Abstract
Dysfunctional self-awareness has been posited as a key feature of drug addiction, contributing to compromised control over addictive behaviors. In the present investigation, we showed that, compared with healthy controls (n=13) and even individuals with remitted cocaine use disorder (n=14), individuals with active cocaine use disorder (n=8) exhibited deficits in basic metacognition, defined as a weaker link between objective performance and self-reported confidence of performance on a visuo-perceptual accuracy task. This metacognitive deficit was accompanied by gray matter volume decreases, also most pronounced in individuals with active cocaine use disorder, in the rostral anterior cingulate cortex, a region necessary for this function in health. Our results thus provide a direct unbiased measurement – not relying on long-term memory or multifaceted choice behavior – of metacognition deficits in drug addiction, which are further mapped onto structural deficits in a brain region that subserves metacognitive accuracy in health and self-awareness in drug addiction. Impairments of metacognition could provide a basic mechanism underlying the higher-order self-awareness deficits in addiction, particularly among recent, active users.
Keywords: Drug addiction, metacognition, self-awareness, anterior cingulate cortex, voxel-based morphometry, magnetic resonance imaging
1. INTRODUCTION
Drug addiction is characterized by pervasive and reliable neurocognitive impairments (Goldstein et al., 2004; Woicik et al., 2009). Recently, we posited that an underappreciated neurocognitive impairment in drug addiction involves dysfunctional self-awareness of higher-order neurocognitive functions including behavioral monitoring and self-perception of illness severity (Goldstein et al., 2009). At the core of this self-awareness deficit may be functional and structural abnormalities of anterior prefrontal cortical (aPFC) structures, including the rostral subregion of the anterior cingulate cortex (rACC) [Brodmann Areas (BA) 24, 32)] extending into the adjacent ventromedial prefrontal cortex (BAs 10, 11, 25) (Moeller and Goldstein, 2014). Support for these hypotheses largely derived from comparing individuals with cocaine use disorder (CUD) to healthy controls on a self-awareness measure that is based on a mismatch between actual ongoing choice for viewing drug-related pictures with retrospective self-report of this choice, with implications for increased drug-seeking behavior and decreased social-emotional functioning (Moeller et al., 2012; Moeller et al., 2014; Moeller et al., 2010). Nevertheless, achieving a mechanistic understanding of this self-awareness deficit requires tapping into basic metacognitive functioning, thereby removing potential ambiguities associated with multifactorial choice behavior and other higher-order constructs. A basic metacognitive task could enable direct translation of self-awareness and insight deficits across psychopathologies (van der Meer et al., 2013) and potentially even across species (Lak et al., 2014; Lucantonio et al., 2014).
In the present study, we tested for metacognitive deficits in individuals with CUD using a basic visuo-perceptual task. In particular, we capitalized on recent computational models of metacognitive accuracy to allow an unbiased measure of the participants’ sensitivity to their own performance (meta-d’), defined as the degree to which participants’ objective performance during basic perceptual judgments maps onto their self-reported confidence in such basic perceptual performance (Fleming et al., 2014; Fleming et al., 2010). These models can circumvent the difficulty in objectively measuring self-awareness in the lab (Fleming and Lau, 2014), reducing reliance on higher-order processes such as long-term memory that could contribute to potentially inaccurate retrospective reporting (Moeller et al., 2010). We hypothesized that CUD participants, particularly active/recent users of cocaine (Moeller et al., 2010), would display impairments in basic metacognitive accuracy, which in turn would be associated with gray matter volume (GMV) decreases in the aPFC (Fleming et al., 2014; Fleming et al., 2010). Structural integrity of this region is of core importance for both self-awareness and basic metacognition (Fleming et al., 2014; Fleming et al., 2010; Moeller et al., 2014).
2. EXPERIMENTAL PROCEDURES
2.1 Participants
Twenty-two individuals with CUD (15 men) and 13 matched healthy controls (7 men) participated in this research (Table 1). Participants were recruited through advertisements, local treatment facilities, and word of mouth; all provided written informed consent in accordance with the Mount Sinai Institutional Review Board. Exclusion criteria were: (A) history of head trauma or loss of consciousness (> 30 min) or other neurological disease of central origin (including seizures); (B) abnormal vital signs at time of screening; (C) history of major medical conditions, encompassing cardiovascular (including high blood pressure), endocrinological (including metabolic), oncological, or autoimmune diseases; (D) history of major psychiatric disorder [for CUD, exceptions to this criterion included other substance use disorders (SUDs) and/or comorbidities that are highly prevalent in this population (e.g., post-traumatic stress disorder); for healthy controls, an exception was nicotine dependence]; (E) pregnancy as confirmed with a urine test in all females; (F) contraindications to the magnetic resonance imaging (MRI) environment; (G) except for cocaine in the CUD participants, positive urine screens for psychoactive drugs or their metabolites (amphetamine or methamphetamine, phencyclidine, benzodiazepines, cannabis, opiates, barbiturates and inhalants); and (H) alcohol intoxication, verified by trained research staff who have extensive experience with recognizing signs of intoxication in CUD participants and confirmed by breathalyzer.
Table 1.
Demographics and drug use of all study participants.
| Between-Group Test | Urine Positive Cocaine (N=8) |
Urine Negative Cocaine (N=14) |
Healthy Controls (N=13) |
|
|---|---|---|---|---|
| Gender: Male/Female | χ2=2.50 | 4/4 | 11/3 | 7/6 |
| Race: African-American/Caucasian/Other | χ2=3.86 | 7/0/1 | 7/4/3 | 9/2/2 |
| Age (years) | F=0.64 | 46.3 ± 9.1 | 44.9 ± 8.5 | 42.4 ± 6.9 |
| Education (years) | F=3.26 | 12.5 ± 1.4 | 13.6 ± 1.5 | 14.4 ± 1.9 |
| Verbal IQ: WRAT III – Scaled Score (Wilkinson, 1993) | F=3.08 | 90.9 ± 9.1 | 99.9 ± 9.8 | 99.7 ± 7.8 |
| Non-Verbal IQ: WASI – Matrix Reasoning Scale (Wechsler, 1999) | F=0.47 | 11.0 ± 1.5 | 10.5 ± 1.8 | 11.1 ± 1.5 |
| Depression: Beck Depression Inventory II (Beck et al., 1996) | F=7.02* | 11.9 ± 7.6C | 5.6 ± 6.0 | 2.5 ± 3.3A |
| Smoking status (smoker/nonsmoker) | χ2=10.32* | 6/2C | 7/7C | 1/12A,B |
| Cocaine diagnosis status: current/partial or sustained remissionD | χ2=1.01 | 4/4 | 4/10 | – |
| Cocaine age of onset (years) | z=−0.14 | 25.3 ± 9.8 | 24.2 ± 6.9 | – |
| Cocaine duration of use (years) | z=−1.99 | 20.0 ± 7.7 | 13.4 ± 6.0 | – |
| Cocaine past month use: days/week | z=−2.85* | 3.1 ± 2.7B | 0.6 ± 1.6A | – |
| Cocaine past month use: $/use | z=−3.36* | 105.0 ± 175.5B | 3.1 ± 7.8A | – |
| Cocaine current abstinence: days (min – max, median) | z=−2.90* | 1–180, 12B | 4–5840, 365A | – |
| Cocaine heaviest use: days/week | z=−0.46 | 6.6 ± 1.2 | 6.4 ± 1.3 | – |
| Cocaine heaviest use: $/use | z=−0.00 | 183.4 ± 179.5 | 222.9 ± 203.2 | – |
| Cocaine longest abstinence: years (min – max, median) | z=−1.93 | 0–4, 1 | 0–16, 4 | – |
| Withdrawal symptoms: CSSA (0–126) | t=2.49* | 29.6 ± 11.9B | 15.2 ± 10.8A | – |
| Severity of Dependence Scale (0–15) | t=0.56 | 4.6 ± 5.2 | 6.1 ± 6.2 | – |
| Cocaine Craving Questionnaire (0–45) | t=6.57* | 30.0 ± 10.5B | 4.7 ± 6.1A | – |
| Alcohol Status (current/past/none)E | χ2=0.73 | 2/2/4 | 3/6/5 | – |
| Alcohol Abstinence: Active or Past Users (days: min–max, median) | z=−0.56 | 5–730, 281 | 1–3650, 183 | – |
| Marijuana Status (current/past/none) | χ2=1.52 | 0/1/7 | 1/4/9 | – |
| Marijuana Abstinence: Active or Past Users (days: min–max, median) | z=−0.88 | 5475 | 120–9855, 365.0 | – |
| Opioid Status (current/past/none) | χ2=1.47 | 0/2/6 | 2/2/10 | – |
| Opioid Abstinence: Active or Past Users (days: min – max, median) | z=−0.47 | 730–4745, 2737.5 | 180–6935, 729 | – |
Note. Values are frequencies or means ± standard deviation;
p<0.05
(mean value differs from that of urine positive cocaine participants;
mean value differs from that of urine negative cocaine participants;
mean value differs from that of controls);
urine negative cocaine participants, despite not having used cocaine within 72 hours, could still meet criteria for current cocaine use disorder; conversely, urine positive cocaine participants, despite having used cocaine within 72 hours, could still meet criteria for cocaine use disorder in partial remission.
Data missing for 2 participants. WRAT = Wide Range Achievement Test; WASI = Wechsler Abbreviated Scale of Intelligence; CSSA = Cocaine Selective Severity Assessment Scale.
Participants underwent a comprehensive diagnostic interview, consisting of: (A) Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996); (B) Addiction Severity Index (McLellan et al., 1992), a semi-structured interview instrument used to assess history and severity of substance-related problems in seven problem areas (medical, employment, legal, alcohol, other drug use, family-social functioning, and psychological status); (C) Cocaine Selective Severity Assessment Scale (Kampman et al., 1998), measuring cocaine abstinence/withdrawal signs and symptoms (i.e., sleep impairment, anxiety, energy levels, craving, and depressive symptoms) 24 hours within the time of interview; (D) Severity of Dependence Scale (Gossop et al., 1992); and (E) Cocaine Craving Questionnaire (Tiffany et al., 1993). This interview identified the following cocaine-related diagnoses: current CUD (n=8) and CUD in sustained or partial remission (n=14). Because in previous studies self-awareness deficits were accentuated in active cocaine users testing positive for cocaine in urine (i.e., active users) (Moeller et al., 2010), we split our cocaine sample a priori by cocaine urine status. These subgroups included those with cocaine positive urine screens (though not currently intoxicated), objectively indicating recent cocaine use within 72 hours (n=8: CUD+), and those with cocaine negative urine screens who did not use cocaine within 72 hours of the study (n=14: CUD−). These sample sizes (including for controls: n=13), albeit relatively small, are consistent with prior studies of this metacognitive accuracy task in a different clinical population with GMV lesions of the aPFC (Fleming et al., 2014). Furthermore, this sample size is consistent with the anticipated effect sizes in CUD participants. Specifically, we previously showed evidence of an insight deficit (i.e., unawareness of drug-choice) in CUD+ and associations of such impairment with drug-relevant outcomes (e.g., withdrawal symptoms indicative of recent use) (Moeller et al., 2010), with effect sizes (Cohen’s d) that averaged 1.05. Detecting these robust effect sizes with 3 groups, and with p<0.05 and 0.80 power, was calculated to require 36 total participants. As expected, the CUD+ participants reported more recent cocaine use, less abstinence, and more current craving and withdrawal; however, the cocaine subgroups did not differ in cocaine use age of onset, duration of use, heaviest use or severity of dependence (Table 1). Thus, differences between these subgroups can be plausibly attributed to recency of drug use rather than severity. Current and remitted substance-related comorbidities, none of which differed between CUD+ and CUD−, included alcohol use disorder, marijuana use disorder, and opioid use disorder (see Table 1 for frequencies and abstinence lengths for these additional substances). One CUD participant also reported remitted use disorder of other stimulants and sedatives (last use of these substances was 9 years prior to the study). Problematic use of more than one substance is quite common for individuals with SUDs (Grant et al., 2015). Finally, one CUD participant met criteria for remitted post-traumatic stress disorder.
2.2 Perceptual Metacognition Task (Figure 1A)
Figure 1.
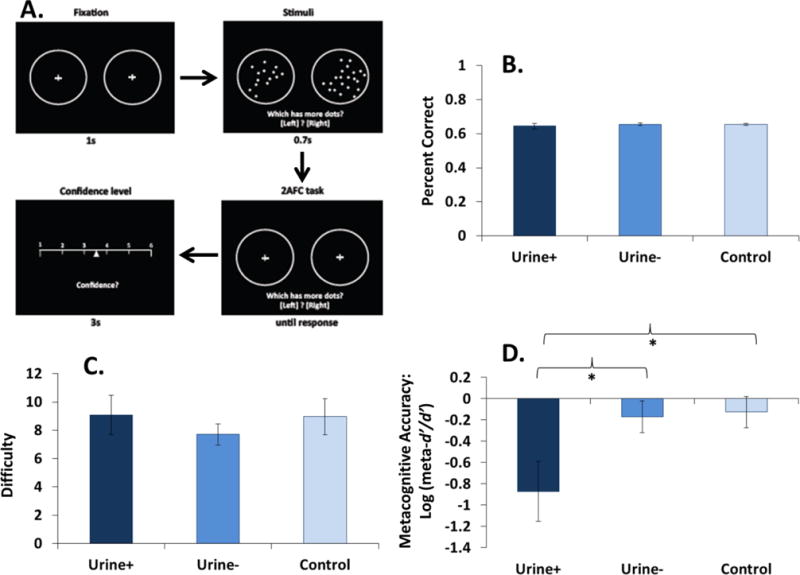
Behavioral effects on the metacognitive accuracy task in CUD+ (n=8), CUD− (n=14), and controls (n=13). (A) Task schematic. (B–C) Although not differing on task accuracy and staircase-adjusted task difficulty, (D) CUD+ had lower metacognitive accuracy than the other groups.
The metacognitive accuracy task was programmed in MATLAB (MathWorks) using Psychtoolbox (Brainard, 1997). Participants sat in front of a computer screen at a comfortable viewing distance and completed a perception task. Participants made a two-choice discrimination judgment about what they had perceived, followed by a confidence rating in their decision. The task consisted of the following sequence of events. Two circles (diameter 5.1 degrees) with small crosshairs in their centers appeared at eccentricities of ± 8.9 degrees for 1 s. The crosshairs then disappeared and a variable number of dots (diameter 0.4 degrees) were displayed inside both circles for 0.7 s. Circles and dots were displayed at maximum contrast (white) on a black background. After stimulus presentation, participants were instructed to guess which circle, left or right, contained more dots. If the left circle contained more dots, the participant pressed the ‘left arrow’ key; if the right circle contained more dots, the participant pressed the ‘right arrow’ key. The number of dots within each circle was bounded between 1 and 100. One randomly selected circle always contained 50 dots; the other circle contained a variable number of dots. The difference in dot number (Δd) between the two circles was titrated such that each participant’s performance was maintained at a constant level using a one-up two-down staircase procedure as used previously (Fleming et al., 2010). After two consecutive correct responses, Δd was decreased by one dot; after one incorrect response, Δd was increased by one dot. The aim of the staircase procedure was to equate the difficulty of the perceptual task between individuals. In total, each participant completed 200 perception trials (8 blocks × 25 trials per block).
To estimate metacognitive efficiency, we computed meta-d’ (Maniscalco and Lau, 2012). In a signal detection theory framework, meta-d’ is a measure of type 2 sensitivity (i.e., the degree to which participants can discriminate their own correct from incorrect judgments) that is expressed in the same units as type 1 sensitivity (d’) (i.e., the degree to which participants can distinguish stimulus alternatives). Meta-d’/d’ is a relative measure of metacognitive efficiency: given a certain level of processing capacity (d’), a meta-d’/d’ value of 1 (equivalently, log(meta-d’/d’) = 0) is metacognitively ideal, whereas meta-d’/d’ < 1 indicates that metacognition is worse than expected based on the model. Using this ratio as a measure of metacognition effectively eliminates performance and response bias confounds that can affect other measures (Barrett et al., 2013). Meta-d’ was fit to each participant’s confidence rating data using maximum likelihood estimation. Before analysis, participants’ continuous confidence ratings were binned into four quantiles. MATLAB code for implementing these fits can be found at http://www.columbia.edu/~bsm2105/type2sdt.
2.3 T1 Structural Scans
T1-weighted anatomical images were acquired on a 3T Skyra (Siemens, Erlangen, Germany) with a 3D MPRAGE sequence [FOV 256 × 256 mm, matrix size 320 × 320, 0.8 mm isotropic resolution, TR/TE/TI = 2400/2.07/1000 ms, flip angle 8° with binomial (1, −1) fat saturation, bandwidth 240 Hz/pixel, echo spacing 7.6 ms, in-plane acceleration (GRAPPA) factor of 2, total acquisition time ~7 min]. Structural T1 images were preprocessed using the “HCP PreFreeSurfer structural pipeline” (based on FSL 5.0.6 and FreeSurfer 5.3.0-HCP) to align the origin to the anterior-posterior commissure line, correct image distortions (bias-field inhomogeneities), and to normalize T1-weighted images to the MNI space using a FLIRT affine linear and then a FNIRT nonlinear registration.
Further preprocessing was conducted with the VBM toolbox (version 8) (C. Gaser, Department of Psychiatry, University of Jena, Jena, Germany; http://dbm.neuro.uni-jena.de/vbm/), which combines bias correction, spatial normalization, and tissue segmentation into a unified model. The structural scans were corrected for bias-field inhomogeneities, spatially normalized by linear (12-parameter affine) and non-linear transformations using the Diffeomorphic Anatomical Registration using Exponential Lie algebra (DARTEL) template in standard MNI space, and segmented into gray matter, white matter, and CSF tissue classes according to a priori tissue probability maps (Ashburner and Friston, 2000, 2005). A hidden Markov random field was used to maximize segmentation accuracy (Cuadra et al., 2005), and non-linear modulation was applied to each tissue type, preserving information about local tissue volumes. Normalized and nonlinear modulated gray matter maps were smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
2.4 Statistical Analyses
2.4.1 Metacognitive Accuracy Task
Parametric statistical tests were conducted on log(meta-d’/d’). A log-transformation weights observations automatically to a ratio scale (Keene, 1995), thus ascribing equal weight to increases and decreases in meta-d’/d’ relative to a theoretically ideal value of 1 (Fleming et al., 2014). Log(meta-d’/d’) was entered into a one-way analysis of variance (ANOVA), with study group as the between-subjects factor (CUD+, CUD−, control). We also used one-way ANOVAs to test for group differences in task accuracy and staircase-adjusted task difficulty. Omnibus group differences were followed by pairwise comparisons to localize the significant differences.
2.4.2 Region of Interest Voxel-Based Morphometry (VBM)
We used MARSBAR (http://marsbar.sourceforge.net) to extract GMV from unbiased bilateral regions of interest (ROIs) in the rACC; these rACC ROIs were defined as 5 mm spheres, centered at peak coordinates taken directly from our prior work in a completely non-overlapping cohort of participants that examined the neural correlates of impaired insight in CUD (x=±12, y=44, z=13) (Moeller et al., 2014). The Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) model from which signal was extracted was a one-way ANOVA. Similarly to the metacognitive accuracy analyses, these ROIs were entered into one-way ANOVAs in SPSS, followed by pairwise comparisons as appropriate. Next, we tested for associations between GMV extracted from rACC ROIs and log(meta-d’/d’), using Pearson correlations across the entire sample and (in more exploratory analyses) as a function of study group.
2.4.3 Effects of Covariates
We examined whether our main variables of interest were correlated with the variables that differed between the groups (depression and cigarette smoking) (Table 1), or were correlated with age (for GMV analyses only, as age is a known predictor of GMV). If significant correlations emerged, analyses of covariance (ANCOVAs) or partial correlations as appropriate were then conducted to ensure that our findings were not driven by the relevant variable(s). Note that current drug use variables that differed between the CUD+ and CUD− groups were not considered as covariates, but rather as reflecting the expected differences that accompany our a priori grouping by urine status.
2.4.4 Mediation
To provide evidence of a structural mechanism for impaired metacognition, we tested whether GMV abnormalities account for the effects of CUD+ and/or CUD− on metacognition. The specific predictors and outcome variables included in these mediation analyses were determined by the results obtained from our main analyses (reported below). Standard criteria for establishing mediation were employed (Baron and Kenny, 1986), with the indirect effect calculated using a bootstrap estimation approach (Preacher and Hayes, 2008).
2.4.5 Exploratory Whole-Brain Correlations
In more exploratory analyses, we inspected for whole-brain correlations between log(meta-d’/d’) and GMV across the entire sample, using multiple regression analysis in SPM8. Age, depression, and cigarette smoking history were entered into the model as covariates of no interest. Significance for these whole-brain regression analyses was set at p<0.05, corrected for family-wise error at the voxel level, although we also examined a reduced threshold of p<0.001 uncorrected (25 voxel minimum). We do not report whole-brain group differences between CUD+, CUD−, and controls; such analyses would require future studies with larger sample sizes.
3. RESULTS
3.1 Metacognitive Accuracy
Results of the metacognition task revealed no group differences on overall task performance (percent accuracy and staircase-adjusted difficulty) (Figure 1B–C). However, as anticipated, there was a significant group difference in metacognitive accuracy [one-way ANOVA: F(2,32)=4.37, p=0.02]. Follow-up pairwise comparisons showed that CUD+ had decreased metacognitive accuracy compared with controls and CUD− (both p=0.01), whereas CUD− did not differ from controls (p=0.85) (Figure 1D). Metacognitive accuracy was uncorrelated with depression (p=0.32) or cigarette smoking (p=0.39), suggesting that these covariates do not explain our findings.
3.2 GMV ROI Analyses
A similar pattern of effects to metacognitive accuracy was observed in the bilateral rACC ROIs. Controlling for age, GMV group differences emerged in the left rACC [F(2,31)=3.24, p=0.05] and right rACC [F(2,31)=7.05, p=0.003]. Follow-up pairwise comparisons showed that CUD+ had lower rACC GMV than controls (left ROI: p=0.02; right ROI: p=0.001) and CUD− (right ROI only: p=0.02) (Figure 2A–B). On these same measures, CUD− did not differ from controls (left ROI: p=0.50; right ROI: p=0.14). Neither rACC ROI was correlated with depression (right: p=0.15; left: p=0.06) or cigarette smoking (right: p=0.25; left: p=0.12).
Figure 2.
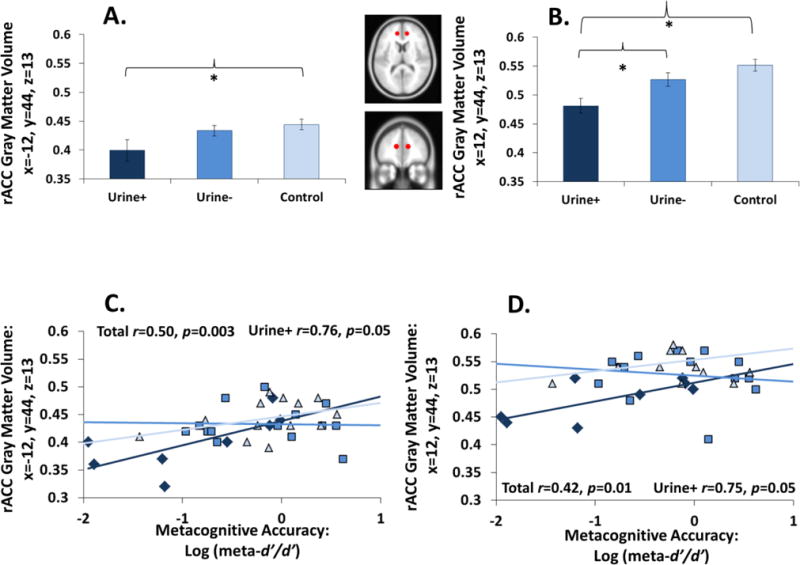
Metacognitive accuracy associations with gray matter volume (GMV). (A–B) CUD+ had reduced GMV in the bilateral rACC. (C–D) The higher the GMV deficit, the lower the metacognitive accuracy in all participants; these correlations further appeared to be driven by CUD+.
Importantly, bilateral rACC GMV was positively correlated with metacognitive accuracy (Figure 2C–D): the lower the left and right rACC volumes (controlling for age), the lower the metacognitive accuracy. Correlations between metacognitive accuracy and GMV remained significant after controlling for depression (left ROI: p=0.005; right ROI: p=0.02) and cigarette smoking (left ROI: p=0.005; right ROI: p=0.02). More exploratory correlations conducted as a function of study group indicated that these metacognition-rACC associations were driven by the CUD+ group (Figure 2C–D).
3.3 Mediation
In these analyses, we tested rACC GMV as a mediator of the association between CUD+ and metacognition. We compared CUD+ versus controls in consideration of our effects showing that CUD+ generally differed from the other two groups. However, we deemed it conceptually inappropriate to combine CUD− and controls into a single group. Also, we did not test for mediation when comparing CUD+ and CUD− because these cocaine subgroups did not differ on left rACC GMV.
First, we established an effect of positive urine status (i.e., CUD+ versus controls) on metacognitive accuracy [F(1,18)=6.61, p=0.02] (Figure 3, c-paths). Second, we found an effect of positive urine status on both the left rACC ROI [F(1,18)=7.47, p=0.01] and the right rACC ROI F(1,18)=15.46, p=0.001] (Figure 3, a-paths). Third, when both positive urine status and the bilateral rACC GMV (examined in two separate analyses) were entered as predictors of metacognitive accuracy, both the left and right rACC GMV were significant predictors [left: F(1,17)=8.21, p=0.01; right: F(1,17)=5.92, p=0.03] (Figure 3, b-paths), whereas the effects of positive urine status on metacognitive accuracy were no longer significant [left: F(1,17)=1.02, p=0.33; right: F(1,17)=0.23, p=0.64] (Figure 3, c′-paths). Results of the bootstrapping procedures with 10000 samples indicated that the indirect coefficient was significant for both models (left rACC: a×b=0.46, SE=0.22, 95% CI=0.11, 1.02; right rACC: a×b=0.52, SE=0.31, 95% CI=0.09, 1.32).
Figure 3.
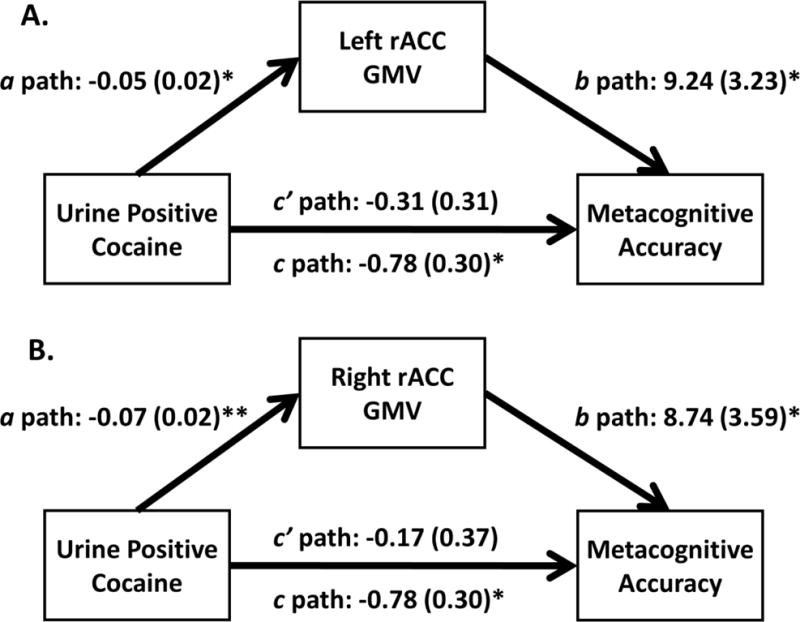
Mediation analyses. First, we established an effect of positive urine status (i.e., CUD+ versus controls) on metacognitive accuracy (c-paths). Second, we found an effect of positive urine status on the bilateral rACC GMV (a-paths). Third, controlling for positive urine status (where effects of CUD+ fell below significance: c′-paths), the (A) left rACC and (B) right rACC ROIs remained significant predictors of metacognitive accuracy (b-paths). Both indirect effects were significant, tested with bootstrapping, indicating mediation (see Results for statistics).
3.4 Exploratory Whole-Brain Correlations
Whole-brain correlations between metacognitive accuracy and GMV did not reach family-wise corrected significance. At a more lenient p<0.001 uncorrected threshold, however, a positive correlation emerged between metacognitive accuracy and (most notably) the rACC (peak: x=−5, y=44, z=10; T=4.79; 197 voxels). Additional positive correlations of note were observed in the precuneus and parahippocampal gyrus (for visualization of all regions emerging in this analysis, see Figure 4). There were no negative whole-brain correlations, at either the family-wise corrected or reduced thresholds. Although these findings are considered preliminary, they are useful for informing future studies on this topic.
Figure 4.
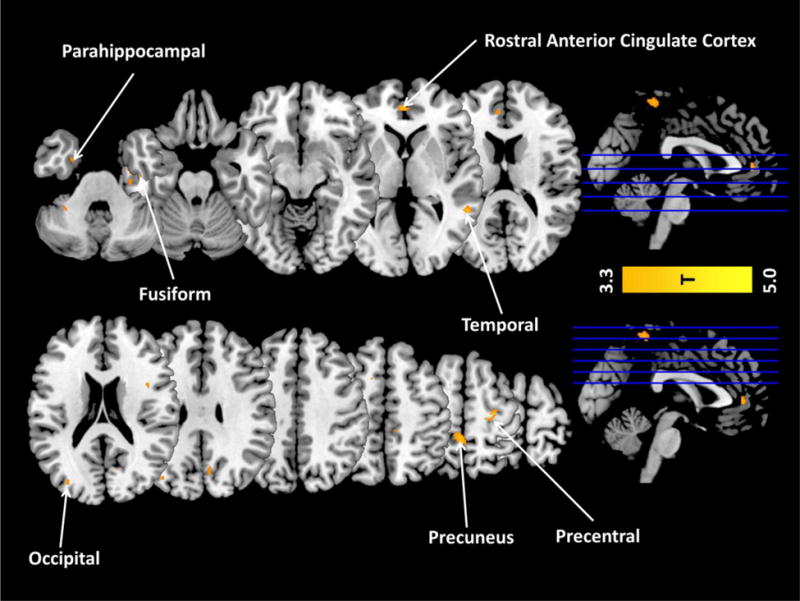
Exploratory whole-brain correlations of GMV with metacognitive accuracy across the entire study sample. At a reduced threshold of p<0.001 uncorrected (T=3.38) (though not at a corrected family-wise error threshold), there was a positive whole-brain correlation in the rostral anterior cingulate cortex (rACC), supporting the ROI analyses. Other regions observed at this reduced threshold included the precuneus; temporal and occipital cortices; and parahippocampal, fusiform, and precentral gyri. There were no whole-brain negative correlations with metacognitive accuracy, at either the corrected or uncorrected thresholds.
4. DISCUSSION
Results of this study demonstrate that metacognitive accuracy on a purely perceptual task (i.e., the mapping between objective performance and self-reported confidence of performance), and associated rACC volumes, are reduced in active cocaine use disorder (CUD+). Because the study groups did not differ on basic task performance (accuracy and staircase-adjusted task difficulty), our results cannot be attributed to gross neuropsychological deficits in CUD+ [see also (Woicik et al., 2009)]. The CUD groups also did not differ on peak drug usage (Table 1), suggesting that our results cannot be attributed to a greater lifetime addiction severity in CUD+. Instead, recent, active CUD may have exerted a deleterious effect on both metacognitive processing and associated rACC GMV, with decreases in rACC GMV potentially mediating the impact of recent CUD on metacognition. Indeed, while GMV abnormalities are reliably observed in drug addiction (Alia-Klein et al., 2011; Ersche et al., 2013; Fein et al., 2002; Franklin et al., 2002; Konova et al., 2012; Moeller et al., 2014; Tanabe et al., 2009), evidence for GMV recovery with abstinence is also beginning to emerge (Connolly et al., 2013).
Our results offer both methodological and theoretical advances in the cognitive neuroscience of active CUD. For the former (methods), we previously defined self-awareness in drug addiction as a categorical mismatch (versus match) between actual ongoing choices for viewing drug-related pictures and retrospective self-report of these choices (Moeller et al., 2012; Moeller et al., 2014; Moeller et al., 2010). In the current task, we (A) obtained a continuous measure of metacognition that (B) removed constituent psychological processes that are possibly invoked during choice behavior in a salient disease-relevant context, which aided in (C) obviating potential demand characteristics and biases of longer-term memory. Thus, this type of design is amenable to direct cross-diagnostic and even cross-species comparisons. For example, a recent study in animals showed that a deficit related to metacognition (imagining future outcomes on the basis of current knowledge) was similarly mediated by a region of the aPFC (orbitofrontal cortex) (Lucantonio et al., 2014). In another study, orbitofrontal cortex lesions in rats produced impairment on a highly similar task that examined “confidence” in decision-making (i.e., the amount of time spent waiting for a reward following a correct choice) (Lak et al., 2014). For the latter (theory), a metacognitive deficit in basic perceptual functions in active CUD may parsimoniously accommodate and reveal mechanistic underpinnings of additional diverse findings in the literature, including deficient self-monitoring of task-related errors (Hester et al., 2007), reduced emotional awareness (Payer et al., 2011), ‘denial’ of illness severity (Dean et al., 2015), and underestimation of one’s drug-related self-control and cognitive-emotional deficits (Ersche et al., 2012; Verdejo-Garcia and Perez-Garcia, 2008). Moreover, the current results with a perceptual task have implications for research that emphasizes the importance of more basic sensory processes in understanding core pathophysiology of addiction (Yalachkov et al., 2010).
Our current focus on the rACC leaned heavily on multiple, converging lines of evidence. First and foremost, prior research demonstrates the importance of the aPFC for metacognitive accuracy on the current task, in both healthy individuals and lesion patients (Fleming et al., 2014; Fleming et al., 2010). More broadly, this region (extending into the adjacent ventromedial prefrontal cortex) has been identified as a core region in a network subserving self-referential processing, in both healthy individuals (Abraham, 2013; D’Argembeau, 2013; de Greck et al., 2008; Sui et al., 2013; van der Meer et al., 2010) and individuals with SUDs (de Greck et al., 2009; Moeller and Goldstein, 2014). Furthermore, the rACC has been implicated in the related constructs of experiencing agency (e.g., when individuals gamble for themselves versus when a computer gambles for them) (Clark et al., 2009) and negative self-conscious emotions (e.g., embarrassment) (Sturm et al., 2013). Thus, this region’s contribution to metacognition impairments in active CUD was highly anticipated, though shown here for the first time.
Nevertheless, regions beyond the rACC are likely to play a role in such metacognitive impairments, as further supported by our exploratory whole-brain analyses. That is, although the rACC emerged in these whole-brain analyses as we expected, other regions were also positively correlated with metacognitive accuracy. The precuneus and the parahippocampal gyrus, for example, were two such regions that deserve mention. For the former, GMV of the precuneus has been positively correlated with a different type of metacognition (metacognitive memory) in healthy individuals (McCurdy et al., 2013). More broadly, the precuneus, similarly to the rACC, has been implicated in self-referential processing (Cavanna and Trimble, 2006) and the processing of close others (friends or relatives) (Zhang et al., 2015). For the latter, resting-state functional connectivity of the parahippocampal gyrus has been positively correlated with dispositional mindfulness (Kong et al., 2015), a construct relevant to self-awareness. In a related vein, a psychophysiological interaction (PPI) analysis showed that, during trait judgments about the self, the parahippocampal gyrus is co-activated with the ventromedial prefrontal cortex (Feyers et al., 2010). In the current study, however, it is important to note that the correlations between metacognitive accuracy and these regions did not reach family-wise corrected significance, and hence they need to be verified in future work with larger sample sizes before firm conclusions can be drawn.
It is also important to note that metacognition is a broad theoretical construct, with multiple facets and behavioral manifestations. Specifically, our meta-d’/d’ measure captures one important domain-specific facet of metacognition: how well one can monitor one’s own decision-making and discriminate between accurate and inaccurate judgments. Another key facet of metacognition not examined here is how the output of this monitoring process is used in the subsequent control of behavior (Nelson and Narens, 1990). Such usage is likely complex, and may depend not only on monitoring efficiency but also on one’s overall level of confidence (Fleming and Lau, 2014), which is likely to be a relatively stable personality trait (Ais et al., 2016; Cesarini et al., 2009). Relating decision confidence, metacognition, and insight to subsequent behavioral control remains an important target for future work. Other types of metacognitive experiences such as feelings-of-knowing (Hart, 1965) or judgments of learning (Koriat, 1997) may also be altered by active cocaine use and/or CUD and similarly remain to be studied.
Taken together, we provide mechanistic evidence that a basic neurocognitive function (perceptual metacognition) is impaired in individuals with active CUD, and map such impairment onto structural integrity of an aPFC region necessary for this function. Accordingly, our results provide a possible foundation for understanding higher-order self-awareness impairments in drug addiction, with the potential for translation to other psychopathologies/species that can ultimately facilitate broad-scale interventions and novel treatments.
Acknowledgments
We thank Anna B. Konova, Prantik Kundu, Junqian Xu, and Chen Yang for early assistance with MRI preprocessing.
Role of the Funding Source: This work was supported by grants from the National Institute on Drug Abuse (K01DA037452 and R21DA040046 to SJM; F32DA033088 to MAP; R21DA034954 to RZG) and the National Institute of Mental Health (R01MH090134 to NAK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support came from: a Sir Henry Wellcome Fellowship from the Wellcome Trust (WT096185 to SMF); and seed funds from the Icahn School of Medicine at Mount Sinai and the Mount Sinai Brain Imaging Center (to SJM, NAK, and RZG). None of these entities had a further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
None declared.
Contributors
SJM, SMF, and RZG designed research; GG, AZ, PM, FDQ, KES, RPC, MAP, TM, and NAK collected data; SJM, SMF, GG, and AZ analyzed data; and SJM, SMF, and RZG wrote the paper. All authors approved the final version.
References
- Abraham A. The world according to me: personal relevance and the medial prefrontal cortex. Frontiers in human neuroscience. 2013;7:341. doi: 10.3389/fnhum.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ais J, Zylberberg A, Barttfeld P, Sigman M. Individual consistency in the accuracy and distribution of confidence judgments. Cognition. 2016;146:377–386. doi: 10.1016/j.cognition.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrett AB, Dienes Z, Seth AK. Measures of metacognition on signal-detection theoretic models. Psychological methods. 2013;18:535–552. doi: 10.1037/a0033268. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cesarini D, Lichtenstein P, Johannesson M, Wallace B. Heritability of overconfidence. J Eur Econ Assoc. 2009;7:617–627. [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One. 2013;8:e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in human neuroscience. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B, Northoff G. Is our self based on reward? Self-relatedness recruits neural activity in the reward system. Neuroimage. 2008;39:2066–2075. doi: 10.1016/j.neuroimage.2007.11.006. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics–a fMRI study. Hum Brain Mapp. 2009;30:1691–1704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 2015;151:84–91. doi: 10.1016/j.drugalcdep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Croudace T, Stochl J. Who Do You Think Is in Control in Addiction? A Pilot Study on Drug-related Locus of Control Beliefs. Addictive disorders & their treatment. 2012;11:173–223. doi: 10.1097/ADT.0b013e31823da151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Current opinion in neurobiology. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyers D, Collette F, D’Argembeau A, Majerus S, Salmon E. Neural networks involved in self-judgement in young and elderly adults. Neuroimage. 2010;53:341–347. doi: 10.1016/j.neuroimage.2010.05.071. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Williams J. Structured Clinical Interview for DSM-IV Axis I disorders – Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fleming SM, Lau HC. How to measure metacognition. Front Hum Neurosci. 2014;8:443. doi: 10.3389/fnhum.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, Blackmon KE. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain. 2014;137:2811–2822. doi: 10.1093/brain/awu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329:1541–1543. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS. Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2015:1–9. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. J Educ Psychol. 1965;56:208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hester R, Simões-Franklin C, Garavan H. Post-error behavior in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Keene ON. The log transformation is special. Statistics in medicine. 1995;14:811–819. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- Kong F, Wang X, Song Y, Liu J. Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Social neuroscience. 2015 doi: 10.1080/17470919.2015.1092469. [DOI] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N, Volkow ND, Goldstein RZ. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. 2012;36:2979–2988. doi: 10.1111/j.1460-9568.2012.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriat A. Monitoring one’s own knowledge during study: A cue-utilization approach to judgments of learning. Journal of experimental psychology General. 1997;126:349–370. [Google Scholar]
- Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron. 2014;84:190–201. doi: 10.1016/j.neuron.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-Chaudhary S, Shaham Y, Lupica CR, Schoenbaum G. Orbitofrontal activation restores insight lost after cocaine use. Nat Neurosci. 2014;17:1092–1099. doi: 10.1038/nn.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cogn. 2012;21:422–430. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- McCurdy LY, Maniscalco B, Metcalfe J, Liu KY, de Lange FP, Lau H. Anatomical coupling between distinct metacognitive systems for memory and visual perception. J Neurosci. 2013;33:1897–1906. doi: 10.1523/JNEUROSCI.1890-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. 2012;135:3481–3494. doi: 10.1093/brain/aws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. 2014;71:61–70. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. Psychol Learn Motiv. 1990;26:125–141. [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry. 2011;68:271–282. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Social cognitive and affective neuroscience. 2013;8:468–474. doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Rotshtein P, Humphreys GW. Coupling social attention to the self forms a network for personal significance. Proc Natl Acad Sci U S A. 2013;110:7607–7612. doi: 10.1073/pnas.1221862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull. 2013;39:1288–1295. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Substance abusers’ self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. The Wide-Range Achievement Test 3- Administration Manual. Wide Range Inc; Wilmington, DE: 1993. [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: Recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Zhang L, Opmeer EM, Ruhe HG, Aleman A, van der Meer L. Brain activation during self- and other-reflection in bipolar disorder with a history of psychosis: Comparison to schizophrenia. Neuroimage Clin. 2015;8:202–209. doi: 10.1016/j.nicl.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


