Abstract
Hypoxic pulmonary hypertension (PH) is a common disease characterized by a disturbance to the balance of apoptosis and cell proliferation in pulmonary artery smooth muscle cells (PASMCs). The anti-apoptotic protein, survivin, has been observed to be upregulated in pulmonary arteries (PAs) of chronic hypoxia-induced PH rats. The present study aimed to investigate the therapeutic potential of sepantronium bromide (YM155), a selective survivin inhibitor, on hypoxic human PASMCs and examine the potential underlying mechanisms. Cultured human PASMCs (HPASMCs) were randomly divided into the following groups: i) Normoxia (N); ii) normoxia + 100 nmol/l YM155 (NY100); iii) hypoxia (H); iv) hypoxia + 1 nmol/l YM155 (HY1); v) hypoxia + 10 nmol/l YM155 (HY10); and hypoxia + 100 nmol/l YM155 (HY100) groups. The cells were exposed to the different conditions for 24 h, according to the group. Cell viability was then determined using a Cell Counting Kit-8 assay, and apoptosis was detected using a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay. The expression levels of survivin were determined using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), immunocytochemistry and Western blot analyses. The expression levels of the voltage-dependent K+ (Kv) channels, Kv1.5 and Kv2.1, were measured using RT-qPCR and Western blotting. Cell proliferation in the hypoxic PASMCs was significantly increased by hypoxia, however, apoptosis of the HPASMCs was suppressed, the expression of survivin were upregulated and the expression levels of Kv1.5 and Kv2.1 were downregulated. YM155 treatment ameliorated the hypoxia-induced increase in cell proliferation and expression of survivin in a concentration-dependent manner, increased apoptosis, and increased the expression levels of Kv1.5 and Kv2.1 (P<0.05). By contrast, YM155 treatment in normoxic HPASMCs had no significant effects on proliferation, apop-tosis, or the expression levels of survivin and Kv channels in the PASMCs. The present study is the first, to the best of our knowledge, to demonstrate that YM155, a selective survivin inhibitor, has a beneficial therapeutic effect on hypoxic HPASMCs, and that YM155 induces a pro-apoptotic effect by downregulating the apoptosis inhibitor, survivin, possibly through a Kv channel-mediated mechanism.
Keywords: hypoxia, pulmonary artery smooth muscle cells, survivin, YM155, Kv1.5, Kv2.1
Introduction
Pulmonary hypertension (PH) is a complex and multifactorial disorder characterized by increased pulmonary arterial pressure and adverse remodeling of pulmonary arteries (PAs), which result in right ventricular failure and premature mortality (1). Excessive proliferation and resistance to apoptosis in pulmonary artery smooth muscle cells (PASMCs) is widely accepted as one of the predominant causes of PA remodeling during hypoxia (2,3). Hypoxia suppresses mitochondria-dependent apoptosis in human PASMCs (HPASMCs) and increases proliferation in these cells (4,5). However, the potential mechanism underlying the proliferation and resistance to apoptosis in hypoxic PASMCs remains to be elucidated, and there remains no curative therapeutic strategy. The overall prognosis of patients with PH remains poor, and further understanding of the mechanisms underlying the development of PH is important to improve patient management and outcomes (6).
Several predisposing and disease-modifying abnormalities, including the de novo expression of survivin and the downregulated expression of the voltage-dependent K+ (Kv)1.5 channel, have been reported to contribute to the cancer-like, proliferative, apoptosis-resistant phenotype of PASMCs (7). Kv channels in PASMCs are inhibited by acute and chronic exposure to hypoxia (8). Survivin is a member of the inhibitor of apoptosis (IAP) protein gene family, which negatively regulates programmed cell death and is well documented to be overexpressed in almost all types of human cancer (9). Additional data has indicated a more selective role of survivin, also a chromosomal passenger protein required for cell division (10), in antagonizing mitochondria-dependent apop-tosis (11). Survivin expression is cell cycle-dependent but it is also regulated by exposure to hypoxia (12). It is almost undetectable in the majority of normal adult tissues, and increased expression of survivin correlates with a poor outcome (13). A previous study by McMurtry et al (14) indicated that survivin was expressed in the PAs of patients with PH, and that the overexpression of survivin coincided with pulmonary vascular remodeling in monocrotaline-induced rat PAH models. In addition, the therapeutic effect of inhibition of survivin was achieved by the induction of mitochondria-dependent apop-tosis and the activation of Kv channels in PASMCs (14). These findings suggested that inducing the expression of survivin may contribute to the abnormal PASMC phenotype observed in PH; therefore, survivin may be an attractive target for PH therapy.
As a novel small-molecule survivin inhibitor, sepantronium bromide (YM155) suppresses the transactivation of survivin via direct binding to its promoter (15) and, therefore, has little effect on the expression levels of other IAP family members or B-cell lymphoma 2-associated proteins (16). It has been demonstrated that YM155 induces tumor cell apoptosis and survivin suppression in various human cancer models (16,17). A previous study by Liu et al (18) demonstrated that survivin was expressed in the PAs of rats with chronic hypoxic pulmonary hypertension, but not in the PAs of normal rats. YM155 treatment downregulated the expression levels of survivin in the distal PAs and lung tissues of the rats exposed to chronic hypoxia, and reduced mean pulmonary arterial pressure and right ventricular hypertrophy, subsequently reversing hypoxia-induced PH. These results suggested that YM155 may be a potential therapeutic agent for hypoxic PH. However, no previous studies, to the best of our knowledge, have evaluated the effects of YM155 on the expression of survivin and apoptosis of HPASMCs exposed to hypoxia, or the potential underlying mechanisms. The present study hypothesized that YM155 may have anti-proliferative effects on hypoxia-induced HP. Therefore, the protective effect of YM155 on hypoxic HPASMCs was investigated, with a focus on the mechanisms of cell proliferation and apoptosis, as well as the activation of Kv1.5 and Kv2.1 channel in the PASMCs during hypoxia.
Materials and methods
Cell culture
Human pulmonary artery smooth muscle cells (HPASMCs) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in smooth muscle cell medium (ScienCell Research Laboratories) supplemented with 2% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, 100 µg/ml streptomycin and 1% smooth muscle cell growth supplement (all ScienCell Research Laboratories) under an atmosphere of 5% CO2 at 37°C. The HPASMCs at passages 4–10 were used in the following experimental assays.
Experimental protocol
The cultured HPASMCs were randomly divided into six groups: i) Normoxia (N) group, in which the cells were cultured in serum-free Dulbecco's modified Eagle's medium (DMEM; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) under normoxic conditions for 24 h at 37°C; ii) normoxia + 100 nmol/l YM155 (NY100) group, in which the cells were cultured in serum-free DMEM with 100 nmol/l YM155 under normoxic conditions for 24 h at 37°C; iii) hypoxia (H) group, in which the cells were cultured in serum-free DMEM under hypoxic conditions for 24 h at 37°C; iv) hypoxia + 1 nmol/l YM155 (HY1) group, in which the cells were cultured in serum-free DMEM with 1 nmol/l YM155 under hypoxic conditions for 24 h at 37°C; v) hypoxia + 10 nM YM155 (HY10) group, in the which cells were cultured in serum-free DMEM with 10 nmol/l YM155 under hypoxic conditions for 24 h; and vi) hypoxia + 100 nmol/l YM155 (HY100) group, in which the cells were cultured in serum-free DMEM with 100 nmol/l YM155 under hypoxic conditions for 24 h at 37°C. YM155 was obtained from Selleck Chemicals (Houston, TX, USA) and dissolved in dimethyl sulfoxide (Beijing Solarbio Science and Technology, Co., Ltd.). The final concentration of DMEM was <0.1% in medium.
The HPASMCs were exposed to the different conditions, according to the group. For all experiments, the cells were rendered quiescent by incubation in serum-free DMEM for 24 h at 37°C prior to incubation in either hypoxic (2.5% O2, 5% CO2) or normoxic (21% O2, 5% CO2) conditions. Hypoxic culture conditions, defined as 2.5% O2, were established using a Heraeus® oxygen-regulated cell culture incubator (Thermo Fisher Scientific, Inc.).
Cell viability
Cell viability was determined using a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) in accordance with the manufacturer's protocol. The HPASMCs were seeded in 96-well plates at 1×104 cells/well (four wells for each group) and cultured for 24 h in complete medium in an atmosphere of 5% CO2 at 37°C. The cells were treated, as described above, and 10 µl/well CCK-8 was added to each well at the end of the experiment and incubated at 37°C for a further 2 h. The absorbance of each well was measured at 450 nm on a microplate reader (HR801; Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the HPASMCs using TRIzol (Tiangen Biotech Co., Ltd., Beijing, China), according to the manufacturer's protocol, and the RNA was treated with RNase-Free DNase I (Tiangen Biotech Co., Ltd.) to eliminate contaminating DNA. The RNA concentration (552 ng/µl) was determined using an ultra-violet spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Inc.), and the quality of RNA was checked by 1.5% agarose gel electrophoresis (Sangon Biotech Co., Ltd., Shanghai, China). cDNA was synthesized from 10 µl total RNA using the QuantScript RT kit (KR106; Tiangen Biotech Co., Ltd.). qPCR was then performed on the ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixture (25 µl) consisted of 6 µl cDNA, 12.5 µl 2X FastFire qPCR PreMix (SYBR Green; Tiangen Biotech Co., Ltd.) and 0.75 µl each of forward and reverse primers. The PCR cycling conditions were as follows: 94°C for 5 min, followed by 45 cycles at 94°C for 40 sec, 58°C for 40 sec and 72°C for 40 sec. The primers were obtained from Sunny Biotech Co., Ltd. (Shanghai, China) and their sequences are presented in Table I. Melting curve analysis was performed to examine the specificity of the amplification reactions. GAPDH served as an internal control. Experiments were performed in triplicate. The relative gene expression levels were calculated for each sample using the 2−ΔΔCq method (19).
Table I.
Sequences of primers used for reverse transcription-polymerase chain reaction analysis.
| Gene | Primer (5′→3′) |
|---|---|
| Survivin | Forward: ACTTGGCCCAGTGGGTTTTT |
| Reverse: CAGAAAGGAAAGCGCAACCG | |
| Kv1.5 | Forward: CTACTTCGACCCCCTGAGGA |
| Reverse: CAGGGTCTCCAAGCAGAAGG | |
| Kv2.1 | Forward: TTTGCCCGGAGCATTGAGAT |
| Reverse: GAACGTTCAGGTGCTGAGGA | |
| GAPDH | Forward: CACCATCTTCCAGGAGCGAG |
| Reverse: AAATGAGCCCCAGCCTTCTC |
Kv, voltage-dependent K+ channel; GAPDH, glyceraldehye 3-phosphate dehydrogenase.
Immunocytochemical analysis
The HPASMCs were seeded onto 96-well plates at a density ~1×105 cells/ml for immunocytochemical analysis. Following washing with phosphate-buffered saline (PBS; Pujingkangli Science & Technology, Beijing, China), the HPASMCs were fixed with 4% paraformaldehyde solution (Beijing Huayueyang Biotechnology Co., Ltd., Beijing, China) and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS. The cells were then incubated with goat anti-human survivin polyclonal antibody (ab27468; Abcam, Cambridge, UK) overnight at 4°C. After washing three times with PBS for 5 min each, the cells were incubated with tetraethyl rhodamine isothiocyanate-conjugated rabbit anti-goat IgG (heavy and light chains) secondary antibody (31650; Thermo Fisher Scientific, Inc.) at room temperature for 30 min. The cells were observed under a fluorescent microscope (Axio Scope.A1; Carl Zeiss AG, Oberkochen, Germany), and the images were acquired and analyzed using Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
The HPASMCs were placed in 200 µl radioimmunoprecipitation assay lysis buffer (Tiangen Biotech Co., Ltd.) supplemented with 1% protease inhibitor cocktail (Pujingkangli Science & Technology). Following centrifugation at 12,000 × g at 4°C for 10 min, the supernatants were collected for Western blot analysis. The amount of protein was quantified using a Bio-Rad Protein Assay kit (500-0116; Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer's protocol. Protein samples (40 µg) were run on a 10% acrylamide gel (Huaxing Biotechnology Co., Ltd., Beijing, China) and transferred onto a nitrocellulose membrane (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skimmed milk powder in Tris-buffered saline and 0.1% Tween 20 (TBST; Huaxing Biotechnology Co., Ltd.) for 1 h at room temperature, followed by washing three times with TBST for 10 min. Subsequently, the membranes were immunoblotted overnight at 4°C with rabbit anti-human survivin polyclonal antibody (ab469; 1:1,000; Abcam, Cambridge, UK), rabbit anti-human Kv1.5 polyclonal antibody (ab181798; 1:1,000; Abcam), mouse anti-human Kv2.1 monoclonal antibody (ab105586; 1:1,000; Abcam) or rabbit anti-human GAPDH monoclonal antibody (14C10; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA). The membranes were washed three times with PBS and then incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (ZB-2305 and ZB-2301; 1:5,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.). Detection was performed using an enhanced chemiluminescence kit (ZLI-9036; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Proteins bands were visualized following exposure of the membrane to X-ray film (Kodak, Rochester, NY, USA). The protein expression levels of survivin, Kv1.5 and Kv2.1 were normalized to the protein expression of GAPDH.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay
Following hypoxia and/or pretreatment, the HPASMCs were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature. The TUNEL assay was performed for apoptotic cell determination using an In situ Cell Death Detection kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer's protocol. Counterstaining of nuclei with DAPI (Thermo Fisher Scientific, Inc.) was performed for 10 min at 20°C, and sealed with nail varnish. All TUNEL-positive cells (indicated by green fluorescence in the nuclei) were counted in each field of view, and subsequently expressed as a percentage of the total number of nuclei in that same field.
Statistical analysis
Data are presented as the mean ± standard deviation and were analyzed using the SPSS 17.0 statistical software package (SPSS, Inc, Chicago, IL. USA). One-way analysis of variance was used for comparison of variance among groups. Comparison between groups was analyzed via Student-Newman-Keuls q test. P<0.05 was considered to indicate a statistically significant difference.
Results
YM155 inhibits the expression of survivin in hypoxic HPASMCs
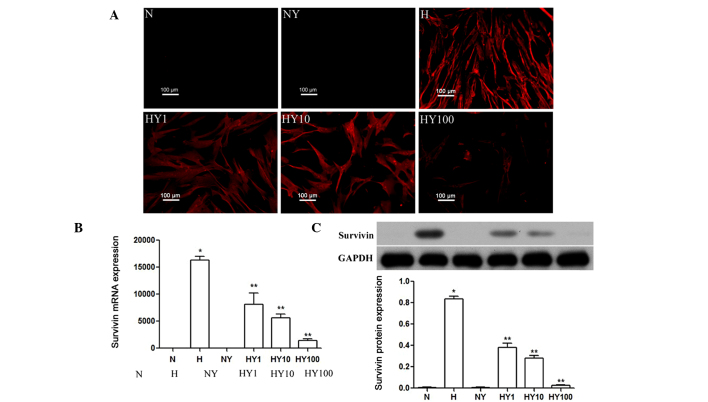
RT-qPCR, immunocytochemistry and Western blot analyses were performed to determine the expression levels of survivin in hypoxic or normoxic HPASMCs with or without YM155 treatment. As shown in Fig. 1A, survivin was expressed in the HPASMCs cultured under hypoxic conditions, but was not expressed in the normoxia-cultured cells. Treatment with YM155 significantly inhibited the hypoxia-induced upregulated gene and protein expression levels of survivin, and this effect was enhanced by increasing the concentration of YM155 (P<0.05; Fig. 1B and C).
Figure 1.
YM155 treatment inhibits the expression of survivin in hypoxic HPASMCs. Expression levels of survivin in hypoxic or normoxic HPASMCs, with or without YM155 treatment, were determined using (A) immunocytochemical (scale bar=100 µm), (B) quantitative reverse transcription-polymerase chain reaction and (C) Western blot analyses. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. N group; **P<0.05 vs. H group. HPAMSCs, human pulmonary artery smooth muscle cells; YM155, sepantronium bromide; N, normoxia; H, hypoxia; NY, normoxia + 100 nmol/l YM155; HY1, hypoxia + 1 nmol/l YM155; HY10, hypoxia + 10 nM YM155; HY100, hypoxia + 100 nmol/l YM155.
YM155 suppresses the cell viability of hypoxic HPASMCs
To investigate the cell viability of the hypoxic or normoxic HPASMCs with or without YM155 treatment, a CCK-8 cell proliferation assay was performed. The results demonstrated that cell proliferation was significantly increased by hypoxia, compared with the normoxia-cultured HPASMCs (P<0.05). By contrast, YM155 treatment ameliorated this hypoxia-induced increase in cell proliferation, in a concentration-dependent manner (P<0.05; Fig. 2). YM155 treatment in the normoxic HPASMCs had no significant effect on the proliferation of the HPASMCs.
Figure 2.

YM155 treatment suppresses the viability of hypoxic human pulmonary artery smooth muscle cells. Cell viability was determined using a Cell Counting Kit-8 assay. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. N group; **P<0.05 vs. H group. YM155, sepantronium bromide; N, normoxia; H, hypoxia; NY, normoxia + 100 nmol/l YM155; HY1, hypoxia + 1 nmol/l YM155; HY10, hypoxia + 10 nM YM155; HY100, hypoxia + 100 nmol/l YM155.
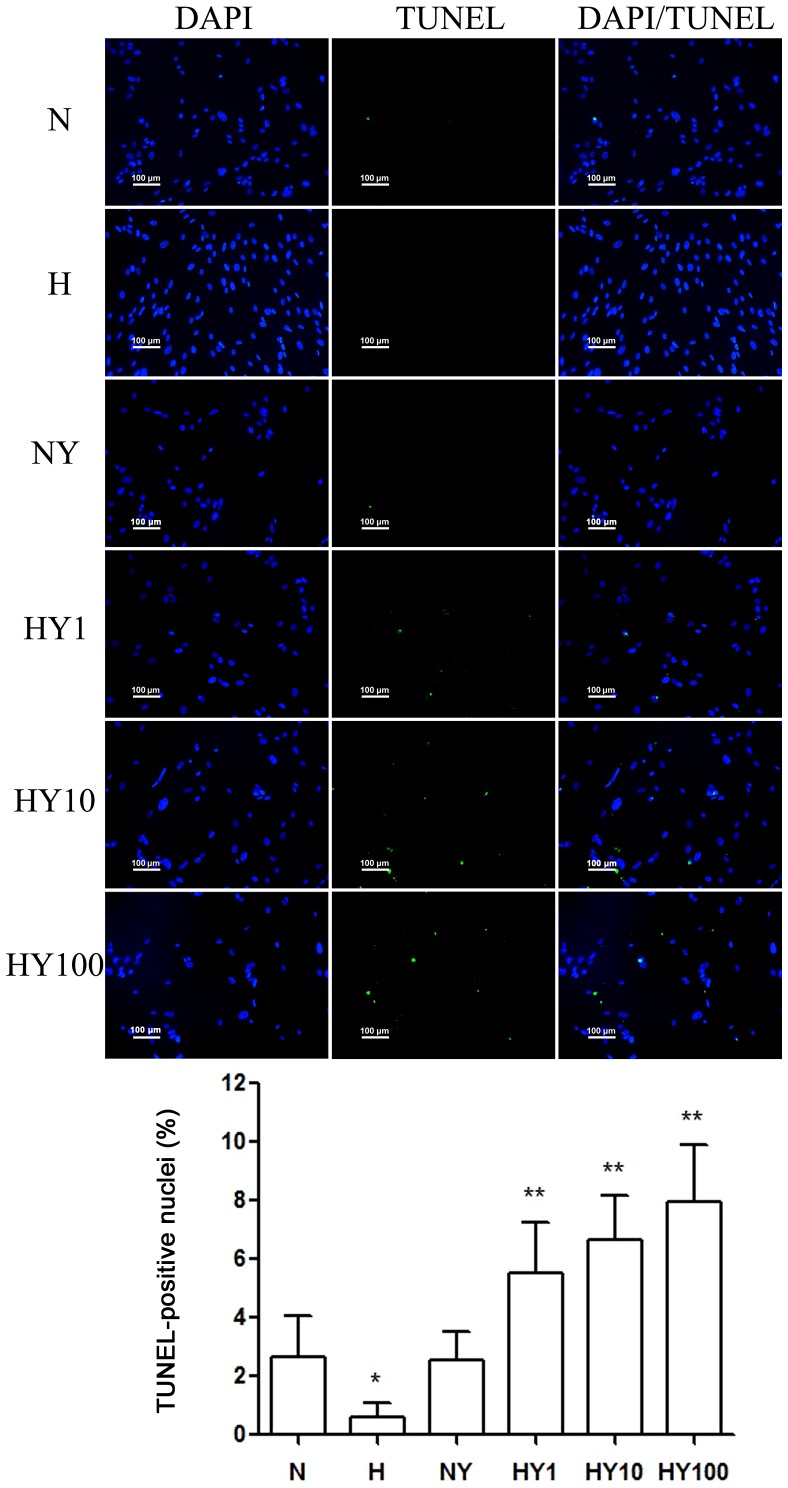
YM155 induced apoptosis of hypoxic HPASMCs
To investigate whether YM155 suppressed cell viability by increasing apoptosis of the HPASMCs during hypoxia, a TUNEL assay was performed (Fig. 3). Hypoxia induced HPASMC proliferation and suppressed apoptosis. Following treatment with YM155, a significant decrease in cell viability was detected in the hypoxic HPASMCs, and the percentage of TUNEL-positive cells decreased in a dose-dependent manner. However, no significant effects were observed in the normoxic HPASMCs treated with 10 nmol/l YM155 for 24 h.
Figure 3.
YM155 induces the apoptosis of hypoxic human pulmonary artery smooth muscle cells. Apoptosis was determined using a TUNEL assay (scale bar=100 µm). Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. N group; **P<0.05 vs. H group. TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling; YM155, sepantronium bromide; N, normoxia; H, hypoxia; NY, normoxia + 100 nmol/l YM155; HY1, hypoxia + 1 nmol/l YM155; HY10, hypoxia + 10 nM YM155; HY100, hypoxia + 100 nmol/l YM155.
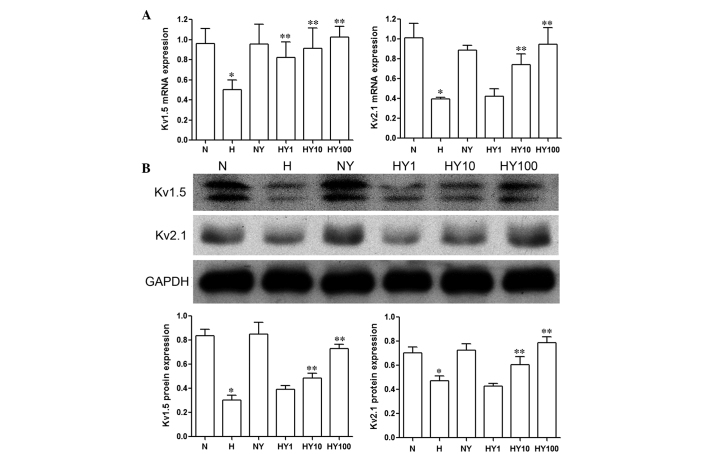
YM155 upregulates the expression levels of Kv1.5 and Kv2.1 in hypoxic HPASMCs
The results of the RT-qPCR and Western blot analyses demonstrated that the expression levels of Kv1.5 and Kv2.1 were markedly inhibited by hypoxia, compared with the HPASMCs cultured under normoxic conditions (P<0.05; Fig. 4). Treatment with YM155 resulted in a concentration-dependent increase in the mRNA (Fig. 4A) and protein (Fig. 4B) expression levels of Kv1.5 and Kv2.1 in the HPASMCs cultured in hypoxia (P<0.05). No significant changes were observed in the expression levels of Kv1.5 and Kv2.1 in the normoxic HPASMCs following YM155 treatment (Fig. 4).
Figure 4.
YM155 decreases the expression levels of Kv1.5 and Kv2.1 in hypoxic human pulmonary artery smooth muscle cells. Expression levels were determined using (A) reverse transcription-polymerase chain reaction and (B) Western blot analyses. Data are presented as the mean ± standard deviation (n=3). *P<0.05 vs. N group; **P<0.05 vs. H group. YM155, sepantronium bromide; N, normoxia; H, hypoxia; NY, normoxia + 100 nmol/l YM155; HY1, hypoxia + 1 nmol/l YM155; HY10, hypoxia + 10 nM YM155; HY100, hypoxia + 100 nmol/l YM155; Kv, K+ voltage-dependent channel.
Discussion
PH is an obstructive vasculopathy characterized by the increased proliferation and suppressed apoptosis of PASMCs. This phenotype is associated with activation of the expression of survivin, which is important in PH pathogenesis (20). In the present study, the potential role for survivin, an inhibitor of apoptosis, was investigated in normoxic and hypoxic HPASMCs. The results demonstrated that survivin was expressed in HPASMCs during hypoxia but not in normoxic conditions. Treatment of the PASMCs under hypoxic condition with YM155, a novel small-molecule inhibitor of survivin, reversed the hypoxia-induced overexpression of survivin at the transcriptional and translational levels, as demonstrated by RT-qPCR, immunocytochemical and Western blot analyses. In addition, the results of the present study also indicated that the proliferation of the PASMCs was significantly induced in the hypoxia-cultured cells, compared with the normoxia-cultured cells. In addition, the apoptosis of the PASMCs was signifi-cantly inhibited, and the activation of Kv1.5 and Kv2.1 were reduced in the hypoxic HPASMCs. The therapeutic benefit of YM155 on HPASMCs during hypoxia was associated with the inhibited growth and increased apoptosis of PASMCs, as well as the reactivation of the expression of Kv1.5 and Kv2.1. These results suggested that survivin may be key in the pathophysi-ology of hypoxia-induced PH, and that the YM155 survivin inhibitor may offer therapeutic potential for use in the treatment of PH, although future investigations in animal models are required.
Gene microarray analysis has demonstrated that dysregulation of apoptotic mediators in the PA wall of patients with PH favored the suppression of apoptosis (21). Survivin is the smallest member of the IAP family, which is structurally unique and involved in essential cellular functions, and is prominently expressed in human cancer (22). High expression levels of survivin in cancer cells are associated with poor patient prognosis and survival rates, as well as resistance to therapy and an increased rate of cancer recurrence (13,23). However, the expression of survivin is not cancer specific, and it is involved in essential cellular functions. It has been observed to be upregulated in the media of small and medium-sized PAs from monocrotaline-induced pulmonary arterial hypertension rats, as well as idiopathic PH patients (14,21). Increasing evidence has indicated that the expression of survivin is increased in PAs of PH (24). A previous study on chronic hypoxia-induced PH demonstrated the overexpression of survivin in the media of the distal PAs of rats following chronic hypoxia (18). Hyperplasia of PASMCs is a characteristic pathological feature of pulmonary hypertension (25). Survivin may facilitate the transition of PASMCs to a proliferative state and inhibit apoptosis by hyperpolarizing mitochondria (14). This would result in the impaired activation of Kv channels and, subsequently, mitochondria-dependent apoptosis (9,26). Increased proliferation of PASMC has been reported in animals with hypoxia-induced pulmonary hypertension, which is often found to be associated with vascular remodeling (27) Consistent with the results of previous studies, the results of the present study indicated that exposure of PASMCs to hypoxia enhanced the proliferation and decreased the apoptosis of PASMCs, significantly upregulated the expression of prosurvival factor, survivin, and downregulated the expression of Kv1.5 and Kv2.1 channel proteins. This is consistent with the results of a previous study by McMurtry et al (14), which indicated that survivin allowed the PASMCs to enter a proliferative phase, parallel with a decrease in Kv1.5 expression. This previous study also provided direct evidence that survivin inhibition led to selective apoptosis of the proliferating PASMCs. This suggested that survivin may be central in the hyperplasia of hypoxia-induced PASMCs, which may also elucidate the therapeutic potential of survivin targeting in PH.
Multiple strategies to modulate the expression and activation of survivin have been developed. As a selective survivin inhibitor, YM155, a small molecule of sepantronium bromide, exerts a marked antiproliferative activity in a large panel of tumor cell models. Preclinical studies have indicated that continuous infusion of YM155 resulted in a reduction of intratumor survivin in tumor-bearing immunodeprived mice leading to marked tumor regression due to an enhanced apoptotic response (28). Survivin was demonstrated to be expressed at high levels in a previous study of chronic hypoxia-induced PH rats, and indicated that YM155 treatment downregulated the expression of survivin in the distal PAs and lung tissues during chronic hypoxia, reversing hypoxia-induced PH (18). This suggests YM155 as an ideal candidate in the treatment of PH. In the present study, the therapeutic effects of YM155 on the proliferation and apop-tosis of HPASMCs during hypoxia were investigated. The cells were treated with different concentrations of the YM155 survivin inhibitor. The results of demonstrated that, in addition to reversing the hypoxia-induced upregulation of survivin in the PASMCs, YM155 treatment significantly promoted apoptosis and suppressed PASMC proliferation, consistent with the results of the survivin-targeting strategy developed by McMurtry et al (14). The disturbance in the balance of apoptosis and proliferation in pulmonary vascular wall cells, favoring proliferation, induces the remodeling of PAs (29,30). Effective therapies require a shift in this balance towards apoptosis (31). The data of the present study indicated that the hypoxia-induced increase in survivin may have been associated with the imbalance of apoptosis and proliferation in the PASMCs, and the downregulation of survivin as a result of YM155 administration in the hypoxic PASMCs resulted in an enhanced apoptotic response.
Kv channels are an important factor in hypoxic PH progression (32,33). The inhibition or lack of Kv channels contributes to resistance to apoptosis (16), whereas Kv upregulation contributes to the promotion of apoptosis via K+ activity of caspases (8). Furthermore, PH is associated with selective inhibition of Kv channels. Prolonged hypoxia inhibits the mRNA and protein expression of Kv channels, and decreases the number of functional Kv channels in PASMCs. The resulting membrane depolarization raises cytoplasmic free calcium concentrations, stimulating PASMC proliferation and, ultimately, increasing pulmonary vascular resistance and PH (34). The upregulated expression of survivin in patients with PH has been suggested to be associated with the impaired activation of Kv channels and consequent mitochondria-dependent apoptosis (9,26). In vitro and in vivo investigations have demonstrated that survivin targeting induces mitochondria-dependent apoptosis of PASMCs, and the activation of K+ channels by survivin targeting may be important in this process (14,35). The present study further investigated the potential therapeutic mechanisms of YM155 on hypoxic HPASMCs, predominantly focussing on the expression of the specific PASMC Kv channels, Kv1.5 and Kv2.1. The results revealed that hypoxia exposure significantly inhibited the expression levels of Kv1.5 and Kv2.1 in the HPASMCs. YM155 treatment reversed the hypoxia-induced downregulation of Kv channel proteins in a dose-dependent manner, suppressed proliferation and enhanced apoptosis of the PASMCs. The expression levels of Kv1.5 and Kv2.1 increased, parallel with the decrease in survivin. By contrast, YM155 had little effect on the expression levels of Kv1.5 and Kv2.1 in the normoxic-cultured HPASMCs. These data suggested that survivin may regulate the balance of cell proliferation and apoptosis in hypoxic PASMCs through a Kv channel-associated mechanism, and YM155 may offer therapeutic potential for use in hypoxia-induced PH.
In conclusion, the present study demonstrated that exposure to hypoxia of HPASMCs significantly increased cell proliferation and suppressed apoptosis, upregulated the mRNA and protein expression levels of survivin, and reduced the activation of the Kv1.5 and Kv2.1 channels. YM155, a selective survivin inhibitor, reversed the hypoxia-induced apoptosis suppression and proliferation enhancement in the HPASMCs, in a dose-dependent manner, which was associated with survivin inhibition and reactivation of the Kv channels. These results are the first, to the best of our knowledge, to demonstrate that YM155 has a beneficial therapeutic effect on hypoxic HPASMCs, and provided evidence that the pro-apoptotic effects induced by YM155 involved downregulation of the apoptosis inhibitor, survivin, possibly via a Kv channel-mediated mechanism.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant no. 81170045).
Abbreviations
- CCK-8
Cell Counting Kit-8
- H
hypoxia
- Kv
voltage-dependent K+ channels
- IAP
inhibitor of apoptosis
- N
normoxia
- PAs
pulmonary arteries
- PASMCs
pulmonary artery smooth muscle cells
- HPASMCs
human PASMCs
- PBS
phosphate-buffered saline
- PH
pulmonary hypertension
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling
- YM155
sepantronium bromide
References
- 1.Guignabert C, Dorfmuller P. Pathology and pathobiology of pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:551–559. doi: 10.1055/s-0033-1356496. [DOI] [PubMed] [Google Scholar]
- 2.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 4.Hu HL, Zhang ZX, Chen CS, Cai C, Zhao JP, Wang X. Effects of mitochondrial potassium channel and membrane potential on hypoxic human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2010;42:661–666. doi: 10.1165/rcmb.2009-0017OC. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Chen C, Wang Z, Wang L, Yang L, Ding M, Ding C, Sun Y, Lin Q, Huang X, et al. Puerarin induces mitochondria-dependent apoptosis in hypoxic human pulmonary arterial smooth muscle cells. PLoS One. 2012;7:e34181. doi: 10.1371/journal.pone.0034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung GL. Evaluation of patients with pulmonary hypertension for lung transplantation. In: Yuan JXJ, Garcia JGN, Hales CA, Rich S, Archer SL, West JB, editors. Textbook of Pulmonary Vascular Disease. Springer; New York, NY: 2011. pp. 1593–1598. [DOI] [Google Scholar]
- 7.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling and fusion: A mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. AM J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 8.Park WS, Firth AL, Han J, Ko EA. Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells. J Smooth Muscle Res. 2010;46:89–105. doi: 10.1540/jsmr.46.89. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC. Validating survivin as a cancer therapeutic target. Nature Reviews Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 10.Zyada MM. Relationship of survivin to clinical drug resistance in Burkitt's lymphoma of the head and neck region. Med Oncol. 2011;28:1565–1569. doi: 10.1007/s12032-010-9581-5. [DOI] [PubMed] [Google Scholar]
- 11.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI200422222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvesen GS, Duckett CS. IAP proteins: Blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 13.Hingorani P, Dickman P, Garcia-Filion P, White-Collins A, Kolb EA, Azorsa DO. BIRC5 expression is a poor prognostic marker in ewing sarcoma. Pediatr Blood Cancer. 2013;60:35–40. doi: 10.1002/pbc.24290. [DOI] [PubMed] [Google Scholar]
- 14.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan BM, O'Donovan N, Duffy MJ. Survivin: A new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka K, Nakata M, Kaneko N, Fushiki H, Kita A, Nakahara T, Koutoku H, Sasamata M. YM155, a selective survivin suppressant, inhibits tumor spread and prolongs survival in a spontaneous metastatic model of human triple negative breast cancer. Int J Oncol. 2011;39:569–575. doi: 10.3892/ijo.2011.1077. [DOI] [PubMed] [Google Scholar]
- 17.Tao YF, Lu J, Du XJ, Sun LC, Zhao X, Peng L, Cao L, Xiao PF, Pang L, Wu D, et al. Survivin selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC Cancer. 2012;12:619. doi: 10.1186/1471-2407-12-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Fan Z, Li J, Liu Y, Wang N, Wang D, Liu Y, Zhang B. Expression of survivin in pulmonary artery of rats exposed to normoxia and hypoxia. International Journal of Respiration. 2013;33:994–998. [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Paulin R, Courboulin A, Meloche J, et al. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: A gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.RES.88.6.555. [DOI] [PubMed] [Google Scholar]
- 22.Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013;332:225–228. doi: 10.1016/j.canlet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: Molecular mechanism, prognostic and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 24.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1798–H1809. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 25.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- 26.Ekhterae D, Platoshyn O, Krick S, Yu Y, McDaniel SS, Yuan JX. Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C157–C165. doi: 10.1152/ajpcell.2001.281.1.C157. [DOI] [PubMed] [Google Scholar]
- 27.Reid L. Vascular remodeling. In: Fishman A, editor. The Pulmonary Circulation: Normal and Abnormal Mechansimc, Management and the National Registry. University of Pennsylvania Press; Philadelphia, PA: 1990. p. p264. [Google Scholar]
- 28.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M, Sasamata M. Broad spectrum and potent antitumor activities of YM155, a novel small-molecule survivin suppressant, in a wide variety of human cancer cell lines and xenograft models. Cancer Sci. 2011;102:614–621. doi: 10.1111/j.1349-7006.2010.01834.x. [DOI] [PubMed] [Google Scholar]
- 29.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: Role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez RA, Sundivakkam P, Smith KA, Zeifman AS, Drennan AR, Yuan JX. Pathogenic role of store-operated and receptor-operated ca(2+) channels in pulmonary arterial hypertension. J Signal Transduct. 2012;2012:951497. doi: 10.1155/2012/951497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurbanov E, Shiliang X. The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. Eur J Cardiothorac Surg. 2006;30:499–507. doi: 10.1016/j.ejcts.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 33.Moudgil R, Michelakis ED, Archer SL. The role of K+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation and apoptosis: Implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13:615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: Role of voltage-gated potassium channels. Respir Res. 2000;1:40–48. doi: 10.1186/rr11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1. 5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]