Abstract
Schizophrenia is a severe psychiatric illness that is characterized by reduced cortical connectivity, for which the underlying biological and genetic causes are not well understood. Although the currently approved antipsychotic drug treatments, which primarily modulate dopaminergic function, are effective at reducing positive symptoms (i.e. delusions and hallucinations), they do little to improve the disabling cognitive and negative (i.e. anhedonia) symptoms of patients with schizophrenia. This review details the recent genetic and neurobiological findings that link N-methyl-d-aspartate receptor (NMDAR) hypofunction to the etiology of schizophrenia. It also highlights potential treatment strategies that augment NMDA receptor function to treat the synaptic deficits and cognitive impairments.
Introduction
Schizophrenia is a chronic, disabling mental disorder that affects 1% of the population worldwide [1]. One of the cardinal pathological features of schizophrenia is a significant and progressive reduction in neuropil resulting in the atrophy of cortico-limbic regions of the brain without comparable loss of neurons [2]. A recent genome-wide association study (GWAS) has revealed over a hundred risk genes of modest effect, many of which encode proteins involved in glutamatergic transmission, particularly with the N-methyl-d-aspartate receptor (NMDAR) [3••].
Here, we describe the function of the NMDAR channel, with a particular focus on how the glycine modulatory site (GMS) regulates its activity. We will then discuss how NMDAR hypofunction contributes to the pathophysiology of schizophrenia. Finally, we will highlight the latest pharmacological treatments being developed to both, directly and indirectly, target the GMS as a means to augment NMDAR function.
Structure and function of the NMDA receptor
The NMDAR is a heterotetrameric, ion channel receptor primarily composed of two GluN1 subunits and two GluN2 subunits. It is widely distributed throughout most of the brain and is a critical postsynaptic mediator of activity-dependent synaptic plasticity. What makes activation of the NMDAR unique is that in addition to the binding of its agonist glutamate to the GluN2 subunit, the neuron must also be contemporaneously depolarized, which relieves the Mg2+ blockade of the channel, and either glycine or d-serine must be bound at the GMS on the GluN1 subunit. Although the concentration of glycine in the cerebrospinal fluid is high, the GMS of the NMDAR is not saturated in vivo [4]. The NMDAR ion channel is highly permeable to Ca2+ that upon entry into the neuron, triggers a cascade of intracellular events that mediate local, acute functional synaptic plasticity and changes in gene expression that influence long-term neural structural plasticity.
The glycine modulatory site: d-serine, glycine, and kynurenic acid
Serine racemase (SR), the enzyme that converts l-serine to d-serine [5], and d-serine itself, are enriched in the forebrain and their regional localization closely parallels that of NMDARs [6,7]. Recent evidence suggests that d-serine is the primary co-agonist for synaptic, but not extra-synaptic NMDARs [8•], via non-vesicular release through alanine-serine-cysteine-1 (Asc-1) transporters [9•]. d-Serine is synthesized almost exclusively by SR [10] using l-serine that is synthesized by the astrocytic enzyme, 3-phosphoglycerate dehydrogenase [11•,12]. d-Serine can be eliminated by the flavoenzyme d-amino acid oxidase (DAAO) or by SR. Initial studies suggested that SR was an astrocytic enzyme and therefore, astrocytes were considered to be the major source of d-serine in the brain [5,6,13]. However, recent studies have demonstrated a predominantly neuronal expression of SR [11•,14]. Using conditional SR−/− mice, the majority (~65%) of SR was shown to be expressed in excitatory forebrain neurons, while only 15% or less appeared to be expressed in astrocytes [15•]. Furthermore, SR is expressed in excitatory and inhibitory neurons in human post-mortem cortex [16•], which is in agreement with the findings in mice. Similar to SR, earlier studies suggested a primary localization of d-serine in astrocytes [6,17,18]. The high concentration of l-serine in astrocytes due to the expression of 3-phosphoglycerate dehydrogenase likely contributed to the artifactual immuno-crossreactivity with d-serine antibodies in these initial immunocytochemical studies. However, recent work utilizing SR−/− mice as a negative control to validate the d-serine immunostaining demonstrated that nearly all the d-serine is in neurons, particularly GABAergic neurons, but not in astrocytes [16•].
The sodium-dependent glycine transporters (GlyT), of which there are two types, GlyT1 and GlyT2, are considered the primary regulators of intra-cellular and extracellular glycine levels [19]. The concentration of glycine in mammalian CSF is high relative to its dissociation constant (KD) for the GMS, but local glycine levels are functionally regulated at the synapse. Although GlyT1 and GlyT2 are both expressed in the cerebellum and spinal cord, where inhibitory glycinergic neurotransmission is concentrated, GlyT1 is also found in the forebrain [20]. GlyT1 is widely expressed in glial cells, as well as in neurons at glutamatergic synapses [21], and is thought to regulate NMDAR activity by affecting availability of this co-agonist [4]. The activity of GlyT1 is itself regulated by the endogenous inhibitor, sarcosine (N-methylglycine), an intermediate and byproduct in glycine synthesis and degradation. GlyT2 is primarily localized to the presynaptic terminals of glycinergic neurons [20] to serve as the main mechanism for synaptic inactivation.
Kynurenic acid, which is present in the human brain in high nanomolar concentrations, is derived from the metabolism of tryphtophan by several unique kynurenine aminotransferases (KATs) that catalyze the irreversible transamination of kynurenine to kynurenic acid. It is a competitive antagonist at the GMS of the NMDAR [22]. In addition, kynurenic acid is a non-competitive antagonist at the a7 nicotinic acetylcholine receptor (a7nAChR) [23] and has antioxidant properties [24].
NMDA receptor hypofunction and schizophrenia
There is substantial pharmacologic, genetic, and bio-chemical evidence to support NMDAR hypofunction as a key etiological component of schizophrenia. Dissociative anesthetics such as ketamine and phencyclidine (PCP) were known since their introduction to produce in adults a syndrome difficult to distinguish from schizophrenia [25]. In healthy individuals, low doses of ketamine that do not cause delirium or dementia produce the full range of schizophrenia symptoms, while those with stabilized schizophrenia exhibit increased sensitivity to ketamine [26]. Individuals with NMDAR autoimmune encephalitis, which is associated with antibodies against the extracellular epitopes of the NR1A subunit, initially present with psychiatric symptoms similar to schizophrenia [27]. It is believed these antibodies cause NMDAR internalization [28•]. A recent study also detected NMDAR antibodies, particularly IgG-NR1A, in acutely ill schizophrenic patients that are different from the antibodies detected in NMDAR encephalitis [29].
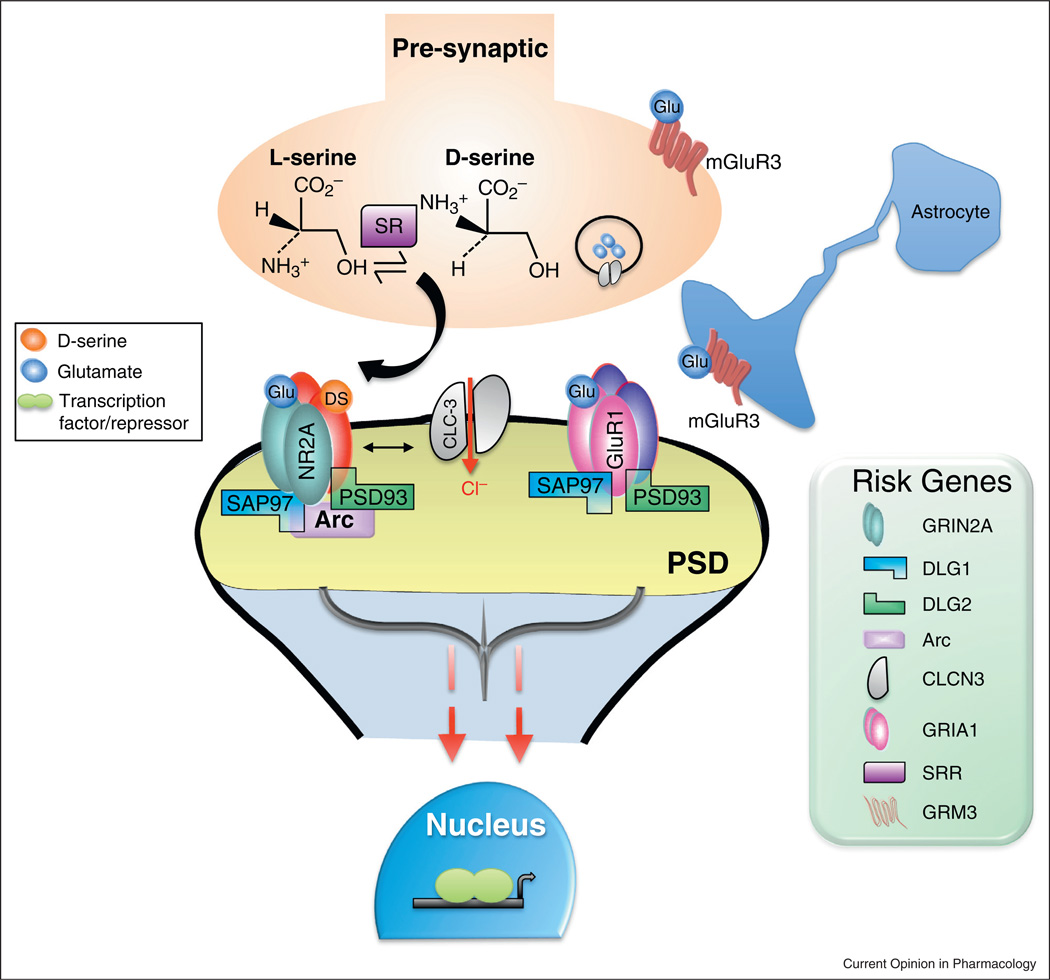
Unbiased meta-analysis of genetic association studies reveal the remarkable concentration of risk genes encoding proteins within 2° of separation of the NMDAR [30]. Furthermore, recent large-scale, copy number variant (CNV) analyses have implicated de novo mutations in genes that encode the NMDAR and proteins associated with the postsynaptic density (PSD) with increased risk of schizophrenia [31–33]. Moreover, the largest genome wide association study (GWAS) of schizophrenia to date (~37,000 cases and ~113,000 controls) identified independent associations [3••], implicating numerous brain-enriched genes that are involved in glutamatergic transmission (Figure 1), including GRIN2A (NMDAR subunit 2A), SRR (serine racemase), the metabotropic 3 glutamate receptor (GRM3) and the glutamate receptor 1 (GRIA1).
Figure 1.
Numerous schizophrenia risk genes are involved in glutamatergic transmission. Recent large-scale schizophrenia GWAS [3••] and exome sequencing [31] studies have identified over 108 genetic loci and de novo mutations, respectively, associated with increased risk for schizophrenia. Many of the genes code for proteins that participate in excitatory transmission, particularly those that interact with the NMDAR. The NMDAR subunit 2A (GRIN2A; NR2A) is an important mediator of synaptic plasticity, with de novo mutations reported in schizophrenia. Discs, large homolog 1 (DLG1; SAP97) and 2 (DLG2; PSD93) belong to the membrane-associated guanylate kinases (MAGUKs) family of scaffolding proteins that regulate NMDAR and AMPAR trafficking, as well as postsynaptic density (PSD) organization [61]. The immediate early gene activity-regulated cytoskeleton-associated protein (ARC; Arc) is expressed almost exclusively in glutamatergic neurons, where it is enriched in the PSD and co-localizes with the NMDAR. Chloride channel, voltage-sensitive 3 (CLC-3; CLCN-3) is a voltage-gated chloride channel that co-localizes with the NMDAR postsynaptically to regulate neuronal excitability [62], and is localized to synaptic vesicles presynaptically where it regulates their size, glutamate content, and release probability [63]. The a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1 (GRIA1; GluR1) is a subunit of the ionotropic glutamate AMPAR that is a critical mediator of fast synaptic transmission. Serine racemase (SRR; SR) is the enzyme that converts l-serine to d-serine, one of the two NMDAR co-agonists. The metabotropic glutamate-3 receptor (GRM3; mGluR3), which is located presynaptically and on astrocytes, provides feedback inhibition of glutamate release from presynaptic terminals. The risk loci and mutations identified in recent genetic studies point to a convergence of perturbations in the PSD complex that would potentially lead to altered intracellular signaling, changes in the transcription of genes important for neuroplasticity, and ultimately impaired connectivity.
Biochemical changes in brain tissue from human post-mortem samples also suggest reduced NMDAR function in schizophrenia. Although there have been mixed results showing NMDAR abnormalities, recent work demonstrated reduced mRNA and protein levels of the NR1 and NR2C subunits in schizophrenia [34•]. In addition to the NMDAR itself, there are numerous abnormalities of GMS modulators not only in the brain, but also the periphery, of patients with schizophrenia. Serine race-mase (SR) and d-serine are reduced in schizophrenia [35–39]. There are also elevated levels of the endogenous GMS antagonist, kynurenic acid, in the cerebral spinal fluid (CSF) and post-mortem brain tissue [40,41]. The latter results suggest that the GMS occupancy is shifted toward antagonism in the disease state.
Finally, there is a wealth of data from diverse animal models, which are beyond the scope of this review, that support the clinical observations of NMDAR hypofunction contributing to the schizophrenia disease process. For example, SR deficient transgenic (SR−/−) mice, which lack d-serine and display NMDAR hypofunction, recapitulate many of the structural and molecular brain abnormalities, as well as cognitive deficits associated with schizophrenia [10,42•,43]. Mice lacking dybsindin (DTNBP1), a risk gene for schizophrenia, have reduced NMDAR function and concomitant deficits in neuroplasticity and cognition [44,45]. Postnatal reduction of the NR1 subunit on GABAergic interneurons produces numerous schizophrenia-related behavioral abnormalities and cortical asynchrony [46].
The glycine modulatory site as drug target for schizophrenia
The GMS of the NMDAR is a potential therapeutic target because the GMS is not saturated in vivo [4], supporting the hypothesis that administration of GMS agonists could benefit patients by enhancing activation of NMDARs. There are several strategies to augment NMDAR function by altering the availability or concentration of GMS co-agonists and antagonists. Furthermore, the efficacy of GMS mediated augmentation of NMDAR function supports the utility of other allosteric interventions at the NMDAR. As such, this is a very active area of research both in the pharmaceutical sector and academia.
There have been more than 70 placebo-controlled clinical trials of GMS agonists in schizophrenia, including d-serine, glycine, d-cycloserine (DCS), and d-alanine. The results have been mixed; many studies reported significant improvements over multiple symptom domains while others did not. Other than intrinsic differences in efficacy among these GMS agonists, methodological factors likely contributed to the variability in results among these trials, most notably small sample sizes, differences in concomitant typical and atypical antipsychotic use, and subject compliance. It should be noted that among the moieties studied glycine has poor efficacy and requires high doses (at least 60 g/day), and the partial GMS agonist DCS, has an inverted-U dose response curve [47] and causes NMDAR desensitization following chronic treatment [48]. Nevertheless, a recent meta-analysis of 26 double-blind placebo controlled clinical trials in which the treatment lasted at least 4 weeks reported that these agonists as a whole significantly improved positive, negative, and cognitive symptoms [49].
The NIMH funded a large, multi-center, placebo controlled clinical trial of d-cycloserine and glycine added on to antipsychotic drugs in subjects with chronic schizophrenia that reported no effects of either drug on SANS or cognition [50]. Although seemingly a definitive rejection of the hypothesis, it was a failed study because there were substantial outcome differences among the sites (p < 0.01); and post hoc analysis revealed significant effects of both d-cycloserine and glycine in subjects tested on inpatient units where there was assured compliance.
When analyzed alone, d-serine improved total psychopathology, negative symptoms, and cognitive symptoms. The potential effects of GMS agonists on positive symptoms may be obscured by the strategy of studying them as an add-on therapy to typical or atypical antipsychotics. Large Phase II trials testing the efficacy of d-serine for treating schizophrenia and the schizophrenia prodrome are ongoing, both as an add-on to antipsychotics and as a monotherapy. Furthermore, this meta-analysis also corroborates the intriguing pattern in the literature that GMS agonists are ineffective when combined with clozapine as opposed to other antipsychotics, which could be explained by an effect of clozapine on GMS occupancy [51].
An alternative approach to directly increasing GMS agonist availability is to block their reuptake. As the uptake and release mechanisms for d-serine are poorly understood, research has focused on inhibiting glycine reuptake via GlyT1 blockade. Sarcosine (N-methylglycine) an intermediate and byproduct in glycine synthesis and degradation, is an endogenous competitive GlyT1 antagonist. The Tsai and Lin [49] meta-analysis found it effective on total psychopathology, negative symptoms, and general psychopathology. However, undesirable side effects of sarcosine-derived GlyT1 inhibitors, such as ataxia, hypoactivity, and decreased respiration, have prompted the development of non-sarcosine-based GlyT1 inhibitors. Unfortunately, the noncompetitive GlyT-1 antagonist from Hoffman-LaRoche, bitoperin, which significantly reduced negative symptoms in a Phase-II trial in schizophrenia, failed to reach its endpoints to improve negative symptoms in two recent Phase III trials (http://www.roche.com/media/media_releases/med-cor-2014-01-21.htm). Perhaps, inhibiting GlyT1 augments NMDAR function primarily at extra-synaptic receptors as suggested by recent electrophysiological studies [8•,52], thereby undercutting therapeutic effects.
Inhibiting DAAO, the enzyme that catabolizes d-amino acids, like d-serine and d-alanine, can increase GMS agonist availability. Genetic association studies of DAAO and DAAO activator (G72) with schizophrenia and the expression and activity of DAAO are increased in patients with schizophrenia [53]. Benzoate is a naturally occurring DAAO inhibitor that is considered a generally safe food preservative by the FDA. In a small randomized, double-blind, placebo-controlled trial, sodium benzoate significantly improved positive, negative, and cognitive symptoms in patients with schizophrenia stabilized on antipsychotics [54]. Although larger studies are needed to validate these findings, this study encourages the search for novel DAAO inhibitors.
Reducing the levels of the endogenous GMS antagonist, kynurenic acid, is another strategy to restore the NMDAR hypofunction observed in schizophrenia. Preclinical studies in normal rodents demonstrate that kynurenine, the immediate precursor to kynurenic acid, impairs cognition. KATII inhibitors, which block the formation of kynurenic acid, prevent the cognitive and auditory-gating deficits in rodents and nonhuman primates caused by drugs such as, ketamine and amphetamine [55,56•]. As noted above, kynurenic acid is also a potent non-competitive antagonist of the a7nAChR, a risk gene for schizophrenia, and disrupts cognitive flexibility [57]. Indeed, Albuquerque and Schwarcz [58] argue that the α-7-nicotinic acetylcholine receptor is the primary site at which kyurenic acid exerts its pro-schizophrenic effects. Regardless, these findings suggest that the modulation of the kynurenine pathway could be a novel mechanism by which to attenuate the cognitive symptoms in schizophrenia.
Conclusions
Data from both preclinical and clinical studies strongly implicate the NMDAR as a point of convergence for many of the signaling pathways that are affected in schizophrenia. Thus, much effort has been made to develop novel therapeutics that target the GMS to stimulate NMDAR activity, a strategy now supported by the discovery that SR is a risk gene for schizophrenia. A more sanguine interpretation of studies to directly or indirectly activate NMDAR GMS function is that the results pro-vide only proof of principle for the therapeutic effects of enhancing NMDAR function in schizophrenia, but not evidence for a viable long-term treatment. Chronic d-serine promotes NMDAR internalization; chronic d-cy-closerine results in desensitization; exogenous glycine may preferentially act at extra-synaptic NMDARs. Nevertheless, other attractive strategies that accomplish enhanced synaptic NMDAR function without the GMS limitations, include positive allosteric NMDAR modulators [59], mGluR5 agonists [57], a7nAChR agonists [60] that indirectly enhance NMDAR function, and muscarinic acetylcholine receptor (M1) positive allosteric modulators [60].
Acknowledgments
Conflict of interest statement
A Phyllis & Jerome Lyle Rappaport Mental Health Research Scholars Award and 1K99MH099252-01A1 (DTB), as well as grants R01MH05190 and P50MH0G0450 (JTC) supported this work.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 2.DeLisi LE, Szulc KU, Bertisch HC, Majcher M, Brown K. Understanding structural brain changes in schizophrenia. Dialogues Clin Neurosci. 2006;8:71–78. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. The largest schizophrenia genome-wide association study to date (36,989 cases and 113,075 controls), identified 128 independent associations that meet genome-wide significance. The associations were enriched among genes expressed in brain, and included genes involved in glutamatergic signaling, the dopamine 2 receptor, and immunity.
- 4.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schell MJ, Molliver ME, Snyder SH. d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 8. Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. Using in vitro hippocampal electrophysiology, the authors report that synaptic and extrasynaptic NMDARs are regulated by different endogenous coagonists, d-serine and glycine, respectively. They also show that long-term potentiation and NMDA-induced neurotoxicity rely on synaptic NMDARs, while long-term depression requires both synaptic and extrasynaptic receptors.
- 9. Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM, et al. Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci. 2013;33:3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013. The authors demonstrate using cell culture and in vitro electrophysiology that the alanine-serine-cysteine transporter-1 (Asc-1) mediates the release of both d-serine and glycine from neurons, thereby modulating NMDAR synaptic activity.
- 10.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, Snyder SH, Wolosker H. d-Serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci. 2013;33:12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013. The authors show that neuronal d-serine is dependent on l-serine that is produced in astrocytes by the enzyme 3-phosphoglycerate dehydrogenase.
- 12.Yang JH, Wada A, Yoshida K, Miyoshi Y, Sayano T, Esaki K, Kinoshita MO, Tomonaga S, Azuma N, Watanabe M, et al. Brain-specific Phgdh deletion reveals a pivotal role for l-serine biosynthesis in controlling the level of d-serine, an N-methyl-d-aspartate receptor co-agonist, in adult brain. J Biol Chem. 2010;285:41380–41390. doi: 10.1074/jbc.M110.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 15. Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. 2012;32:613–624. doi: 10.1007/s10571-012-9808-4. Using conditional mutant mice, the authors show that serine racemase is predominantly expressed in excitatory forebrain neurons.
- 16. Balu DT, Takagi S, Puhl MD, Benneyworth MA, Coyle JT. d-Serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol. 2014;34:419–435. doi: 10.1007/s10571-014-0027-z. Using immunohistochemistry and serine racemase deficient mice, the authors demonstrate that serine racemase and D-serine are localized primarily in neurons. Interestingly, a significant amount of D-serine is concentrated in cortical and hippocampal inhibitory neurons.
- 17.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, et al. Glial d-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex. 2012;22:595–606. doi: 10.1093/cercor/bhr130. [DOI] [PubMed] [Google Scholar]
- 18.Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP. Storage and uptake of d-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci. 2013;33:3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V. Glycine transporters: essential regulators of synaptic transmission. Biochem Soc Trans. 2006;34:55–58. doi: 10.1042/BST0340055. [DOI] [PubMed] [Google Scholar]
- 20.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 21.Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 22.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 23.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugo-Huitron R, Blanco-Ayala T, Ugalde-Muniz P, Carrillo-Mora P, Pedraza-Chaverri J, Silva-Adaya D, Maldonado PD, Torres I, Pinzon E, Ortiz-Islas E, et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol. 2011;33:538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 26.Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 27.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. This review describes the cases and clinical manifestations of encephalitis associated with NMDAR antibodies since their discovery in 2007. The patients’ antibodies cause a titre-dependent, yet reversible, decrease of synaptic NMDARs by a mechanism of crosslinking and internalization.
- 29.Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, Kastner A, Skalej M, Jordan W, Schiltz K, et al. Increased prevalence of diverse N-methyl-d-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-d-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 30.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 31.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:178–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kahler A, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT, Newell KA, Pellen D, Huang XF, Catts SV, et al. Molecular evidence of N-methyl-d-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–1192. doi: 10.1038/mp.2012.137. The authors find that the NR1 subunit (mRNA and protein) and NR2C (mRNA) are decreased in post-mortem dorsolateral prefrontal cortex from people with schizophrenia. They also show in post-mortem brain that the NR2B single nucleotide polymorphism (rs1805502; T5988C)C allele was associated with reduced NR1 mRNA and protein in schizophrenia.
- 35.Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Goltsov AY, Loseva JG, Andreeva TV, Grigorenko AP, Abramova LI, Kaleda VG, Orlova VA, Moliaka YK, Rogaev EI. Polymorphism in the 5′-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry. 2006;11:325–326. doi: 10.1038/sj.mp.4001801. [DOI] [PubMed] [Google Scholar]
- 37.Labrie V, Wang W, Barger SW, Baker GB, Roder JC. Genetic loss of d-amino acid oxidase activity reverses schizophrenia-like phenotypes in mice. Genes Brain Behav. 2009;9:11–25. doi: 10.1111/j.1601-183X.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, et al. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry. 2007;61:1200–1203. doi: 10.1016/j.biopsych.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M. Reduced d-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 41.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 42. Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A. 2013;110:E2400–E2409. doi: 10.1073/pnas.1304308110. The authors demonstrate that genetically altered mice that lack serine racemase exhibit many of the same neurochemical and morphological brain, as well as cognitive abnormalities that occur in schizophrenia. The deficiencies in serine racemase knockout mice are reversed with d-serine that was administered during adulthood.
- 43.DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, Eichenbaum H. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–222. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–E970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. Reduced dysbindin expression mediates N-methyl-d-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry. 2011;69:28–34. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2009;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goff DC, Tsai G, Manoach DS, Coyle JT. Dose-finding trial of d-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry. 1995;152:1213–1215. doi: 10.1176/ajp.152.8.1213. [DOI] [PubMed] [Google Scholar]
- 48.Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- 49.Tsai GE, Lin PY. Strategies to enhance N-methyl-d-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 50.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 51.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Sacchi S, Pollegioni L, Basu AC, Coyle JT, Bolshakov VY. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat Commun. 2013;4:1760. doi: 10.1038/ncomms2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verrall L, Burnet PW, Betts JF, Harrison PJ. The neurobiology of d-amino acid oxidase and its involvement in schizophrenia. Mol Psychiatry. 2010;15:122–137. doi: 10.1038/mp.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lane HY, Lin CH, Green MF, Hellemann G, Huang CC, Chen PW, Tun R, Chang YC, Tsai GE. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry. 2013;70:1267–1275. doi: 10.1001/jamapsychiatry.2013.2159. [DOI] [PubMed] [Google Scholar]
- 55.Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, Chapin DS, McGinnis D, Abbott AL, Roberts BM, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–10602. doi: 10.1523/JNEUROSCI.1107-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. This review details the kynurenine pathway, which is an enzymatic cascade that regulates the metabolism of tryptophan into neuroactive compounds. Perturbations in this pathway are associated with neurodegenerative, as well as psychiatric diseases such as depression and schizophrenia.
- 57.Herman EJ, Bubser M, Conn PJ, Jones CK. Metabotropic glutamate receptors for new treatments in schizophrenia. Handb Exp Pharmacol. 2012;29:7–365. doi: 10.1007/978-3-642-25758-2_11. [DOI] [PubMed] [Google Scholar]
- 58.Albuquerque EX, Schwarcz R. Kynurenic acid as an antagonist of alpha7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol. 2013;85:1027–1032. doi: 10.1016/j.bcp.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul SM, Doherty JJ, Robichaud AJ, Belfort GM, Chow BY, Hammond RS, Crawford DC, Linsenbardt AJ, Shu HJ, Izumi Y, et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-d-aspartate receptors. J Neurosci. 2013;33:17290–17300. doi: 10.1523/JNEUROSCI.2619-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones CK, Byun N, Bubser M. Muscarinic nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin EI, Jeyifous O, Green WN. CASK regulates SAP97 conformation and its interactions with AMPA and NMDA receptors. J Neurosci. 2013;33:12067–12076. doi: 10.1523/JNEUROSCI.0816-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang XQ, Deriy LV, Foss S, Huang P, Lamb FS, Kaetzel MA, Bindokas V, Marks JD, Nelson DJ. CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons. Neuron. 2006;52:321–333. doi: 10.1016/j.neuron.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 63.Guzman RE, Alekov AK, Filippov M, Hegermann J, Fahlke C. Involvement of ClC-3 chloride/proton exchangers in controlling glutamatergic synaptic strength in cultured hippocampal neurons. Front Cell Neurosci. 2014;8:143. doi: 10.3389/fncel.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]