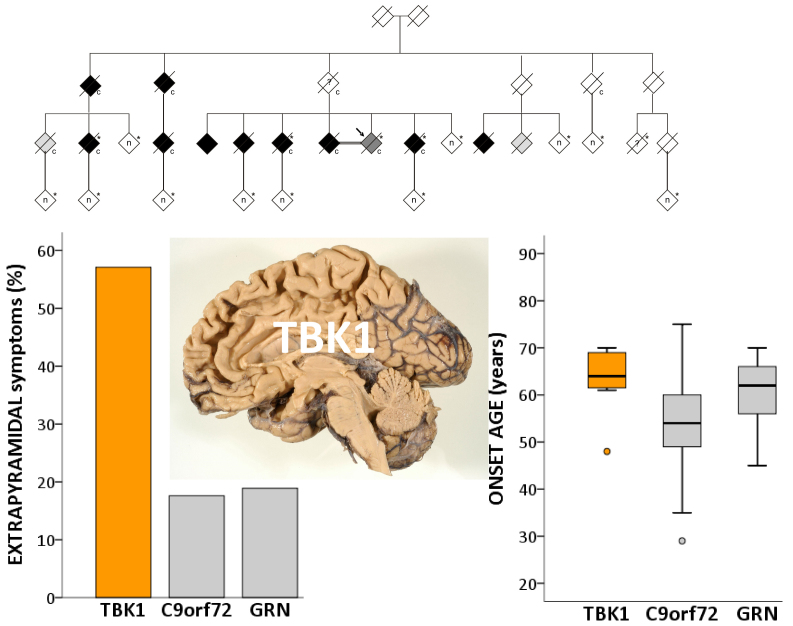
Loss-of-function mutations in TBK1 have been identified in frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Van Mossevelde et al. compare TBK1-mutation carriers with FTD, ALS or FTD-ALS to patients carrying GRN or C9orf72 mutations. Differences are seen in age of onset, extrapyramidal symptoms, and in memory, language and behaviour.
Keywords: TBK1, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, C9orf72, genotype–phenotype correlations
Loss-of-function mutations in TBK1 have been identified in frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Van Mossevelde et al. compare TBK1-mutation carriers with FTD, ALS or FTD-ALS to patients carrying GRN or C9orf72 mutations. Differences are seen in age of onset, extrapyramidal symptoms, and in memory, language and behaviour.
Abstract
We identified in a cohort of patients with frontotemporal dementia (n = 481) or amyotrophic lateral sclerosis (n = 147), 10 index patients carrying a TBK1 loss of function mutation reducing TBK1 expression by 50%. Here, we describe the clinical and pathological characteristics of the 10 index patients and six of their affected relatives carrying a TBK1 mutation. Six TBK1 carriers were diagnosed with frontotemporal dementia, seven with amyotrophic lateral sclerosis, one with both clinical phenotypes and two with dementia unspecified. The mean age at onset of all 16 TBK1 carriers was 62.1 ± 8.9 years (range 41–73) with a mean disease duration of 4.7 ± 4.5 years (range 1–13). TBK1 carriers with amyotrophic lateral sclerosis had shorter disease duration than carriers with frontotemporal dementia. Six of seven TBK1 carriers were diagnosed with the behavioural variant of frontotemporal dementia, presenting predominantly as disinhibition. Memory loss was an important associated symptom in the initial phase of the disease in all but one of the carriers with frontotemporal dementia. Three of the patients with amyotrophic lateral sclerosis exhibited pronounced upper motor neuron symptoms. Overall, neuroimaging displayed widespread atrophy, both symmetric and asymmetric. Brain perfusion single-photon emission computed tomography or fluorodeoxyglucose-positron emission tomography showed asymmetric and predominantly frontotemporal involvement. Neuropathology in two patients demonstrated TDP-43 type B pathology. Further, we compared genotype–phenotype data of TBK1 carriers with frontotemporal dementia (n = 7), with those of frontotemporal dementia patients with a C9orf72 repeat expansion (n = 65) or a GRN mutation (n = 52) and with frontotemporal dementia patients (n = 259) negative for mutations in currently known causal genes. TBK1 carriers with frontotemporal dementia had a later age at onset (63.3 years) than C9orf72 carriers (54.3 years) (P = 0.019). In clear contrast with TBK1 carriers, GRN carriers were more often diagnosed with the language variant than the behavioural variant, and presented in case of the diagnosis of behavioural variant, more often than TBK1 carriers with apathy as the predominant characteristic (P = 0.004). Also, TBK1 carriers exhibited more often extrapyramidal symptoms than C9orf72 carriers (P = 0.038). In conclusion, our study identified clinical differences between the TBK1, C9orf72 and GRN carriers, which allows us to formulate guidelines for genetic diagnosis. After a negative result for C9orf72, patients with both frontotemporal dementia and amyotrophic lateral sclerosis should be tested first for mutations in TBK1. Specifically in frontotemporal dementia patients with early memory difficulties, a relatively late age at onset or extrapyramidal symptoms, screening for TBK1 mutations should be considered.
Introduction
Frontotemporal lobar degeneration (FTLD) is a term covering a heterogeneous group of neurodegenerative disorders with predominant atrophy of the frontal and/or the temporal lobes of the brain. It is the third most common form of dementia after Alzheimer’s disease and vascular dementia, and accounts for 5–10% of all dementia patients (Prince et al., 2014). In individuals younger than 65 years, it is the second most common cause of dementia after Alzheimer’s disease.
FTLD is remarkably heterogeneous in clinical presentation, neuropathological characteristics and underlying genetic causes. Clinically, frontotemporal dementia (FTD) is characterized by a progressive deterioration in behaviour, personality and/or language with relative preservation of memory function. It can be divided in two clinical subtypes: a behavioural variant, and a language variant or primary progressive aphasia (PPA). PPA can be further subdivided into progressive non-fluent aphasia, semantic dementia and logopenic progressive aphasia, although the latter is frequently associated with underlying atypical Alzheimer’s disease pathology (Gorno-Tempini et al., 2011). Pathologically, FTLD is divided in different subclasses according to the major inclusion protein that is found in protein depositions in degenerating neurons: TAR DNA binding protein 43 (TDP-43, encoded by TARDBP; FTLD-TDP), microtubule associated protein tau (MAPT, FTLD-tau), FUS RNA binding protein (FUS, FTLD-FUS) or no inclusions (FTLD-ni) (Mackenzie et al., 2010). Clinical and population-based studies showed that FTD is familial in 30–50% of patients, with an autosomal dominant inheritance in up to 40% (Goldman et al., 2005). About 17–40% of Mendelian inherited forms of FTD are explained by a mutation in GRN, MAPT or C9orf72 and more rarely by a mutation in VCP or CHMP2B (Sieben et al., 2012).
About 15% of patients with FTD also develop amyotrophic lateral sclerosis (ALS), a motor neuron disease clinically characterized by progressive muscle weakness, muscular atrophy, fasciculations and spasticity (Lomen-Hoerth et al., 2002). Also common neuropathological findings and genetic causes suggest that FTD and ALS are two extremes of a continuum of neurodegenerative disorders. Both ALS and FTD have pathological subgroups characterized by inclusions of the TDP-43 or FUS proteins (van Langenhove et al., 2012). Genetically, C9orf72 repeat expansions are the most common cause of FTD and ALS, explaining 25% of familial FTD and 37% of familial ALS patients (Majounie et al., 2012). Up to 88% of familial patients with FTD and ALS (FTD-ALS) are explained by the C9orf72 repeat expansion (Cruts et al., 2013).
Most recently, TANK-binding kinase 1 (TBK1) was identified as a gene for ALS and FTD in which loss of function mutations result in 50% loss of TBK1 protein (Cirulli et al., 2015; Freischmidt et al., 2015; Gijselinck et al., 2015a; Pottier et al., 2015). TBK1 is an important serine/threonine kinase of the IκB kinase family. It phosphorylates a wide range of substrates, including optineurin (OPTN) (Gleason et al., 2011; Weidberg and Elazar, 2011) and p62 (SQSTM1), two proteins involved in autophagy and associated with FTD and ALS (Maruyama et al., 2010; Fecto et al., 2011; Rubino et al., 2012; van der Zee et al., 2014). These findings emphasized once more a major role of defective autophagy in neurodegeneration (Mizushima et al., 2008).
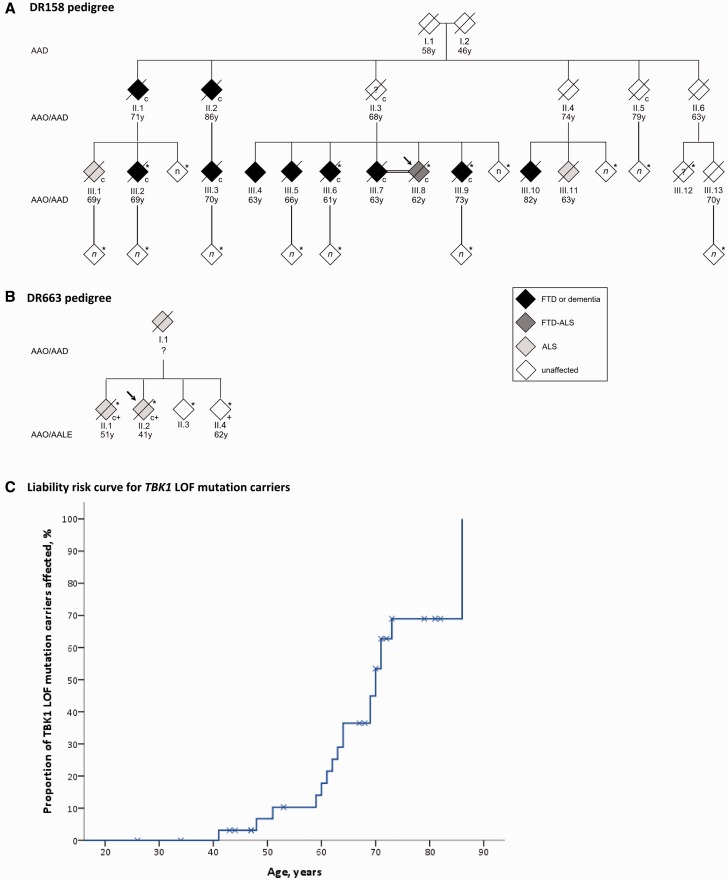
We sequenced the coding sequence of TBK1 in a Belgian cohort of 481 unrelated FTD and FTD-ALS patients, and 147 ALS patients, and identified 10 patients carrying a loss of function mutation. The overall TBK1 loss of function mutation frequency in the Belgian FTD and ALS patient cohort was 1.7%, of which 1.1% in FTD, 4.5% in FTD-ALS and 3.4% in ALS patients. This made TBK1 loss of function mutations the third most frequent cause of FTD and the second most frequent cause of ALS in the Belgian patient cohorts (Gijselinck et al., 2015a). One loss of function mutation, p.Glu643del, segregated in an extended FTD-ALS family, DR158 (Fig. 1A). In two TBK1 carriers, brain and spinal cord was investigated and the pathological diagnosis was TDP type B (Gijselinck et al., 2015a). Here, we describe the clinical characteristics of TBK1 carriers with FTD or ALS. We also compared the genotype–phenotype data of FTD patients with a TBK1 mutation with those of FTD patients with a causal mutation in GRN or C9orf72, two other genes implicated in FTLD TDP-43 (Sieben et al., 2012), and those of 259 patients with FTD without identified mutations. With the current study we aimed to discover specific clinical characteristics which can suggest the presence of a TBK1 mutation. In particular for patients with (a familial history of) both FTD and ALS, specific clinical characteristics could help in a clinically based differentiation with C9orf72 carriers.
Figure 1.
Families segregating the TBK1 mutation p.Glu643del. (A) DR158 pedigree; (B) DR663 pedigree. To protect the privacy of the family members, the gender of each person was masked, the order of sibs was scrambled and the number (n) of tested at-risk individuals shown in white diamonds was not specified. Filled symbols are clinically affected patients with their age at onset (AAO) in years (y) below the symbol. Age at death (AAD) is shown for individuals who died at old age without symptoms. Age at last evaluation (AALE) is shown for currently unaffected mutation carriers that are still alive. An asterisk identifies the family members of whom genomic DNA was available and a ‘c’ identifies the TBK1 p.Glu643del mutation carriers. A ‘+’ sign indicates which DR663 members are carriers of the C9orf72 repeat expansion. The arrow identifies the proband of the family. Only the clinically affected patients known to carry the mutation and of whom clinical records were available, were described in this paper. Other family members pictured as clinically affected were not described because the clinically affected state was only based on oral information obtained from relatives or because their mutation status was not known. (C) Liability risk curve for TBK1 loss of function (LOF) mutation carriers. This curve shows the proportion (%) of TBK1 loss of function mutation carriers (y-axis) that is affected at a certain age (years) (x-axis). The crosses stand for censoring of the patients that are clinically still unaffected at the last time of clinical evaluation or at death. The crosses are placed on the curve at the age of last evaluation or at the age of death.
Materials and methods
Patients and relatives
The FTD, FTD-ALS and ALS patients were part of larger cohorts recruited since 1998 by neurologists of different university and general hospitals nationwide, collaborating in the framework of the Belgian Neurology (BELNEU) consortium. Index patients were evaluated using a standard protocol, which included a detailed clinical and family history, clinical neurological examination, neuropsychological testing, biochemical analyses, and neuroimaging. The diagnosis of possible or probable FTD was made according to the international consensus criteria (Gorno-Tempini et al., 2011; Rascovsky et al., 2011). Patients with a combination of the behavioural and the language variant of FTD at presentation were denoted as mixed FTD. Diagnosis of FTD-ALS was made in patients with FTD who also met the criteria for clinical possible or probable ALS according to the revised El Escorial criteria (Brooks et al., 2000; de Carvalho et al., 2008). Autopsy and neuropathological examinations had been performed for two TBK1 carriers. Additional relatives of mutation carriers for whom detailed neurological records were available were included in the current study. All data records, medical records, neuroimaging studies, and autopsy reports were reviewed, and the demographic, clinical and pathological characteristics of each patient were summarized in a standardized format. Among relatives of Family DR158, segregating the TBK1 p.Glu643del mutation, there were two affected relatives of the index patient with FTD-ALS who were diagnosed with dementia unspecified (Gijselinck et al., 2015a). These patients with unspecified dementia were combined with the group of FTD/FTD-ALS patients in the clinical studies of TBK1. More detailed information on the clinical evaluation and technical investigations can be found in the Supplementary material.
For molecular genetic studies, index patients and their relatives were contacted by trained research nurses. Detailed information on family history of dementia and/or ALS was gathered, and additional patients and unaffected family members were asked to participate in genetic studies. A positive family history was defined as the occurrence of at least one affected relative with dementia or motor neuron disease. Criteria for autosomal dominant inheritance were defined as the occurrence of at least three affected individuals with dementia or motor neuron disease in the pedigree in two or more generations. Written informed consent for participation in the clinical and genetic studies, and for brain autopsy when appropriate, was obtained from participants and/or from their legal guardians. The clinical study protocol and the informed consent forms for patient ascertainment were approved by the local ethics committees of each of the collaborating neurological centres. The genetic and pathological study protocols and informed consent forms were approved by the Ethics committee of the University Hospital of Antwerp and the University of Antwerp, Belgium.
Molecular genetic analysis
The coding region of TBK1 was sequenced using massive parallel sequencing as described in the Supplementary material and in Gijselinck et al. (2015a). All carriers of a repeat expansion mutation in C9orf72 or a loss of function mutation in GRN (Cruts et al., 2006), were included in the current genotype–phenotype correlation study performed in the Belgian FTD and FTD-ALS cohort. C9orf72 repeat expansion testing was performed by repeat-primed PCR, as described (Gijselinck et al., 2012). GRN loss of function mutations were detected using Sanger sequencing or next-generation sequencing. Data of affected relatives of index patients were included if they or their offspring carried the mutation. The genetic and clinical characteristics of the C9orf72 and GRN carriers are summarized in Supplementary Table 1. The mutation-negative FTD patients (n = 259) were analysed for mutations in GRN, C9orf72, MAPT and VCP, as well as for 10 other Mendelian genes associated with other major neurodegenerative brain diseases, i.e. Alzheimer’s disease, prion disease, ALS and Parkinson’s disease (APP, PSEN1, PSEN2, PRNP, TARDBP, FUS, SOD1, LRRK2, PARK2, SNCA). The genetic profiling of the known genes was performed by use of a next-generation sequencing gene panel based on high-level multiplex PCR amplification of targeted exons using the Multiplex Amplification of Specific Targets for Resequencing (MASTR) technology (www.multiplicom.com), followed by sequencing on a MiSeq platform (Illumina). The non-mutation carriers in this paper were also negative for the C9orf72 repeat expansion and TBK1 loss of function mutations.
Statistical analysis
Average onset age and average disease duration were compared between ALS and FTD patients carrying a TBK1 mutation, using a Mann-Whitney U-test because of the small sample group. Comparison of average onset age and average disease duration between carriers of a mutation in one of the three genes (TBK1, C9orf72 or GRN), or non-mutation carriers, was performed using the non-parametric Kruskal Wallis test and Mann-Whitney U-test because onset age and disease duration were not normally distributed in all groups (Shapiro-Wilk test) and because of the small group of TBK1 mutation carriers. Clinical characteristics were compared using a Fisher exact test. All tests were two-tailed and the level of significance was set at P < 0.05.
Results
Demographic characteristics
Demographic, genetic and clinical data of all TBK1 carriers diagnosed with FTD, FTD-ALS or ALS are summarized in Table 1. The mean age at onset of TBK1 carriers was 62.1 ± 8.9 years (range 41–73) with a mean disease duration of 4.7 ± 4.5 years (range 1–13). Three TBK1 carriers also had a C9orf72 repeat expansion: Patient DR1044 and two members of Family DR663 (Fig. 1B and Table 1). When excluding these three double mutation carriers, the mean onset age of TBK1 carriers was 64.5 ± 6.7 years (range 48–73) and the mean disease duration 5.7 ± 4.8 years (range 1–13). Patients with ALS, including the three C9orf72 carriers, had an earlier mean age at onset (58.1 ± 9.4 years) than patients with FTD (65.5 ± 8.0 years) and a shorter mean disease duration (1.7 ± 1.0 years and 7.1 ± 4.8 years, respectively), almost reaching the level of significance (P = 0.054 and P = 0.052, respectively). However, when we excluded the three C9orf72 repeat expansion carriers, the difference in onset age was much smaller (65.5 ± 8.0 years in FTD versus 63.0 ± 4.5 years in ALS) (P = 0.214). The disease duration was still much shorter in ALS patients than FTD patients (1.6 ± 1.3 years and 7.1 ± 4.8 years’ respectively), but not significantly (P = 0.143).
Table 1.
Genetic, demographic and clinical data of TBK1 loss of function mutation carriers
| TBK1 mutation | Patients/relativesa | Gender | Familial history | Age at onset, y | Age at death, y | Disease duration, years | Clinical diagnosis | Diagnosis subtype | |
|---|---|---|---|---|---|---|---|---|---|
| p.Glu643del c.1927_1929delGAA | DR158 | III.1 | Male | F-AD | 69 | 72 | 3 | ALS | Spinal onset |
| III.2 | Male | F-AD | 69 | 75 | 6 | FTD | BvFTD | ||
| III.3 | Male | F-AD | 70 | 73 | 3 | D | |||
| III.6 | Female | F-AD | 61 | 74 | 13 | FTD | BvFTD | ||
| III.8b | Female | F-AD | 62 | 74 | 11 | FTD-ALS | BvFTD, spinal onset | ||
| III.9 | Female | F-AD | 73 | 84 | 11 | D | |||
| DR467 | Female | F | 64 | > 9 | FTD | BvFTD | |||
| DR663 | II.1c | Male | F-AD | 51 | 53 | 2 | ALS | Bulbar onset | |
| II.2b,c | Male | F-AD | 41 | 41 | < 1 | ALS | Bulbar onset | ||
| DR1044c | Male | S | 63 | 66 | 3 | ALS | Unknown | ||
| DR1121 | Male | F | 70 | > 6 | FTD | PPA | |||
| DR1122 | Female | S | 69 | > 7 | FTD | BvFTD | |||
| p.Gly272_Thr331del c.992+1G>T | DR189d | Male | F | 48 | 50 | 2 | FTD | BvFTD | |
| p.Ser398Profs*11 c.1192delT | DR1123 | Male | F | 59 | > 5 | ALS | Bulbar onset | ||
| p.Ser518Leufs*32 c.1551_1552insTT | DR1124d | Female | U | 64 | 64 | < 1 | ALS | Bulbar onset | |
| p.Asp167del c.499_501delGAT | DR1127 | Male | S | 60 | 61 | 1 | ALS | Unknown | |
aRoman numbers indicate affected relatives in Families DR158 and DR663 (Fig. 1).
bPatient III.8 is the proband of Family DR158, Patient II.2 of Family DR663.
cCarriers of a C9orf72 repeat expansion mutation.
dPatients with a pathological diagnosis of TDP-43 proteinopathy.
D = dementia unspecified; F = familial; F-AD = familial-autosomal dominant; S = sporadic.
cDNA is numbering based on NM_013254.3.
A liability risk curve based on onset age or age at last clinical evaluation of TBK1 carriers and relatives (n = 34; 18 affected, 16 unaffected), showed that 50% of the TBK1 carriers were affected by the age of 70 (Fig. 1C). Conversely, four TBK1 carriers were still unaffected at ages of 73, 79, 81 and 82 years. Six carriers were members of the same family, Family DR158, in which the TBK1 mutation, p.Glu643del, co-segregates with disease (Gijselinck et al., 2015a) (Fig. 1A). In Families DR158 and DR663 (Fig. 1B), the segregation pattern was compatible with autosomal dominant inheritance. Further, four index patients had at least one affected relative with dementia or motor neuron disease, but the criteria we used for autosomal dominant inheritance were not met. Three index patients did not have a documented familial history of neurodegenerative diseases, and for one index patient we had no information.
Behavioural, language and cognitive dysfunction
Of the TBK1 carriers, five had behavioural variant FTD, one PPA, one behavioural variant FTD plus ALS and two unspecified dementia. Details on their behavioural, cognitive, and clinical characteristics are shown in Table 2. In the patients with behavioural variant FTD, behavioural disinhibition and social inappropriate behaviour was present in more patients than apathy, especially in the early disease stage. However, some patients such as Patients DR189 and III.2 in Family DR158, presented concomitantly with apathy or passivity as well as elements of disinhibition such as aggressiveness and inappropriate laughing. Aggressive behaviour and motor agitation with runaway behaviour were frequent in TBK1 carriers. Also echolalia and perseverations were quite common in these patients. Visual hallucinations were present in one patient of Family DR158 (Patient III.6).
Table 2.
Detailed behavioural, cognitive and clinical characteristics of TBK1 carriers
| Patients/relativesa | A | BD | C/S | HO | MoA | RM | ED | OA/A | PP | EoS | CD | IR | SP | WR | ML | PA | OD | AP | PS | H | FRS | T | BK/HK | R | S | HR | BS | F | MuA | LP | DPh | DA | DP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DR158 | III.1 | + | + | + | + | + | + | + | + | +* | |||||||||||||||||||||||||
| III.2 | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| III.3 | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||
| III.6 | +* | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||
| III.8b | + | + | +* | + | + | + | + | + | + | + | |||||||||||||||||||||||||
| III.9 | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||||
| DR467 | +* | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||
| DR663 | II.1c | +* | +* | + | + | ||||||||||||||||||||||||||||||
| DR1121 | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||||
| DR1122 | +* | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||
| DR189d | + | +* | + | + | + | + | + | + | +* | ||||||||||||||||||||||||||
| DR1123 | + | + | + | + | + | +* | + | + | + | ||||||||||||||||||||||||||
| DR1124d | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||||
aRoman numbers indicate affected relatives in Families DR158 and DR663 (Fig. 1).
bPatient III.8 is the proband of Family DR158.
cCarrier of a C9orf72 repeat expansion mutation.
dPatients with a pathological diagnosis of TDP-43 proteinopathy.
A = apathy; AP = apraxia; BD = behavioural disinhibition; BK/HK = brady/hypokinesia; BS = Babinski sign; C/S = compulsive or stereotyped; CD = comprehension deficits; DA = dysartria; DP = dyspnoea; DPh = dysphagia; ED = executive dysfunctions; EoS = economy of speech; F = fasciculations; FRS = frontal release signs; H = hallucinations; HO = hyperorality; HR = hyperreflexia; IR = impaired repeating; LP = limb paresis; ML = memory loss; MoA = motor agitation; MuA = muscle atrophy; OA/A = oral apraxia or agrammatism; OD = orientation difficulties; PA = prosopagnosia; PP = phonetic paraphasia; PS = psychiatric symptoms; R = rigidity; RM = repetitive movements; S = spasticity; SP = semantic paraphasia; T = tremor; WR = word retrieval difficulties. Asterisk indicates feature appeared later in the disease.
PPA Patient DR1121 had a clinical picture characterized by an important reduction in spontaneous speech with severe difficulties in word retrieval. No clear comprehension problems were reported in hetero-anamnesis, although he scored 0/3 on the ACE-R (Addenbrooke’s Cognitive Examination-Revised) word comprehension test. He produced some semantic paraphasias. No oral apraxia was present. The patient became more apathetic and passive, and developed severe problems in short-term memory. Five other patients with behavioural variant FTD or patients with unspecified dementia also suffered from word retrieval difficulties. Furthermore, one behavioural variant FTD carrier, Patient DR1122, exhibited impaired repeating of function words as well as an important reduction of spontaneous speech later in the evolution of the disease. This last feature was also present in two patients of Family DR158: the patient with FTD-ALS (Patient III.8) and a patient with unspecified dementia (Patient III.9). This latter patient was also known to have alcoholism. Initially, this patient had especially anterograde memory deficits as well as mild visuoconstructive apraxia. A clinical diagnosis of Korsakoff dementia was considered. During follow-up, the patient developed a more global cognitive deterioration with deficits in short-term memory, executive dysfunctions and behavioural disturbances including apathy and hyperorality. As the patient initially presented with early prominent memory difficulties, the criteria for FTD (Rascovsky et al., 2011) were not met, although the clinical evolution was suggestive of FTD. The alcohol abuse can be considered as an early behavioural change that might have been related to FTD as it only occurred during the 5 years before disease onset. The other patient in Family DR158 with unspecified dementia (Patient III.3) was previously (at the age of 47) diagnosed with multiple sclerosis. The first reported cognitive problems consisted of progressive short-term memory problems, disorientation, apathy and episodes of confusion. These cognitive problems were attributed to further progression of multiple sclerosis and no detailed neuropsychological examination was performed.
Memory and encoding difficulties were remarkably present in TBK1 carriers: seven of eight patients with FTD or unspecified dementia, one patient with ALS and the FTD-ALS patient, had complaints of episodic memory loss relatively early in the disease. Also disorientation in time and/or space was present in five patients. Because of the occurrence of an early amnestic syndrome, one patient, Patient DR1122, received initially a diagnosis of amnestic mild cognitive impairment, and another patient (Patient III.2) in Family DR158, was diagnosed with Alzheimer’s disease. In this last patient, doubt persisted in the differential diagnosis between the frontal variant of Alzheimer’s disease and FTD. However, all patients with FTD also developed important behavioural problems early in the disease.
One patient, Patient DR1124, who was eventually diagnosed with bulbar ALS, presented initially with memory problems and a score of 24/30 on MMSE (Mini-Mental State Examination). The patient also exhibited signs of behavioural disinhibition and impulsivity. However, this patient soon developed a rapidly progressive form of bulbar ALS that overruled the cognitive symptoms and no further diagnostic workup was performed concerning cognition.
Psychiatric symptoms were present in seven TBK1 carriers: six of nine patients with FTD, FTD-ALS or unspecified dementia and one ALS patient. Six of these patients were members of Family DR158. In this family, we observed a very high load of psychiatric diseases (relapsing depressions, alcohol abuse, bipolarity and schizophrenia) in TBK1 carriers as well as non-mutation carriers.
Motor neuron disease and extrapyramidal symptoms
Seven TBK1 carriers had a diagnosis of pure ALS and one a diagnosis of co-occurring ALS and FTD. Four ALS patients had a bulbar onset and two a spinal onset, and of two we had no records or information on the site of onset. We had detailed clinical information of five ALS patients. The index patient of the Family DR158 (Patient III.8) presented with initial symptoms of progressive gait problems, aggressive behaviour and reduced spontaneous speech. Signs of upper motor neuron disease as hyperreflexia and spasticity, especially of the right leg and the left arm, were most prominent at presentation. The re-evaluation 7 years post-diagnosis, in which the patient had not been followed by a neurologist, revealed a totally mute patient, bed-ridden in decorticate posture with gastrostomy tube. Further, Patients DR1123 and DR1124 had most prominent upper motor neuron signs. Patient DR1123 initially presented with bulbar dysarthria, and later, progressive gait disturbances appeared and fine motor skills decreased. One and a half years after disease onset, he developed important respiratory weakness with need for BiPAP (bilevel positive airway pressure) and dysphagia with placement of PEG (percutaneous endoscopic gastrostomy). Discrete muscle atrophy was present. After 3.5 years he had developed quadriparesis with spasticity and hyperreflexia. Patient DR1124 developed severe bulbar speech with important bulbar affect lability, over a period of 8 weeks. Discrete atrophy of the tongue with fasciculations was present. The patient was diagnosed with a rapidly progressive ALS and developed a spastic quadriparesis over a period of only 3 months. In contrast, Patient II.1 of Family DR663 did not present with any pyramidal signs. His first complaints consisted of dysarthria and dysphagia. First, the diagnosis of myasthenia gravis was assumed and a beneficiary effect of pyridostigmine treatment on the dysphagia was noted. During follow-up, the patient developed slight limb muscle atrophy. Patient III.1 of Family DR158 had clear upper motor neuron as well as lower motor neuron signs. The patient presented with gait disturbances, balance problems and extrapyramidal symptoms (poker face, hypokinesia and positional tremor). Subsequently, he rapidly developed limb paresis, fasciculations, muscle atrophy and hyperreflexia.
One patient with FTD (Patient III.6) of Family DR158 displayed hypertonia of the left hemicorpus with Babinski sign on the left side and a central left facial nerve paresis. These clinical symptoms were hypothesized to be related to the pronounced right frontal lobe atrophy on brain MRI. Patient III.3 of Family DR158 had a diagnosis of multiple sclerosis since the age of 47. He exhibited paresis of the lower limbs with spasticity, hyperreflexia and Babinski signs, which were attributed to his diagnosis of secondary progressive multiple sclerosis. Later in the disease he developed paresis and spasticity of the upper limbs as well.
In four patients with FTD and one patient with ALS, extrapyramidal symptoms were reported. In all FTD patients rigidity was present, one also exhibited bradykinesia and another, a resting tremor. The patient with ALS presented with positional tremor and bradykinesia. All patients with extrapyramidal symptoms were carriers of the TBK1 p.Glu643del mutation.
Technical investigation findings
Records of performed structural imaging were available for all TBK1 carriers with a clinical diagnosis of FTD or FTD-ALS, two patients with unspecified dementia and two patients with ALS (Table 3). Various patterns of atrophy were observed on visual inspection. When atrophy was localized, the temporal region was most frequently involved. Asymmetric atrophy was seen in five patients. In four, the right hemisphere was more severely affected than the left hemisphere. On the MRI images in Patient III.3 of Family DR158, demyelinating lesions were present at the semioval centre, in the periventricular regions and infratentorial at the level of the brainstem, compatible with his diagnosis of multiple sclerosis.
Table 3.
Imaging findings in TBK1 carriers
| Patients/relativesa | Structural imaging |
Functional imaging(FDG PET/SPECT) |
|||
|---|---|---|---|---|---|
| Localization atrophy | Symmetry | Localization hypometabolism/hypoperfusion | Symmetry | ||
| DR158 | III.1 | No clear atrophy | |||
| III.2 | H, O > G (incl. C) | R > L | |||
| III.3 | FR | BI | |||
| III.6 | T > G (incl. C) | (T) R > L | |||
| III.8b | T, C | (T) R > L | |||
| III.9 | G | BI | |||
| DR467 | H, T | (T) R > L | |||
| DR663 | II.1c | ||||
| DR1121 | FR, T > G | L > R | FR, T > P | L > R | |
| DR1122 | No clear atrophy | FR, T > P | L > R | ||
| DR189d | T > FR, P | BI | FR, T, P | (FR, P) BI R > L (T) | |
| DR1123 | |||||
| DR1124d | T, H | BI | |||
aRoman numbers indicate affected relatives in Families DR158 and DR663 (Fig. 1).
bPatient III.8 is the proband of Family DR158 .
cCarrier of a C9orf72 repeat expansion mutation.
dPatients with a neuropathological diagnosis of TDP-43 proteinopathy.
BI = bilateral; C = cerebellar; FDG = fluorodeoxyglucose; FR = frontal; G = global; H = hippocampal; L = left; O = occipital; P = parietal; R = right; SPECT = single-photon emission computed tomography; T = temporal.
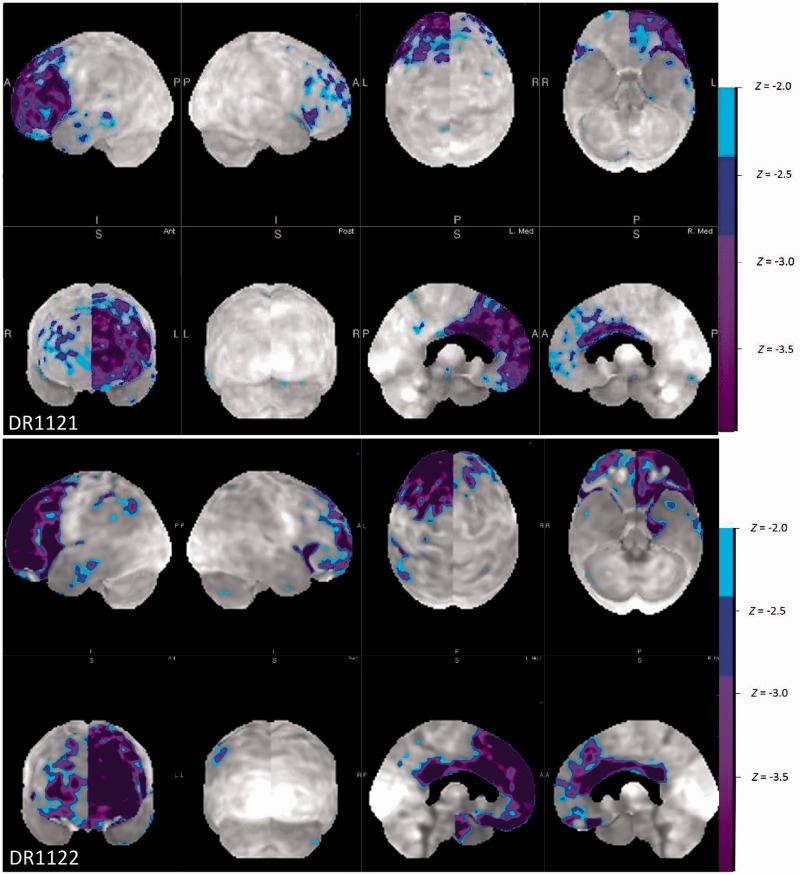
Functional imaging was performed in three TBK1 carriers with a FTD diagnosis, consisting of single-photon emission computed tomography (SPECT) in one patient and PET with 18F-fluorodeoxyglucose (FDG-PET) in two patients. In all, predominant hypoperfusion or glucose hypometabolism was seen in the frontal and temporal regions. The parietal regions were also involved, but to a lesser extent. Also, in all, the pattern of hypoperfusion or glucose hypometabolism was asymmetric. In two patients, the left side was more severely affected and in one patient the right side. FDG-PET images of the two patients with predominant left side involvement (Patients DR1121 and DR1122) are illustrated in Fig. 2. Both patients displayed a similar pattern of frontal, anterior and medial temporal and, to a lesser extent, parietal hypometabolism. However, the pattern was less extended in Patient DR1121, who was clinically diagnosed with PPA. The other patient, Patient DR1122, clinically diagnosed with behavioural variant FTD, also exhibited language difficulties such as word retrieval difficulties, impaired repeating and loss of spontaneous speech. In Patient DR1122, amyloid PET imaging was also performed, which was not compatible with Alzheimer’s disease. Lumbar puncture with evaluation of the levels of β-amyloid, total tau and phospho-tau181P in CSF was performed in three TBK1 carriers with FTD. In all three, the CSF biomarkers profile was not compatible with Alzheimer’s disease. In one, there was an isolated increased total tau.
Figure 2.
Neuroimaging using FDG-PET. These images are z-map renderings of the FDG-PET in Patients DR1121 and DR1122. The z-maps are calculated by comparing the individual glucose metabolic pattern to the normal age-matched control. Both imaging series show a similar pattern of remarkable asymmetry with a severely decreased metabolism in the left frontal, and anterior and medial temporal lobe. These results are much more asymmetric than age could explain, do not follow typical age-related patterns, and are much more intense.
We had detailed information on EMG investigations in five TBK1 carriers with ALS. In three, chronic denervation signs such as giant potentials and reduced interference patterns were reported. In one, active denervation signs and fasciculations were also observed. Remarkably, EMG in Patients DR1123 and DR1124 did not show obvious neurogenic changes, compatible with their clinical presentation of predominantly upper motor neuron signs.
Neuropathology
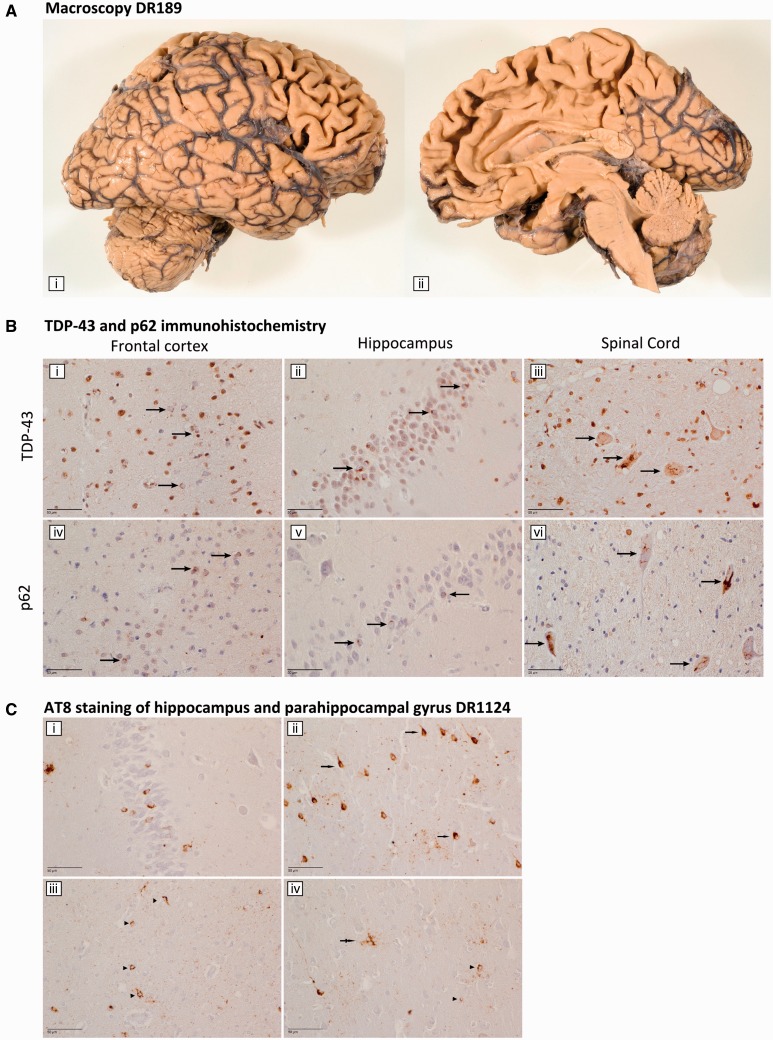
The clinical diagnosis of FTD was autopsy-confirmed in one TBK1 carrier, Patient DR189. Macroscopically, there was a mild atrophy of the frontal cortices and to a lesser extent of the temporal neocortex (Fig. 3A). Furthermore, mild hippocampal atrophy was present. Microscopically, a moderate number of TDP-43 neuronal cytoplasmic inclusions and short dystrophic neurites were detected throughout all layers of the frontal and temporal neocortices, in the hippocampal dentate gyrus, the parahippocampal transentorhinal cortex, the neostriatum and the pallidum [Fig. 3B(i and ii)]. No neuronal intranuclear inclusions were observed. In the internal capsule, a moderate amount of glial cytoplasmic inclusions were present. These lesions stained positive for p62 [Fig. 3B(iv and v)], and to a lesser extent for ubiquitin. These findings were compatible with FTLD TDP type B proteinopathy (Mackenzie et al., 2011).
Figure 3.
Neuropathology. (A) Macroscopy Patient DR189. Macroscopic picture of the right cerebral and cerebellar hemisphere of Patient DR189, from a lateral view (i) and a medial view (ii). (B) TDP-43 and p62 immunohistochemistry. Staining for TDP-43 and p62 of the frontal cortex (i and iv) and the hippocampus (ii and v) of FTLD Patient DR189 and for the spinal cord of ALS Patient DR1124 (iii and vi). TDP-43 and p62 positive neuronal cytoplasmic inclusions are indicated with an arrow. (C) AT8 staining of the hippocampus and parahippocampal gyrus DR1124. AT8 staining showed many neuronal cytoplasmic inclusions in the dentate gyrus of the hippocampus (i). In the parahippocampal cortex, neuronal neurofibrillary tangles or neuronal cytoplasmic inclusions were found (arrow, ii), whereas in the white matter, glial astrocytic cytoplasmic inclusions (arrowhead, iii and iv) and tufted astrocytes (double arrow, iv) were present.
In Patient DR1124, the diagnosis of ALS was autopsy-confirmed. Macroscopically, mild cortical frontal and mild hippocampal atrophy was noted. Pathological signs of lower motor neuron disease were present, manifesting as neuronal loss in the hypoglossal nucleus as well as in the ventral horns of the cervical and thoracic spinal cord. Additionally, loss of Betz cells and severe demyelination of the pyramidal tract in mesencephalon, pons, medulla oblongata and the entire spinal cord confirmed the presence of upper motor neuron disease. The TDP-43- and p62-positive neuronal cytoplasmic inclusions were mainly restricted to the lower motor neurons, i.e. the hypoglossal nucleus and the ventral horn neurons of cervical and thoraco-lumbar spinal cord [Fig. 3B(iii–vi)]. The findings of TDP-43 staining in the cortex were most suggestive for TDP type B proteinopathy. Besides the TDP-43 pathology, a moderate amount of AT8-immunoreactive tufted astrocytes and ‘neurofibrillary tangle’-like neuronal cytoplasmic inclusions was found in the frontal and temporal neocortex (Fig. 3C). More technical details on the performed neuropathological investigations can be found in the Supplementary material.
Genotype–phenotype correlations in patients with FTD
Demographic and clinical data of the seven TBK1 carriers with FTD were compared to 65 C9orf72 carriers, 52 GRN carriers and 259 non-mutation carriers (Table 4). The mean onset age in the TBK1 carriers was 63.3 ± 7.7 years, whereas C9orf72 carriers were significantly younger at disease onset (54.3 ± 9.2 years, P = 0.019) (Fig. 4A). When only taking into account the index patients, the significance remained (P = 0.049). The mean disease duration of TBK1 carriers was relatively long (8.2 ± 4.9 years), but not significantly different than that of the other patient groups.
Table 4.
Demographic and clinical characteristics of TBK1 carriers versus C9orf72, GRN or non-mutation carriers
| TBK1 | C9orf72 | GRN | No mutation |
||||
|---|---|---|---|---|---|---|---|
| Familial | Sporadic | Unknown | |||||
| FTD patients (index), n | 7 (5) | 65 (51) | 52 (21) | 76 (76) | 102 (102) | 81 (81) | |
| Male, n (%) | 3 (42.9) | 36 (55.4) | 23 (44.2) | 44 (57.9) | 57 (55.9) | 48 (59.3) | |
| Age at onset, y | Mean (SD) | 63.3 (7.7) | 54.3 (9.2) | 61.0 (6.5) | 63.9 (9.2) | 61.7 (11.0) | 68.5 (11.4) |
| Median (range) | 64 (48–70) | 54 (29–75) | 62 (45–70) | 65 (29–79) | 63 (29–83) | 72 (42–82) | |
| Disease duration, y | Mean (SD) | 8.2 (4.9) | 6.0 (4.8) | 5.6 (2.1) | 6.0 (3.14) | 7.1 (4.2) | 8.8 (3.0) |
| Median (range) | 8.7 (2–13) | 4.7 (1–19) | 5.4 (2–11) | 6.0 (1–14) | 7.0 (1–22) | 9.0 (5–12) | |
| Familial history, n (%) | F-AD | 3 (42.9) | 35 (53.8) | 18 (34.6) | 15 (19.7) | ||
| F | 3 (42.9) | 21 (32.3) | 16 (30.8) | 61 (80.3) | |||
| S | 1 (14.3) | 3 (4.6) | 5 (9.6) | 102 (100.0) | |||
| U | 0 | 6 (9.2) | 13 (25.0) | 81 (100.0) | |||
| Clinical subtype, n (%*) | BvFTD | 6 (85.7) | 51 (83.6) | 19 (45.2) | 41 (63.1) | 62 (67.4) | 19 (65.5) |
| PNFA | 0 | 5 (8.2) | 16 (38.1) | 5 (7.7) | 13 (14.1) | 4 (13.8) | |
| SD | 1 (14.3) | 3 (4.9) | 4 (9.5) | 14 (21.5) | 7 (7.6) | 4 (13.8) | |
| LA | 0 | 0 | 0 | 0 | 2 (2.2) | 0 | |
| PPA U | 0 | 0 | 0 | 1 (1.5) | 0 | 0 | |
| Mixed FTD | 0 | 2 (3.3) | 3 (7.1) | 4 (6.2) | 8 (8.7) | 2 (6.9) | |
| Behavioural subtype, n (%*) | Apathetic | 1 (16.7) | 14 (31.1) | 14 (87.5) | 13 (36.1) | 25 (41.7) | 6 (66.7) |
| Disinhibition | 5 (83.3) | 31 (68.9) | 2 (12.5) | 23 (63.9) | 35 (58.3) | 3 (33.3) | |
| FTD-ALS , n (%*) | Total | 1 (14.3) | 15 (23.1) | 0 | 1 (1.3) | 5 (4.9) | 3 (3.7) |
| Bulbar onset | 0 | 3 (4.6) | 0 | 0 | 1 (1.0) | 1 (1.2) | |
| Spinal onset | 1 (14.3) | 3 (4.6) | 0 | 1 (1.3) | 0 | 0 | |
| Bulbospinal onset | 0 | 5 (7.7) | 0 | 0 | 3 (2.9) | 0 | |
| Unknown onset | 0 | 4 (6.2) | 0 | 0 | 1 (1.0) | 2 (2.5) | |
| Extrapyramidal symptoms, n (%*) | 4 (57.1) | 9 (17.6) | 7 (18.9) | 22 (33.3) | 24 (27.9) | 10 (37.0) | |
| Psychiatric symptoms, n (%*) | Total | 4 (57.1) | 16 (30.7) | 7 (18.9) | 15 (24.6) | 28 (33.3) | 5 (25.0) |
| Psychosis | 2 (28.6) | 8 (15.3) | 2 (5.4) | 4 (6.6) | 12 (14.3) | 2 (10.0) | |
| Depression | 2 (28.6) | 7 (13.4) | 5 (13.5) | 7 (11.5) | 14 (16.7) | 3 (15.0) | |
| Abuse | 0 | 4 (7.7) | 0 | 4 (6.6) | 4 (4.8) | 1 (5.0) | |
BvFTD = behavioural variant of FTD; F = familial; F-AD = familial-autosomal dominant; LA = logopenic progressive aphasia; PNFA = progressive non-fluent aphasia; PPA U = primary progressive aphasia unspecified subtype; S = sporadic; SD = standard deviation ; U = unknown.
*Percentage (%) within group of patients for whom we have information.
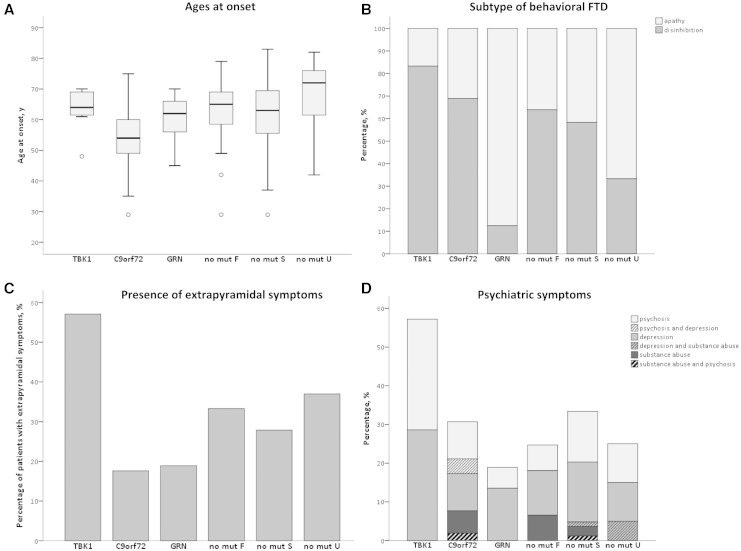
Figure 4.
Genotype–phenotype correlations in FTD patients. In these graphs, FTD patients are divided in groups based on their causal gene mutation, i.e. TBK1, C9orf72 and GRN carriers and non-mutation carriers. The latter group is further divided by familial history in familial and sporadic, and patients with unknown familial history. (A) Ages at onset. The boxplots illustrate the distribution of ages at onset in each FTD patient group. For each genetic subtype a boxplot is created in which the middle horizontal line represents the median age at onset. The upper and lower border of the box represent the value of the first quartile (25%) and the third quartile (75%) of the data. The whiskers represent the minimal and maximal age at onset with exception of the outliers. The outliers are presented as circles and refer to ages at onset that are greater than 1.5 interquartile ranges away from the 25th or the 75th percentile. (B) Subtype of behavioural FTD. This bar graph illustrates which proportion of the behavioural variant FTD patients of each patient group has frontal disinhibition or apathy as predominant feature, respectively. (C) Presence of extrapyramidal symptoms. In this bar graph, the proportion of patients who exhibit extrapyramidal symptoms are pictured for each patient group. (D) Psychiatric symptoms. This bar graph shows the proportion of FTD patients with psychiatric symptoms for each patient group. The bars are subdivided for the proportion of the major psychiatric characteristic.
Of the seven TBK1 carriers with FTD, one was also diagnosed with ALS (14.3%). ALS was so far not reported in any FTD patient with a GRN mutation. C9orf72 carriers had the highest proportion of FTD-ALS patients with 23.1%. This was a significantly higher proportion than in familial non-mutation FTD patients (1.3%, P < 0.001), sporadic non-mutation FTD patients (4.9%, P = 0.001) and non-mutation FTD patients with unknown familial history (3.7%, P = 0.001). Similar results were obtained when only including the index patients (P < 0.001, P = 0.002 and P = 0.001, respectively). One of seven TBK1 carriers was diagnosed with PPA, which was a similar proportion as in FTD patients caused by a C9orf72 repeat expansion (14.3% versus 13.1%). The GRN carriers were the only patient group in which there was a higher proportion of PPA diagnoses in comparison to behavioural variant FTD diagnoses (47.6% PPA versus 45.2% behavioural variant FTD). GRN carriers presented significantly more often with PPA (mostly progressive non-fluent aphasia) compared to C9orf72 carriers (13.1%, P < 0.001) and sporadic non-mutation carriers (23.9%, P = 0.009). In the index patients-only analysis, there was only a significant difference in proportion of PPA patients between GRN carriers (7/16, 43.8%) and C9orf72 carriers (7/47, 14.9%) (P = 0.033).
Behavioural disinhibition and social inappropriate behaviour was more frequent than apathy in TBK1 carriers with behavioural variant FTD (83.3% and 16.7%, respectively). This is in contrast with GRN carriers who present significantly more often with apathy as the most prominent behavioural characteristic (87.5%, P = 0.004) (Fig. 4B). In the behavioural variant FTD index patients-only analysis, 75.0% (3/4) of the TBK1 carriers had behavioural disinhibition and social inappropriate behaviour as most prominent behavioural feature in comparison with only 16.7% (1/6) of the GRN carriers, although comparative analysis did not reveal significant differences (P = 0.190). TBK1 carriers presented often with extrapyramidal symptoms (57.1%), which was significantly more often than C9orf72 carriers (17.6%, P = 0.038) and borderline significantly more often than GRN carriers (18.9%, P = 0.054) (Fig. 4C). In the index patients-only analysis, significance was reached for TBK1 index carriers (3/5, 60.0%) in comparison with GRN carriers (1/14, 7.1%) (P = 0.037), but lost for TBK1 carriers in comparison with C9orf72 carriers (8/42, 19.0%) (P = 0.076). A high incidence of psychiatric symptoms such as psychosis, depression and substance abuse, was observed in TBK1 carriers (57.1%). In all other patient groups, the proportion of patients with psychiatric symptoms was smaller, but in comparative analyses no significance was reached, although a borderline result was obtained in comparison with GRN carriers (18.9%) (P = 0.054) (Fig. 4D). In the index patients-only analysis, a smaller proportion of TBK1 carriers had psychiatric symptoms (2/5, 40.0%), which was not significantly more than in any other group.
Discussion
Loss of function mutations in TBK1 are the third most frequent cause of clinical FTD after C9orf72 and GRN in the Belgian patient cohort, and the second most frequent cause of clinical ALS after C9orf72 (Gijselinck et al., 2015a). Here, we described the detailed clinical characteristics of 16 TBK1 carriers, of which six were clinically diagnosed with FTD, seven with ALS, one with FTD-ALS and two with unspecified dementia. Also, we provided the results of the genotype–phenotype characteristics of TBK1 carriers compared to those of C9orf72 and GRN carriers, and non-mutation carriers with FTD.
The age at onset of TBK1 carriers was variable with a mean age at onset of 62 years. However, 75% of the TBK1 carriers developed the disease after the age of 62, with two mutation carriers still unaffected at an age above 80. The latter carriers are members of Family DR158, in which the TBK1 p.Glu643del mutation co-segregated with disease (Gijselinck et al., 2015a). This family has a high load of patients with psychiatric symptoms that may have complicated the diagnosis of FTD. Consequently, it is not excluded that early symptoms of FTD might have been wrongly interpreted in some carriers as psychiatric disease symptoms, or vice versa. However, our data suggest that the clinical penetrance of TBK1 loss of function mutations might be incomplete as three carriers had no documented familial history of neurodegenerative disease and only 50% of the TBK1 carriers from our cohort of index patients and relatives were affected by the age of 70.
The TBK1 carriers with FTD developed the first symptoms significantly later than C9orf72 carriers with FTD. However, this finding should be cautiously interpreted as the p.Glu643del mutation was over-represented among the TBK1 carriers. Carriers of this specific mutation exhibited a milder phenotype with significantly later onset age (66.3 ± 3.9 years) and longer disease duration (8.3 ± 1.8 years) than carriers of another TBK1 mutation (Gijselinck et al., 2015a). Two p.Glu643del carriers with ALS were siblings in Family DR663 and had remarkably young onset ages (Patient II.1, 51 years and Patient II.2, 41 years; Fig. 1). These two ALS patients had also inherited a C9orf72 expansion mutation as did a third sibling (Patient II.4) who only carried the C9orf72 repeat expansion and was still unaffected at the age of 62 (Gijselinck et al., 2015a). This observation seems to support an interaction between the TBK1 and C9orf72 genes in patients carrying a mutation in both genes, lowering onset age (Cady et al., 2015). Another ALS carrier of p.Glu643del, Patient DR1044, (Table 1), carried a ‘short’ C9orf72 expansion of 59 repeats units and had a disease onset at 63 years. We showed that C9orf72 carriers of a short repeat expansion (range 45 to 80 repeat units), had a significantly later age at onset than those with long repeats (Gijselinck et al., 2015b).
The disease duration of TBK1 carriers is relatively long, particularly in patients with FTD (8.2 ± 4.9 years). Though not significant, the disease duration was much shorter in TBK1 carriers with ALS. These observations were in line with earlier observations in C9orf72 carriers with ALS (Cruts et al., 2013).
The majority of patients with FTD with a TBK1 mutation were diagnosed with the behavioural variant of FTD. We found a comparable fraction of behavioural variant FTD in TBK1 carriers as in C9orf72 carriers, the latter known to present most often with behavioural variant FTD (Van Langenhove et al., 2013; Cooper-Knock et al., 2014). Similar to these C9orf72 carriers, behavioural disinhibition and social inappropriate behaviour was more frequent than apathy in TBK1 carriers. This is in contrast with GRN carriers who more often presented with the language variant, mostly with progressive non-fluent aphasia (Cruts et al., 2006; Cruts and Van Broeckhoven, 2008; Gijselinck et al., 2008), and in case of behavioural variant FTD, with apathy as the dominant feature (Beck et al., 2008).
Psychiatric symptoms were present in >50% of the TBK1 carriers. However, as six of the seven TBK1 carriers with psychiatric symptoms belonged to the same Family DR158, we cannot draw firm conclusions regarding overall occurrence of psychiatric symptoms in TBK1 carriers. As only five TBK1 carriers with FTD were index patients, the non-significant result of the comparison with other mutation carriers only taking into account index patients is not sufficient to reject the hypothesis that TBK1 carriers present more frequently with psychiatric symptoms. Therefore, research in larger cohorts of unrelated TBK1 carriers will be needed.
Interestingly, memory loss and disorientation in time and/or space were common early in the disease course of TBK1 carriers with FTD. Therefore, technical investigations were more often necessary to allow a clear differentiation between FTD and Alzheimer’s disease. In Patient III.2 of Family DR158 (Table 3), doubt persisted in the clinical diagnosis between the frontal variant of Alzheimer’s disease or FTD. This is in line with the observations by Pottier et al. (2015), who described a TBK1 mutation in four patients with pathological diagnosis of FTLD or FTLD-MND of which two had a clinical diagnosis of Alzheimer’s disease. Together, these observations suggest that screening of clinically diagnosed Alzheimer’s disease patients for mutations in TBK1 might be advisable. In Patient DR1124 (Table 1), who clinically presented with cognitive deficits and subsequently developed a rapidly evolving ALS phenotype, at autopsy we observed TDP-43 proteinopathy in the motor neurons while hyperphosphorylated tau pathology was present in the hippocampus (Fig. 4) in the absence of other proteinopathies. The question remains whether this hippocampal tauopathy might explain the cognitive decline in the patient, or whether it is merely a sign of ageing associated tauopathy. Three TBK1 carriers with ALS exhibited prominent upper motor neuron signs and in two of them no obvious electrophysiological evidence of lower motor neuron disease was present upon disease presentation. However, neuropathological examination in Patient DR1124 showed that TDP-43 and p62 positive neuronal cytoplasmic inclusions were mainly restricted to the lower motor neurons. Only one of the TBK1 carriers was diagnosed with FTD-ALS, preventing a genotype–phenotype comparison with FTD-ALS patients carrying a C9orf72 expansion mutation. ALS occurred in the current study in 23.1% of the C9orf72 carriers in the Belgian FTD cohort, a similar result as the previous observation of 27% by Van Langenhove et al. (2013). In the same FTD cohort, none of the GRN carriers had ALS. TBK1 carriers with FTD presented significantly more often with extrapyramidal symptoms than C9orf72 carriers and, when only taking into account index patients, than GRN carriers. Noticeably, all TBK1 carriers with extrapyramidal symptoms were carriers of the p.Glu643del mutation. Parkinsonism is said to occur in 20–30% of FTD patients (Park and Chung, 2013), particularly linked to MAPT and GRN mutations (Hutton et al., 1998; Baker et al., 2006; Boeve and Hutton, 2008). Future studies in larger cohorts of carriers of different TBK1 mutations are needed to examine whether the association with extrapyramidal symptoms holds true.
Structural neuroimaging in TBK1 carriers showed various patterns of cortical atrophy, which could be global or localized with frontotemporal predominance but also with involvement of the parietal regions, the hippocampus or the cerebellum. These variable findings are reminiscent of findings in C9orf72 carriers who exhibited widespread patterns of atrophy with predominant atrophy of the frontal and, to a lesser extent, of the anterior temporal lobes, without regional specificity, and with involvement of the parietal lobes and the cerebellum (Whitwell et al., 2012). In contrast with the generally symmetric pattern of atrophy in C9orf72 carriers (Boeve et al., 2012; Mahoney et al., 2012), more than half of our TBK1 carriers displayed an asymmetric pattern of atrophy (most frequently with right predominance). The variable widespread patterns are in contrast with the more characteristic patterns observed in GRN carriers who have most prominent atrophy in the temporal and parietal lobes (Whitwell et al., 2009a; Rohrer et al., 2010) or in MAPT carriers who present with most prominent atrophy in the anteromedial temporal lobes (Whitwell et al., 2009b; Rohrer et al., 2010). As in C9orf72 carriers and GRN carriers, neuropathology of TBK1 carriers is characterized by TDP-43 pathology. Neuropathological examination had been carried out in one FTD and one ALS patient and was compatible with TDP-43 type B proteinopathy, as was reported in other studies (Freischmidt et al., 2015; Pottier et al., 2015). One study also reported TDP-43 type A in TBK1 carriers with FTD (Pottier et al., 2015).
Our study has some limitations, as we identified in the Belgian patients a relatively low number of TBK1 carriers and also lacked detailed clinical information on three of the carriers with ALS. Consequently, our observations in the genotype–phenotype comparisons with C9orf72 carriers, GRN carriers and non-mutation carriers were often not or borderline significant and need to be examined in larger groups of TBK1 carriers. Also, not all TBK1 carriers received the same investigations, since this study was largely performed retrospectively. The fact that six TBK1 carriers were members of the same family might have influenced some of our results, such as the high load of psychiatric symptoms, which might be due to other genetic factors shared among relatives.
In conclusion, carriers of a TBK1 loss of function mutation have a variable, but on average late onset age (58.1 years in ALS patients and 63.3 years in FTD patients). Most patients with FTD present with behavioural variant FTD, with disinhibition as the most prominent feature. Memory loss is remarkably present in the initial phase of the disease. Extrapyramidal symptoms occur rather often, especially in carriers of the p.Glu643del mutation. On neuroimaging, various patterns of atrophy as well as of hypoperfusion or hypometabolism can be seen. Neuropathologically, TBK1 carriers are characterized by TDP-43 pathology. Based on the observed genotype–phenotype correlations and the frequency of causal mutations in C9orf72, GRN and TBK1 in our Belgian cohort, we propose the following guidelines for the genetic diagnostic setting. After a negative result for the C9orf72 repeat expansion, which still remains the major cause for FTD-ALS and behavioural variant FTD, patients with FTD-ALS or behavioural variant FTD should preferably be tested first for loss of function mutations in TBK1, specifically in FTD patients with early memory difficulties in combination with behavioural problems. Furthermore, in patients with a relatively late onset age or in patients who present with extrapyramidal symptoms, TBK1 loss of function mutations, especially the TBK1 mutation p.Glu643del, should be considered.
Supplementary Material
Acknowledgements
The authors thank the personnel of the Genomics Service Facility of the VIB Department of Molecular Genetics (http://www.vibgeneticservicefacility.be) and the Antwerp Biobank of the Institute Born-Bunge for their expert support. The authors also acknowledge the contribution of the neurologists Dr Marie-Claire De Poorter (General Hospital Oudenaarde), Dr Marc Bruyland (General Hospital Glorieux Ronse) and Dr Frederik Clement and Dr Marie-Christine Hasenbroeckx (General Hospital Roeselare) to the clinical evaluation of patients. Peter P. De Deyn is also affiliated to the Department of Neurology and Alzheimer research Center, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- PPA
primary progressive aphasia
- TDP-43
TAR DNA binding protein 43
Appendix 1
We acknowledge the following members of the Belgian Neurology (BELNEU) consortium for their contribution to the clinical and pathological phenotyping and follow-up of the Belgian patient cohorts: Dirk Nuytten (Hospital Network Antwerp, Antwerp); Katrien Smets (Antwerp University Hospital, Edegem); Wim Robberecht and Philip Van Damme (University Hospitals Leuven Gasthuisberg, Leuven); Patrick Santens and Bart Dermaut (University Hospital Ghent, Ghent); Olivier Deryck and Bruno Bergmans (General Hospital Sint-Jan Brugge, Bruges); Jean Delbeck (General Hospital Sint-Maria, Halle); Jan Versijpt and Alex Michotte (University Hospital Brussels, Brussels); Christiana Willems (Jessa Hospital, Hasselt); Adrian Ivanoiu (Saint-Luc University Hospital, Brussels); and Eric Salmon (University of Liège and Memory Clinic, CHU Liège, Liège).
Funding
This study was funded in part by the MetLife Foundation for Medical Research Award, USA; the Belgian Science Policy Office Interuniversity Attraction Poles program, the Flanders government initiated Impulse Program on Networks for Dementia Research (VIND), the Flemish government initiated Methusalem Excellence Program, the Research Foundation Flanders (FWO), the University of Antwerp Research Fund, Belgium. The FWO provided a postdoctoral fellowship to I.G. and a clinical investigator fellowship to A.S. and J.B.
Supplementary material
Supplementary material is available at Brain online.
References
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–19. [DOI] [PubMed] [Google Scholar]
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 2008; 131: 706–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 2012; 135: 765–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Hutton M. Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN) [Review]. Arch Neurol 2008; 65: 460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. [Review]. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–9. [DOI] [PubMed] [Google Scholar]
- Cady J, Allred P, Bali T, Pestronk A, Goate A, Miller TM, et al. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol 2015; 77: 100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion A, Leblond CS, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 2015; 62: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Knock J, Shaw PJ, Kirby J. The widening spectrum of C9ORF72-related disease; Genotype/phenotype correlations and potential modifiers of clinical phenotype [Review]. Acta Neuropathol 2014; 127: 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006; 442: 920–4. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Loss of progranulin function in frontotemporal lobar degeneration [Review]. Trends Genet 2008; 24: 186–94. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum [Review]. Trends Neurosci 2013; 36: 450–9. [DOI] [PubMed] [Google Scholar]
- De Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS [Review]. Clin Neurophysiol 2008; 119: 497–503. [DOI] [PubMed] [Google Scholar]
- Fecto F, Yan J, Vemula SP, Liu E, Yang Y, Chen W, et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol 2011; 68: 1440–6. [DOI] [PubMed] [Google Scholar]
- Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Müller K, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 2015; 18: 631–6. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Van Broeckhoven C, Cruts M. Granulin mutations associated with frontotemporal lobar degeneration and related disorders: an update. Hum Mutat 2008; 29: 1373–86. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 2012; 11: 54–65. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B, et al. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology 2015a; 85: 2116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Engelborghs S, De Bleecker J, et al. The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol Psychiatry 2015b. Advance Access published on October 20, 2015, doi: 10.1038/mp.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Ordureau A, Gourlay R, Arthur JSC, Cohen P. Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon β. J Biol Chem 2011; 286: 35663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 2005; 65: 1817–19. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–5. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002; 59: 1077–9. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011; 122: 111–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 2012; 135: 736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Dopper EGP, Waite A, Rollinson S, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 2012; 11: 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 2010; 465: 223–6. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451: 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HK, Chung SJ. New perspective on parkinsonism in frontotemporal lobar degeneration [Review]. J Mov Disord 2013; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Bieniek KF, Finch N, van de Vorst M, Baker M, Perkersen R, et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol 2015; 130: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Albanese E, Guerchet M, Matthew P. World Alzheimer Report 2014 Dementia and Risk Reduction. An analysus of protective and modifiable factors. London: Alzheimer's Disease International; 2014. [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage 2010; 53: 1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino E, Rainero I, Chiò A, Rogaeva E, Galimberti D, Fenoglio P, et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 2012; 79: 1556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben A, Van Langenhove T, Engelborghs S, Martin JJ, Boon P, Cras P, et al. The genetics and neuropathology of frontotemporal lobar degeneration [Review]. Acta Neuropathol 2012; 124: 353–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J, Van Langenhove T, Kovacs GG, Dillen L, Deschamps W, Engelborghs S, et al. Rare mutations in SQSTM1 modify susceptibility to frontotemporal lobar degeneration. Acta Neuropathol 2014: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration–amyotrophic lateral sclerosis spectrum [Review]. Ann Med 2012; 44: 817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove T, van der Zee J, Gijselinck I, Engelborghs S, Vandenberghe R, Vandenbulcke M, et al. Distinct clinical characteristics of C9orf72 expansion carriers compared with GRN, MAPT, and nonmutation carriers in a Flanders-Belgian FTLD cohort. JAMA Neurol 2013; 70: 365–73. [DOI] [PubMed] [Google Scholar]
- Weidberg H, Elazar Z. TBK1 mediates crosstalk between the innate immune response and autophagy [Review]. Sci Signal 2011; 4: pe39. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Senjem ML, Baker M, Rademakers R, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 2009a; 72: 813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Senjem ML, Baker M, Ivnik RJ, et al. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology 2009a; 73: 1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, Dejesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 2012; 135: 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.