Abstract
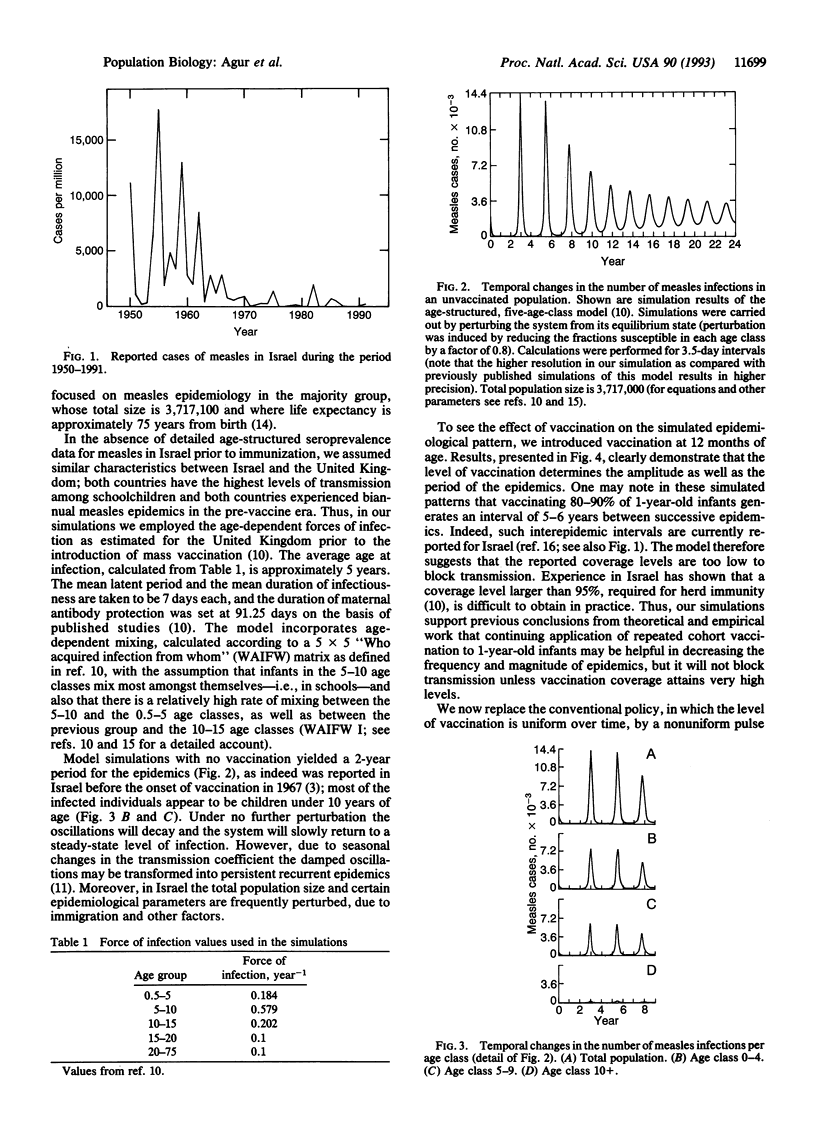
Although vaccines against measles have been routinely applied over a quarter of a century, measles is still persistent in Israel, with major epidemics roughly every 5 years. Recent serological analyses have shown that only 85% of Israelis aged 18 years have anti-measles IgG antibodies. Considering the high transmissibility of the virus and the high level of herd immunity required for disease eradication, the Israeli vaccination policy against measles is now being reevaluated. Motivated by theoretical studies of populations in perturbed environments, we examined the possibility of replacing the conventional cohort vaccination strategy by a pulse strategy--i.e., periodic vaccination of several age cohorts at the same time. Numerical studies of a deterministic age-structured model suggest that vaccination, which renders immunity to no more than 85% of the susceptible children aged 1-7 years, once every 5 years will suffice to prevent epidemics in Israel, where infection rate is highest amongst schoolchildren. The model suggests that by using such a strategy the density of susceptible individuals is always kept below the threshold above which recurrent epidemics will be maintained. Analysis of simpler, non-age-structured, models serves to clarify the basic properties of the proposed strategy. Our theoretical results indicate that the advantages and disadvantages of a pulse strategy should be seriously examined in Israel and in countries with similar patterns of measles virus transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agur Z., Danon Y. L., Anderson R. M., Cojocaru L., May R. M. Measles immunization strategies for an epidemiologically heterogeneous population: the Israeli case study. Proc Biol Sci. 1993 May 22;252(1334):81–84. doi: 10.1098/rspb.1993.0049. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Age-related changes in the rate of disease transmission: implications for the design of vaccination programmes. J Hyg (Lond) 1985 Jun;94(3):365–436. doi: 10.1017/s002217240006160x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi M., Schulman A., Kedem R., Danon Y. L. Measles in adults: a prospective study of 291 consecutive cases. Br Med J (Clin Res Ed) 1987 Nov 21;295(6609):1314–1314. doi: 10.1136/bmj.295.6609.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethcote H. W. Measles and rubella in the United States. Am J Epidemiol. 1983 Jan;117(1):2–13. doi: 10.1093/oxfordjournals.aje.a113511. [DOI] [PubMed] [Google Scholar]
- May R. M. Vaccination programmes and herd immunity. Nature. 1982 Dec 9;300(5892):481–483. doi: 10.1038/300481a0. [DOI] [PubMed] [Google Scholar]
- Pannuti C. S., Moraes J. C., Souza V. A., Camargo M. C., Hidalgo N. T. Measles antibody prevalence after mass immunization in São Paulo, Brazil. Bull World Health Organ. 1991;69(5):557–560. [PMC free article] [PubMed] [Google Scholar]
- Schenzle D. An age-structured model of pre- and post-vaccination measles transmission. IMA J Math Appl Med Biol. 1984;1(2):169–191. doi: 10.1093/imammb/1.2.169. [DOI] [PubMed] [Google Scholar]
- Slater P. E. Measles containment in Israel. Isr J Med Sci. 1991 Jan;27(1):19–21. [PubMed] [Google Scholar]



