin D1; DKK1, dickkopf Wnt signaling pathway inhibitor 1. *P<0.05 and **P<0.01 vs. non-SP cells.
Knockdown of β-catenin suppresses the SP cell phenotype
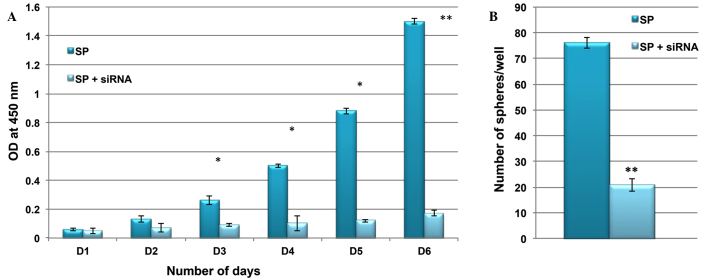
It was then investigated whether the inactivation of Wnt/β-catenin signaling was able to suppress the rapid proliferation and self-renewal of SP cells. Using siRNA technology, β-catenin was inactivated in SP cells, and the rate of cell proliferation and ability to form tumor spheres was compared between control and siRNA SP cells. As presented in Fig. 6, the cell proliferation rate and sphere generation capacity of SP cells were markedly reduced following knockdown of the Wnt/β-catenin signaling pathway. These results indicated that Wnt/β-catenin signaling and its target genes are involved in the tumorigenic properties of CSCs.
Figure 6.
Knockdown of β-catenin suppresses the SP cell phenotype. Following siRNA knockdown of β-catenin, SP cells exhibited reduced (A) cell proliferation and (B) clone formation efficiency. SP, side-population; siRNA, small interfering RNA; OD, optical density. *P<0.05 and **P<0.01 vs. SP.
Knockdown of Wnt/β-catenin in SP cells reduces invasion
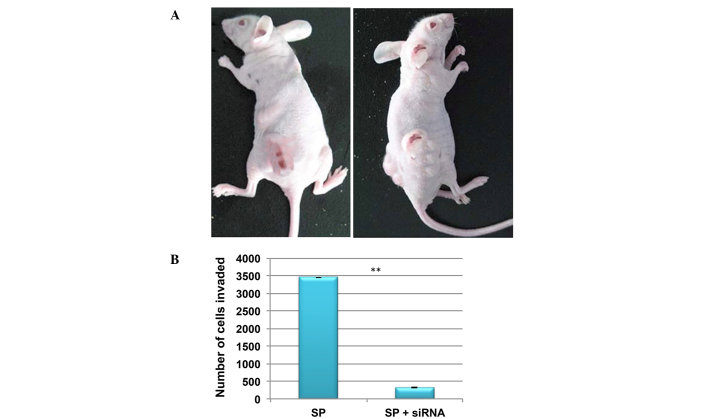
In order to investigate tumorgenicity, a low density (4×105 cells) of SP and non-SP cells were injected into NOD/SCID mice. At this cell concentration, SP cells were able to induce tumor growth in NOD/SCID mice, whereas the non-SP cells were not (Fig. 7A). In addition, the in vitro invasion assay demonstrated that the number of SP cells invading the Matrigel was significantly reduced following the knockdown of β-catenin (Fig. 7B). Therefore, this suggests that Wnt/β-catenin signaling may additionally contribute to CSC-mediated tumor invasion and metastasis.
Figure 7.
Inactivation of Wnt/β-catenin signaling attenuates the invasion of SP cells. (A) SP cells initiate tumor growth in NOD/SCID mice more rapidly in vivo than non-SP cells. (B) siRNA knockdown of β-catenin in SP cells results in reduced invasivity. SP, side-population; siRNA, small interfering RNA. **P<0.01 vs. SP cells.
Discussion
To date, clinical and experimental studies in several solid tumors have revealed that the presence of CSCs is a major obstacle for treatment and the complete eradication of refractory cancer (5,6,20–22). Studies in HCC cell lines have additionally demonstrated the existence of a small subset of cancer stem-like SP cells that are resistant to chemotherapeutic drugs and are highly tumorigenic (20–22). A noteworthy characteristic feature of SP cells is the elevated expression of drug efflux transporter proteins, such as ABCG1 and MDR1, which are actively involved in expelling drugs out of the cell and thus result in therapy failure and tumor recurrence (23–25). Therefore, it is high time to improve the treatment strategies that may efficiently target the tumor cell of origin.
In the current study, liver cancer samples were observed to contain a small population of tumor initiating SP cells which shared the features of CSCs. The liver cancer SP cells were observed to induce the rapid formation of tumor spheres, due to the increased expression of stem cell surface proteins. In addition, increased transcriptional regulation of the levels of drug efflux genes (ABCG2, MDR1 and ABCB5) were observed in the present study. Previously, a crucial role of ABC transports in resistance to chemotherapeutic drugs has been reported (7,8,23). The current study demonstrated that liver cancer SP cells were resistant to numerous DNA targeting drugs including 5-FU, gemcitabine, oxaliplatin, paclitaxel, cisplatin, etoposide and oxaliplatin. This drug resistance property of SP cells is hypothesized to be partly due to the presence of ABC transporters. However, there may be additional signaling factors involved in the tumorgenicity of CSCs.
The Wnt/β-catenin pathway is an important signal transduction pathway, and serves a key role in the tumorigenesis, invasion and metastasis of CSCs (26–28). Increased activation of Wnt/β-catenin signaling has been reported in CSCs, and as a consequence the CSCs possess unlimited cell proliferation and therefore, the Wnt/β-catenin pathway is crucial for the maintenance of self-renewal (15). Notably, the current study suggested that the elevated expression of β-catenin in liver cancer SP cells leads to increased activation Wnt/β-catenin targeted genes including CCND1, DKK1 and AXIN2. This drives the SP cells to proliferate at a higher rate, rapidly form tumor spheres and become highly invasive, as demonstrated by reduction in these phenotypes following the siRNA-mediated knockdown of β-catenin SP cells. Further studies to elucidate the different processes and factors involved in the cross talk between Wnt/β-catenin signaling and CSC-induced tumor relapse may aid in the innovation of novel cancer therapies.
In conclusion, increased Wnt/β-catenin signaling contributes to the ability of SP cells to proliferate, rapidly generate tumor spheres and become highly invasive. Therefore, further investigation of novel anticancer drugs which target and suppress Wnt/β-catenin signaling may aid in the improvement of cancer treatment strategies.
Acknowledgments
The current study was supported by the National Natural Science Foundation of China (grant nos. 81000959 and 81201781), the Guangdong Natural Science Foundation (grant no. S2013010016023), the Science and Technology Planning Project of Guangdong Province, China (grant no. 2009B030801007), the Fundamental Research Funds for the Central Universities (grant no. 12ykpy47) and the National 12th Five-Year Science and Technology Plan Major Projects of China (grant nos. 2012ZX10002017-005, 2012ZX10002016-023 and 2012ZX10002010-001-007).
The authors would like to thank Dr Wanshan Li (Department of Oral and Maxillofacial Surgery, Children's Hospital, Chongqing Medical University), Dr Wei-Dong Han (Department of Oncology, Zhejiang University) and Dr Nan Jiang (Department of Hepatic Surgery, The Third Affiliated Hospital of Sun Yat-sen University) for their collaboration, sharing of reagents and protocols, and their contribution.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: Mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 5.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct 'side population' of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuring-Buske M, Hogge DE. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34(+)CD38(−) progenitor cells from patients with acute myeloid leukemia. Blood. 2001;97:3882–3889. doi: 10.1182/blood.V97.12.3882. [DOI] [PubMed] [Google Scholar]
- 8.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.V98.4.1166. [DOI] [PubMed] [Google Scholar]
- 9.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 10.Lee TK, Castilho A, Ma S, Ng IO. Liver cancer stem cells: Implications for a new therapeutic target. Liver Int. 2009;29:955–965. doi: 10.1111/j.1478-3231.2009.02040.x. [DOI] [PubMed] [Google Scholar]
- 11.Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA. 2002;99:365–370. doi: 10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam JS, Hirohashi S, Wakefield LM. Dysadherin: A new player in cancer progression. Cancer Lett. 2007;255:161–169. doi: 10.1016/j.canlet.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royds JA, Iacopetta B. p53 and disease: When the guardian angel fails. Cell Death Differ. 2006;13:1017–1026. doi: 10.1038/sj.cdd.4401913. [DOI] [PubMed] [Google Scholar]
- 14.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Teng Y, Wang X, Wang Y, Ma D. Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392:373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: Highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5:e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding 72. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Fu X, Hua Y, Han Y, Lu Y, Wang J. The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells. PLoS One. 2012;7:e33358. doi: 10.1371/journal.pone.0033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 22.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 23.Bunting KD, Zhou S, Lu T, Sorrentino BP. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000;96:902–909. [PubMed] [Google Scholar]
- 24.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 25.Norwood K, Wang RY, Hirschmann-Jax C, Andreeff M, Brenner MK, Goodell MA, Wulf GG. An in vivo propagated human acute myeloid leukemia expressing ABCA3. Leuk Res. 2004;28:295–299. doi: 10.1016/j.leukres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi-Ihara N, Murohashi I, Nara N, Tohda S. Promotion of the self-renewal capacity of human acute leukemia cells by Wnt3A. Anticancer Res. 2008;28(5A):2701–2704. [PubMed] [Google Scholar]
- 27.Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138:338–348. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- 28.Ysebaert L, Chicanne G, Demur C, De Toni F, Prade-Houdellier N, Ruidavets JB, Mansat-De Mas V, Rigal-Huguet F, Laurent G, Payrastre B, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20:1211–1216. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]