Abstract
We have previously shown that acute intravenous (i.v.) administration of cocaine increases Fos immunoreactivity in rats under isoflurane anesthesia. Given that Fos expression is a marker of neural activation, the results suggested that isoflurane is appropriate for imaging cocaine effects under anesthesia. However, most imaging research in this area utilizes subjects with a history of repeated cocaine exposure and this drug history may interact with anesthetic use differently from acute cocaine exposure. Thus, this study further examined Fos expression under isoflurane in rats with a history of repeated i.v. cocaine administration. Rats received daily injections of either saline or cocaine (2 mg/kg, i.v.) across 7 consecutive days, followed by 5 days of no drug exposure. On the test day, rats were either nonanesthetized or anesthetized under isoflurane and were given an acute challenge of cocaine (2 mg/kg, i.v.). Additional saline-exposed controls received a saline challenge. Ninety min after the drug challenge, the rats were perfused under isoflurane anesthesia and their brains were processed for Fos protein immunohistochemistry. We found that challenge injections of cocaine following a regimen of repeated cocaine exposure resulted in Fos expression in the prefrontal cortex and striatum roughly equivalent to that found in rats who had received the cocaine challenge after a history of vehicle injections. Additionally, isoflurane anesthesia resulted in a heterogeneous attenuation of cocaine-induced Fos expression, with the most robust effect in the orbital cortex but no effect in the nucleus accumbens core (NAcC). These results indicate that cocaine-induced Fos is preserved in the NAcC under isoflurane, suggesting that isoflurane can be used in imaging studies involving cocaine effects in this region.
Keywords: chronic exposure, isoflurane, c-fos, Fos, cocaine, immediate early gene, anesthesia, sensitization
1. Introduction
Functional magnetic resonance imaging (fMRI) is a popular technique for the study of drug effects throughout the brain in animal models(Ferrari et al., 2012; Salmeron and Stein, 2002). In vivo fMRI in animals requires immobilization and therefore almost always involves the use of general anesthesia (Steward et al., 2005). Isoflurane anesthesia is often used in brain imaging studies that require immobile animals (Liu et al., 2004; Masamoto et al., 2007) mainly because it is administered by the noninvasive route of inhalation and post-experiment recovery is rapid (Hanusch et al., 2007; Lukasik and Gillies, 2003). However, concerns remain over its impact on the coupling between neural activity and measurable physiological effects (Austin et al., 2005; Sicard et al., 2003). With the careful measurement of physiological variables, isoflurane was recently shown to allow robust fMRI examination of cocaine activation in drug-naïve rats (Schmidt et al., 2006). Additionally, we found that cocaine-induced Fos expression is partially maintained under isoflurane anesthesia in cocaine-naïve rats (Kufahl et al., 2009). Fos is the protein product of the immediate early gene, c-fos. Intracellular signaling in response to a variety of stimuli transiently increases Fos expression, and its expression is therefore considered a biomarker of neuronal activity in response to a stimulus (Morgan and Curran, 1995). Detection of Fos expression under isoflurane distinguishes this anesthetic from various injectable anesthetics, which have been shown to largely abolish cocaine-induced Fos expression (Kreuter et al., 2004; Kufahl et al., 2009; Ryabinin, 2000; Torres and Rivier, 1993).
An important issue that remains to be addressed is whether neural signaling that increases Fos expression is also preserved under isoflurane anesthesia in animals that have a history of repeated exposure to cocaine, given that animal models of cocaine dependence employ chronic administration regimens that often sensitize the behavioral and neurochemical responses to cocaine (Post and Rose, 1976; Shuster et al., 1977; White and Kalivas, 1998). For instance, daily cocaine administration reliably increases locomotor activity responses to a subsequent challenge dose, relative to an identical cocaine challenge in drug-naïve rats (Kalivas and Duffy, 1990). This sensitized cocaine-induced locomotor behavior persists up to two months after discontinuing a regimen of repeated cocaine injections (Henry and White, 1995) and is thought to be an effective model for examining neurobiological changes that may lead to addictive behaviors (see for review Everitt and Wolf, 2002; Koob and Le Moal, 1997; Robinson and Berridge, 1993). Furthermore, repeated cocaine administration is associated with long-lasting adaptations within multiple neurotransmitter systems (White and Kalivas, 1998; Wolf, 1998), including glutamate and GABA systems that are also affected by isoflurane anesthesia (Westphalen et al., 2011; Westphalen et al., 2013).
In the present study, we assessed the effect of isoflurane anesthesia on cocaine-induced Fos expression in rats that had undergone repeated injections of cocaine or saline. In addition, Fos expression of the anesthetized rats was compared to that of nonanesthetized rats with identical drug histories. Additionally, locomotor activity was measured in the nonanesthetized control rats to confirm that the repeated cocaine administration produced behavioral sensitization.
2. Results
2.1. Locomotor sensitization to cocaine
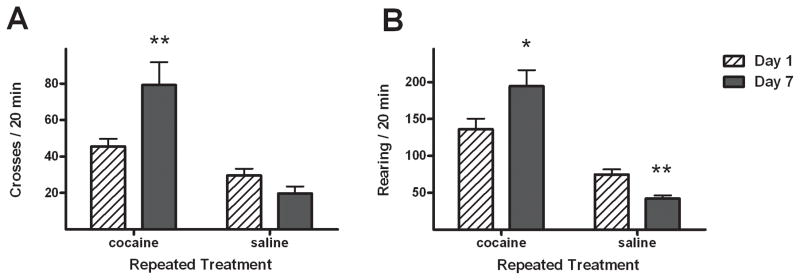
For seven consecutive days, rats were given daily intravenous (i.v.) injections of 2 mg/kg cocaine (n = 13) or saline vehicle (n = 18) and released into rectangular locomotor cages for 90 min. Horizontal crossovers between cage halves and vertical rearing were recorded from video and accumulated over the first 20 min of each session (Fig. 1). Repeated exposure to i.v. cocaine resulted in increased locomotor behavior from Day 1 to Day 7 of treatment of the cocaine-treated rats, as revealed by the presence of repeated treatment × day interactions in both crossovers (F1,23 = 7.1, p < 0.05) and rearing (F1,23 = 37.8, p < 0.0001). Post hoc comparisons confirmed that Day 7 crossovers (paired t-test, t5 = 2.7, p < 0.05) and rearing (t5 = 5.7, p < 0.005) were significantly greater in the cocaine-treated rats. In contrast, Day 7 rearing was significantly reduced in saline-treated rats (t8 = 7.3, p < 0.005), but the reduction in crossovers was not significant.
Figure 1.
Effects of repeated treatment (cocaine, n=14 or saline, n=17) on horizontal crossovers (A) and rearing behavior (B), as assessed by comparisons between measurements made on Day 1 and Day 7. Values are presented as mean ± standard error of measurement (SEM). * p < 0.05 and ** p < 0.005 difference between Day 1 and Day 7 (paired t-tests).
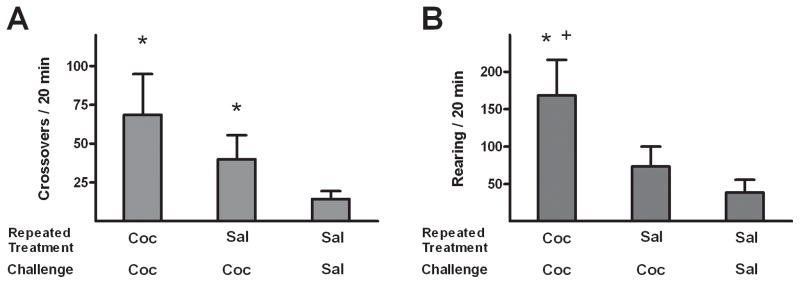
Three groups of rats were tested without anesthesia (NoA) and received the following repeated and challenge treatments: repeated and challenge (2 mg/kg) cocaine (CC-NoA, n = 8), repeated saline and challenge cocaine (SC-NoA, n = 6), and repeated and challenge saline (SS-NoA, n = 6). Among these subjects, cocaine exposure resulted in changes in cocaine-induced motor behavior, as revealed by the presence of significant effects of repeated/test treatment in both the crossover (F2,19 = 13.9, p < 0.0005) and rearing (F2,19 = 26.2, p < 0.0001) data (Fig. 2). In post hoc tests, crossovers and rearing were higher in CC-NoA rats than the other groups (Newman-Keuls tests, p < 0.05), and the crossovers, but not rearing, were greater in SC-NoA relative to SS-NoA rats (Newman-Keuls, p < 0.05).
Figure 2.
Effects of challenge injections on horizontal crossovers (A) and rearing (B) in non-anesthetized rats (i.e., all NoA groups) with histories of repeated cocaine or saline treatments. Rats received repeated daily treatments of either cocaine (C; 2 mg/kg, i.v.) or saline (S), followed by a challenge injection of C or S, with group names representing these sequential treatments. Values are presented as mean ± SEM. * p < 0.05 different from SS-NoA (Newman-Keuls). + p < 0.05 different from SC-NoA. Group sizes are CC-NoA: n=9, SC-NoA: n=6, SS-NoA: n=5.
2.2. Fos protein expression
Two groups of rats were tested under isoflurane anesthesia (ISO) that received the following chronic and challenge treatments: chronic and challenge cocaine (CC-ISO), and chronic and challenge saline (SS-ISO). All groups of rats were perfused 90 min after challenge injection and their brain tissue was processed for Fos protein immunohistochemistry. Group differences in Fos expression were evaluated by 2 × 2 factorial ANOVAs with anesthesia (ISO vs. NoA) and drug history/challenge (CC vs. SS) conditions as between group factors. The possible tolerance effect imposed by a history of repeated cocaine treatment was evaluated by planned t-test comparisons between SC-NoA and CC-NoA groups.
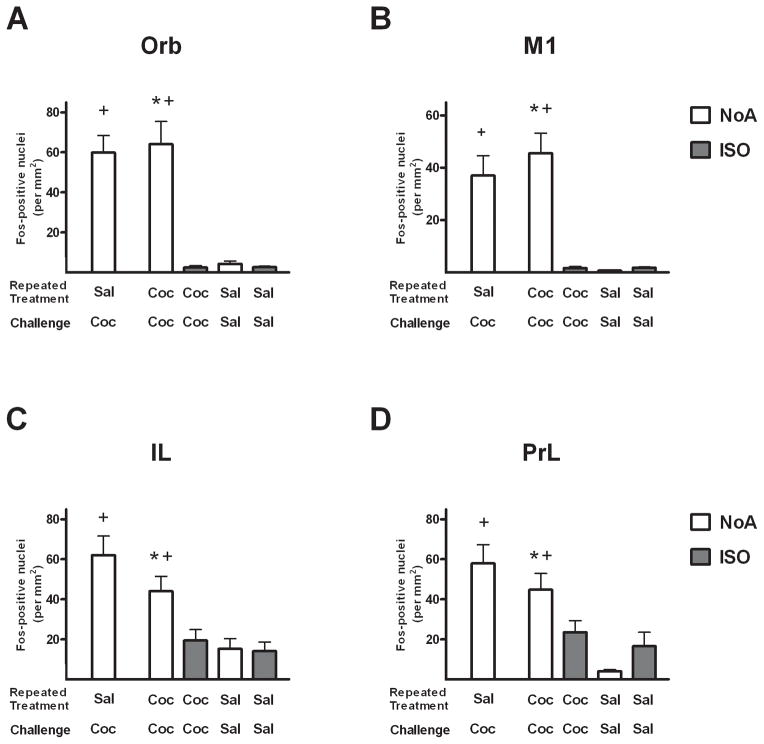
In cortical regions of rats with a history of repeated cocaine treatment, Fos protein expression was elevated in response to cocaine challenge, but this effect was robustly attenuated by anesthesia (Fig. 3). This was confirmed by the presence of significant anesthesia × drug history/challenge interactions in Orb (F1,21 = 11.8, p < 0.005), M1 (F1,21 = 15.0, p < 0.001) and PrL (F1,21 = 5.6, p < 0.05), and a trend toward a significant interaction in IL (F1,21 = 3.8, p = 0.06). Significant main effects of anesthesia were found in Orb (p < 0.005) and M1 (p < 0.001), and significant main effects of drug history/challenge were found in all four cortical regions (p < 0.05). Fos expression in the CC-NoA group was significantly greater than in the CC-ISO group in all cortical regions (Newman-Keuls tests, p < 0.01), indicating a suppressive effect of isoflurane on cocaine-induced Fos. Significant differences in Fos expression were not found between the CC-NoA and SC-NoA groups in any of the cortical regions (t-tests), indicating the lack of tolerance effects on cocaine-induced Fos among the non-anesthetized animals.
Figure 3.
Mean number of Fos-positive nuclei/mm2 (±SEM) in the orbital (A), primary motor (B), infralimbic (C) and prelimbic (D) cortex regions of rats following challenge injections under isoflurane (ISO groups, shaded) or no anesthesia (NoA groups, white). Rats received repeated daily treatments across 7 days of either cocaine (C; 2 mg/kg, i.v.) or saline (S), followed by a challenge injection of C or S, with group names representing these sequential treatments. * p < 0.05 different from CC-ISO (Newman-Keuls). + p < 0.05 different from SS-NoA. Group sizes are SC-NoA: n=6, CC-NoA: n=9, CC-ISO: n=5, SS-NoA: n=5, SS-ISO: n=6.
The effects of isoflurane anesthesia and drug on cocaine-induced Fos expression in the basal ganglia were less uniform than in the cortex (Fig. 4). Significantly greater Fos expression was found in the SC-NoA group than the CC-NoA group in the dCPu (t10 = 3.2, p < 0.05), indicating a possible tolerance effect. However, this difference was not found in the other basal ganglia regions. Additionally, only NAcS exhibited a significant anesthesia × drug interaction (F1,21 = 6.9, p < 0.05) among the basal ganglia regions. In this region, the cocaine-induced increase in Fos expression was not observed in anesthetized rats, although the anesthesia itself appeared to increase Fos expression in the SS-ISO group which may have obscured detection of cocaine-induced Fos expression in this region. A main effect of drug was found in NAcC (F1,21 = 17.3, p < 0.001), indicating a cocaine-induced increase in Fos expression in this region regardless of whether or not the animals were anesthetized. Post-hoc comparisons of group differences in Fos expression in NAcS revealed Fos expression was greater in the CC-NoA group than the CC-ISO group (Newman-Keuls test, p < 0.05), similar to the anesthesia-induced loss of Fos expression revealed in the cortical areas. However, NAcC Fos was elevated in CC-ISO rats relative to SS-ISO rats (Newman-Keuls tests, p < 0.05), indicating a preservation of cocaine-elicited Fos expression under anesthesia conditions in this region.
Figure 4.
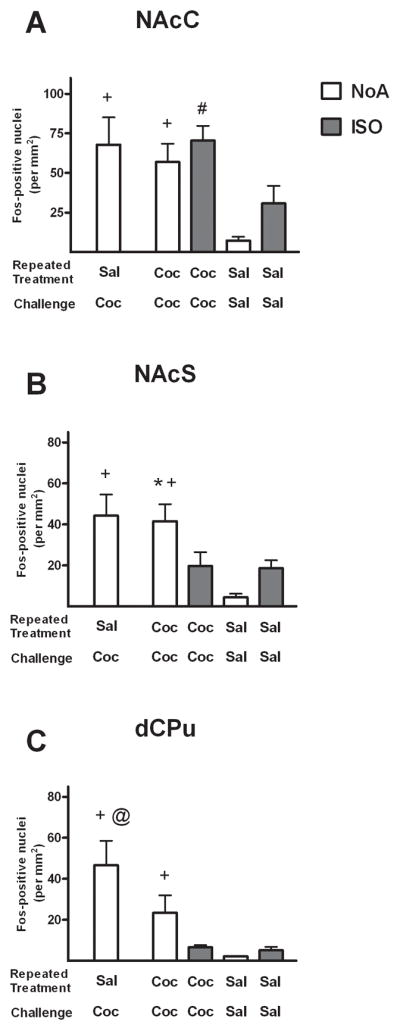
Number of Fos-positive nuclei/mm2 in the nucleus accumbens shell (A), nucleus accumbens core (B) and dorsal caudate/putamen (C) regions of rats following challenge injections under isoflurane (ISO groups, shaded) or no anesthesia (NoA groups, white). Rats received repeated daily treatments across 7 days of either cocaine (C; 2 mg/kg, i.v.) or saline (S), followed by a challenge injection of C or S, with group names representing these sequential treatments. Values are presented as mean ± SEM. * p < 0.05, different from CC-ISO (Newman-Keuls). + p < 0.05, different from SS-NoA. # p < 0.05, different from SS-ISO. @ p < 0.05, different from CC-NoA. Group sizes are SC-NoA: n=6, CC-NoA: n=9, CC-ISO: n=5, SS-NoA: n=5, SS-ISO: n=6.
Lastly, SC-NoA Fos was significantly greater than SS-NoA Fos in all cortical and basal ganglia regions (Newman-Keuls tests, p < 0.05), replicating previous observations of i.v. cocaine-induced Fos expression in cocaine-naïve animals (e.g., Harlan and Garcia, 1998; Kufahl et al., 2009; Pich et al., 1997; Samaha et al., 2004).
3. Discussion
The present results suggest that the effects of isoflurane anesthesia on cocaine-induced Fos expression in rats that have previously received repeated cocaine exposure varies depending on the brain region examined. Isoflurane had no effect on cocaine-induced Fos in the NAcC, but nearly abolished it in the motor cortex, significantly attenuated it in the other cortical regions and in the NAcSh, and produced a nonsignificant decrease in the dCPu. We verified that the repeated cocaine administration regimen sensitized horizontal (cage crossovers) and vertical (rearing) motor behaviors evident both as an increase in these behaviors in rats across days of cocaine treatment and as an increase in response to acute cocaine challenge between the groups given repeated cocaine versus repeated saline administration. These behavior results are consistent with previous studies of sensitization after repeated i.v. cocaine treatment in both tethered (Browman et al., 1998; Lecca et al., 2007; Samaha et al., 2002; Samaha et al., 2004) and freely-moving rats (O’Dell et al., 1996; Orona et al., 1994; Wallace et al., 1996). We also verified that nonanesthetized controls that received either repeated cocaine or saline demonstrated an increase in Fos expression in response to cocaine challenge in all regions examined.
The acute cocaine-induced increases in Fos were expected based on previous studies of the nucleus accumbens (Crombag et al., 2002; Hope et al., 2006; Mattson et al., 2008), medial prefrontal cortex (Todtenkopf et al., 2002) and orbital cortex (Kufahl et al., 2009). All of these regions play a role in stimulant effects of cocaine and drug-conditioned effects (Neisewander et al., 2000). Among the groups of non-anesthetized rats given the cocaine challenge, the previous repeated cocaine regimen attenuated Fos expression relative to repeated saline in the dCPu only. Previous research has also shown that the robust c-fos and Fos response to acute cocaine in the CPu of drug-naïve rats (Graybiel et al., 1990; Young et al., 1991) is attenuated following repeated cocaine administration (Hope et al., 1992; Moratalla et al., 1996). Similarly, Zahm and colleagues (2010) found that Fos expression in response to the first cocaine self-administration session was attenuated after 6 self-administration sessions in the NAcSh, CPu, and anterior cingulate cortex as well as subregions of lateral habenula, hypothalamus, and brainstem. However, we did not expect to observe such attenuation in the present experiment because we employed a 72-h drug-free period prior to the final cocaine challenge on the test day. Previous research has shown that this period of time is sufficient for Fos expression induced by repeated cocaine administration to recover from tolerance (Crombag et al., 2002). The apparent persistence of tolerance to cocaine-induced Fos expression in the dCPu of the present study is also surprising given that Zahm et al. (2010) found that control rats given experimenter-delivered intravenous cocaine exhibited either no difference or higher amounts of Fos expression compared to self-administering rats. This apparent discrepancy may be due to a number of experimental differences including the doses and timing of cocaine administration and the amount of time since the last exposure to cocaine from the exposure on the final day.
The region-specific pattern of changes in cocaine-induced Fos observed with isoflurane in this study has similarities and differences compared to the pattern of changes we observed previously without a history of repeated drug administration (Kufahl et al., 2009). In both studies, isoflurane had no significant effect on Fos expression in response to acute cocaine administration in the dCPu but did attenuate Fos expression in the orbital frontal cortex. The latter effect appeared more robust in the present study, perhaps due to changes in sensitivity to the cocaine challenge as a result of previous repeated cocaine exposure. Differences in response to acute cocaine challenge across studies were that isoflurane increased Fos expression in the prelimbic cortex and in the core and shell of NAc in the previous study of drug-naïve rats, but decreased expression in PrL and NAcSh and failed to alter expression in NAcC in the present study of rats repeatedly exposed to cocaine. Given that the decreases in Fos were only observed in rats under isoflurane anesthesia and not in nonanethetized rats, the results suggest that neuronal signaling activation is more disrupted by isoflurane in rats with a history of repeated cocaine exposure than in rats that have not been exposed previously to cocaine. Nevertheless, the preserved Fos expression in response to cocaine in the NAcC in isoflurane-anesthetized rats with previous cocaine exposure suggests that neuronal signaling remains intact in this region even under isoflurane anesthesia.
Several anesthetic and analgesic drugs, including ketamine, sodium pentobarbital (Torres et al., 1994) and buprenorphine (Placenza et al., 2008), have been shown to interfere with neurobiological cocaine effects, but isoflurane is a popular choice for investigations of drug action in fMRI neuroimaging (Steward et al., 2005) as well as other in vivo experiments (Du et al., 2006). Another popular fMRI anesthetic, the injectable compound α-chloralose, was previously shown to abolish cocaine-induced Fos expression in drug-naïve rats throughout the prefrontal cortex and striatum (Kufahl et al., 2009). A similar effect has also been reported for sodium pentobarbital (Ryabinin et al., 2000) and (in the striatum) ketamine, chloral hydrate and urethane anesthesia regimens (Kreuter et al., 2004; Torres and Rivier, 1993). These severe reductions in cocaine-induced Fos expression may be attributable to the loss of downstream perceptional and motor functions that are sensitive to cocaine, rather than the direct pharmacological effects (Ryabinin, 2000). Additionally, all of the general anesthetics discussed above have a wide variety of neurobiological substrates for their actions that are incompletely understood (Austin et al., 2005; Campagna et al., 2003; Ishizawa, 2007).
Recently, an in vivo fMRI method for studying cocaine effects in nonanesthetized rats has been developed (Febo et al., 2005), although this technique still uses isoflurane repeatedly to condition the animal to the fMRI apparatus (Febo et al., 2004). Febo et al. (2005) reported that chronic exposure to i.p. cocaine decreased the cerebral blood flow response to a cocaine challenge dose administered into the cerebral ventricles, with significant reductions in the medial prefrontal cortex, dCPu and nucleus accumbens (Febo et al., 2005). In contrast, the present study, using the same number of repeated cocaine injections and incubation days prior to the cocaine challenge, found no effect of repeated cocaine exposure on cortical and nucleus accumbens cocaine-induced Fos expression, in agreement with a previous report (Todtenkopf et al., 2002). The discrepancies across studies may be due to differences in cocaine delivery methods or dose. Fos immunochemistry has been used to validate the spatial distribution of fMRI results for several animal experiments (Dashti et al., 2005; Di Chiara et al., 2004; Lawrence et al., 2004; Liu et al., 2004), and a similar evaluation of cocaine exposure in nonanesthetized rats would be useful.
While behavioral sensitization to cocaine was not accompanied by increases in Fos expression in the brain regions examined, adaptations in the prefrontal cortex may have made the Fos expression sensitive to reduction by isoflurane anesthesia. The practical use of isoflurane to study the effects of chronic cocaine exposure on cocaine-elicited neuronal activity is therefore limited to nucleus accumbens and other areas whose cocaine response is not dominated by extrapharmacological effects. Given the complex array of neurochemical targets of general anesthetics and drugs of abuse, feasibility of isoflurane use in the study of amphetamine, methamphetamine or other drugs that induce behavioral sensitization would have to be evaluated because it is unclear whether the findings in the present study would generalize to effects of these drugs.
While Fos immunoreactivity is a widely used biomarker for cocaine activation of neural tissue, the exact nature of stimulus-Fos expression coupling is an area of uncertainty given the existence of a variety of mechanisms that result in Fos activation discovered from in vitro experimentation (Rhodes and Crabbe, 2005; Sheng and Greenberg, 1990). In behaving rodents, the expression of Fos protein is mediated by a pathway that is initiated by calcium influx into neuronal cell bodies via glutamate-activated N-methyl-D-aspartate (NMDA) receptors and voltage-sensitive calcium channels, and requires a consistent influx of calcium to translate c-fos (Cohen and Greenberg, 2008). In dopamine target fields, such as within the nucleus accumbens and prefrontal cortex, Fos expression may not be a direct consequence of drug-induced dopamine release, since in the absence of glutamate signaling (i.e. non-activated neurons), dopaminergic agonists usually hyperpolarize neurons and thereby suppress Fos (Cruz et al., 2013; O’Donnell, 2003). In activated neurons, cocaine-elicited dopamine release enhances the ongoing effect of glutamate neurotransmission, increasing calcium influx to levels sufficient for significant Fos expression (Cohen and Greenberg, 2008). Under anesthesia, the pathways by which cocaine elicits Fos expression have the potential to change, but the amount of anesthesia-induced Fos suppression appears to be more dependent on glutamate than dopamine neurotransmission effects (Kreuter et al., 2004). Experimental results indicating drug-induced c-fos gene expression under anesthesia may therefore benefit from verification by analyses of other brain activity-related immediate early genes, such as zif268 and arc (Harlan and Garcia, 1998). Nonetheless, the existence of regionally-specific neural responses to isoflurane and other anesthetics (Kufahl et al., 2009; Westphalen et al., 2011), as well as the variegated glutamate responses elicited by different drugs of abuse (Gass and Olive, 2008), suggest that the brain activity response to procedural pairings of drugs of abuse with anesthetic regimen should be carefully investigated.
4. Experimental Procedure
4.1. Animals
Male Sprague-Dawley rats weighing 350–450 g were housed individually in a temperature-controlled colony room with a 12-h reversed light/dark cycle and allowed free access to food and water. Animal care and housing conditions were consistent with the specifications of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Experimental procedures were approved by the Institutional Animal Care and Use Committee at Arizona State University. Animals were acclimated to these housing conditions and experimenter handling for 5 days prior to surgery.
4.2. Surgery
Atropine sulfate (10 mg/kg i.p., Sigma) was administered before surgery to reduce bronchial secretions. The rats were anesthetized with isoflurane (4% induction, 2.5% maintenance) and a catheter was inserted into the jugular vein as described in detail previously (Neisewander et al., 2000). Catheters were constructed from Silastic tubing (10 cm length, 0.012 in inner diameter, 0.025 in outer diameter, Dow Corning, Midland, MI) connected to a 22 gauge nonferrous metal cannula encased within a plastic screw connector (Plastics One, Roanoke, VA). A flexible obturator made from Tygon tubing was fitted over the cannula to prevent infection when not in use. Patency of the catheters was maintained throughout the experiment by daily flushing with 0.1 ml bacteriostatic saline solution containing heparin (70 U/ml, Elkins-Sinn, Cherry Hill, NJ) and ticarcillin disodium (20 mg/ml GlaxoSmithKline, Philadelphia, PA). Rats also received 0.67 mg/ml urokinase (Astra USA, Westerborough, MA) daily for 1 week after surgery. Catheter patency was tested every five days with 0.8 mg methohexital sodium (Brevital, Sigma), a dose that produces loss of muscle tone only when administered intravenously Behavioral experiments were performed at least 14 days after surgery.
4.3. Drugs
Cocaine hydrochloride (Research Triangle Institute) was dissolved in 0.9% saline and filtered for i.v. administration. All injections were delivered at a volume of 1 ml/kg body weight over five seconds. These timed infusions were performed by hand, with the aid of a subsecond timer to ensure consistent delivery of drug. The investigators were trained with the timer for several sessions in preparation for this procedure. Liquid isoflurane (Abbott Laboratories, North Chicago, IL) was mixed with oxygen gas and delivered through a plastic nose cone connected to a vaporizer/mixer with a charcoal filtration system (SurgiVet, Waukesha, WI).
4.4. Repeated administration of drugs
Thirty-one rats were divided into five groups (n = 5–9 per group). After two days of 90-min habituation sessions in the locomotor testing chambers, whose size (18×10×8 in), shape and bedding type (Sani-chip, Harlan-Teklad, Madison, WI) matched their home cages. During the subsequent treatment sessions, all rats were moved to the testing environment (while in their home cage) for 30 min, attached to an extended piece of flexible tubing for IV infusion, placed into the locomotor chambers for 2 min, and then given a controlled (5-sec) IV infusion. Two groups (14 rats total) were injected with cocaine dissolved into 0.9% saline (2 mg/kg in 1 ml/kg i.v.), and the other three groups (17 rats total) were injected with 0.9% saline (1 ml/kg i.v). The animals remained in the locomotor chambers for 90 min, while their movements were recorded by video cameras mounted above the chambers and driven by TopScan software (Clever Systems, Reston, VA). Video of the first 20 min of the Day 1 and Day 7 of drug administration sessions for each rat were later visually analyzed for locomotor behavior. Horizontal crossovers were defined as movement of the entire rat from one half of the chamber to the opposite half. Rearing was defined as the rat raising both forepaws off of the floor, either in the air or against the chamber wall (Lever et al., 2006). Rats were brought back to their home cages following each session. Rats were given one session each day for seven consecutive days. All injections of cocaine or vehicle were administered over 5 sec, which has been determined to be the optimal rate for locomotor sensitization (Samaha et al., 2002).
4.5. Testing of nonanesthetized animals
To avoid the attenuation of Fos expression known to occur in the ventral striatum after repeated psychostimulant administration (Hope et al., 1992; Persico et al., 1993; Steiner and Gerfen, 1993; Todtenkopf et al., 2002), all rats were given cocaine or saline challenge injections 6 days after the last of the repeated administration sessions. Habituation of cocaine-evoked c-fos mRNA and Fos expression has been shown to reverse after three days (Nye et al., 1995; Persico et al., 1995).
Rats were divided into anesthetized and non-anesthetized testing groups. Rats in the cocaine-conditioned and cocaine-tested non-anesthetized (CC-NoA, n = 8) group and the saline-conditioned and cocaine-tested non-anesthetized (SC-NoA, n = 6) group were moved to the testing environment, remained inside their home cages for 30 min, attached to an extended piece of flexible tubing for IV infusion, placed into the locomotor chambers for 2 min, and then injected with cocaine dissolved into 0.9% saline (2 mg/kg in 1 ml/kg i.v.). Rats in the saline-conditioned and saline-tested non-anesthetized (SS-NoA, n = 6) group were injected with 0.9% saline. All challenge injections were administered over 5 sec, the optimal speed for inducing peak c-fos and dopamine neurotransmitter responses for awake animals (Ferrario et al., 2008; Samaha et al., 2004). Immediately after the challenge injections, rats were placed into their locomotor activity chambers for 90 min and their movements were recorded as described previously.
4.6. Testing under isoflurane anesthesia
On the test day, rats in the cocaine-conditioned cocaine-tested anesthetized (CC-ISO, n = 5) and saline-conditioned saline-tested anesthetized (SS-ISO, n = 6) groups were placed in a Plexiglas induction chamber and administered 3% isoflurane. After induction of anesthesia was confirmed by tail-pinch, the rat was placed on an electric heating blanket and the isoflurane concentration was reduced to 2%. Breathing was visibly monitored and core temperature was continually measured with a flexible rectal probe (YSI, Dayton, OH) connected to a digital thermometer (Cole-Parmer, Vernon Hills, IL). Expired gases were collected from the rat’s nose by a cannula connected to a low-flow capnograph (model V90041, SurgiVet) via a plastic sample line (Smiths Medical, Waukesha, WI). Heart rate and oxygen saturation (SpO2) were monitored using a pulse oximetry clip (SurgiVet) secured to the rear foot and connected to the same capnograph system. Thirty min after reducing the isoflurane, rats in the CC-ISO group received an i.v. infusion of cocaine (2 mg/kg in 1 ml/kg saline) over a 5–sec period. Rats in the SS-ISO group received an i.v. infusion of saline at the same time and volume (1 ml/kg). Rats were then maintained under isoflurane anesthesia for an additional 90 min. Physiological parameters (end-tidal CO2, SpO2, breathing rate, heart rate and core temperature) were recorded every 15 min under anesthesia. The maintenance dose of isoflurane was continually adjusted (between 1.4% and 2.5%) in order to keep SpO2 > 95% and breathing rate > 68 cycles/min, and the power of the electric heating blanket was adjusted to keep the core temperature at 37 ± 1 °C.
4.7. Tissue preparation
Ninety min after the challenge cocaine or saline administration, all rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.). The circulatory system was perfused with 200 ml of ice-cold saline followed by 250 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). The brain was removed and post-fixed in paraformaldehyde for 24 h, and then cryoprotected by submersion in 30% sucrose for at least 24 h. The brains were then sectioned using a sliding microtome (Microm International, Walldorf, Germany) connected to a freezing stage (Physitemp, Clifton, NJ). Serial coronal 40 μm sections were collected, separated by 120 μm, centered at anatomical locations corresponding to bregma +3.2 and +1.6 (anterior) mm (Paxinos and Watson, 1998). The tissue sections were then frozen and stored at 4° C in a cryoprotectant solution comprised of 0.02 M PBS (pH 7.2), 30% sucrose, 10% polyvinyl pyrrolidone and 30% ethylene glycol.
4.8. Fos protein immunohistochemistry
Tissue from all rats for each section was processed in the same immunohistochemistry assay. Free floating tissue sections were first washed in 0.02 M PBS (pH 7.2, seven times for 10 min each), incubated for 1 h in 0.4% Triton X-100 and 1.5% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) in 0.02 M PBS. The tissue was then incubated for 48 h at 4°C with rabbit polyclonal anti-Fos serum (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:10,000 in 0.02 M PBS containing 1% NGS and 0.4% Triton X-100. Following incubation, the sections were washed in 0.02 M PBS (five times for 5 min each) and then incubated for 1 h at room temperature in biotinylated goat anti-rabbit IgG antibody (Vector Laboratories), diluted 1:400 in 0.02 M PBS. The tissue was then washed in 0.02 M PBS (three times for 10 min each) and then incubated for 1 h in avidin-biotinylated horseradish peroxidase complex (ABC Elite Kit; Vector Laboratories) diluted 1:100 in 0.02 M PBS. The sections were then washed with 0.05 M Tris buffer (pH 7.6, three times for 10 min each) and incubated in 0.05 M Tris buffer containing 0.02% 3,3′-diaminobenzidine (DAB; Sigma), 2.5% nickel ammonium sulfate and 0.005% H2O2. The DAB reaction was terminated after 3 min by rinsing the tissue three times for 10 min in 0.02 M PBS. All of the washes and incubations described above were performed on an orbital shaker (Cole-Parmer, Vernon Hills, IL) operating at 90 rpm. The sections were mounted onto gelatin chromium-coated slides, air-dried, dehydrated and protected with a coverslip of light-microscopic inspection.
4.9. Fos immunoreactivity analysis
Fos immunoreactivity was examined using a Nikon Eclipse E600 (Nikon Instruments, Melville, NY) microscope set at 20× magnification and counted by an observer blind to treatment conditions using the ImageTool software package (Version 3.0, University of Texas Health Science Center, San Antonio, TX). The anatomical locations and boundaries of each region were determined using a rat brain atlas (Paxinos and Watson, 1998) and are illustrated in Fig 5. Sections taken at +3.2 mm from bregma contained the prelimbic (PrL), infralimbic (IL), orbital (Orb) and M1 region of the motor cortex (M1). Sections taken at +2.24 mm from bregma contained the Cg2 region of the anterior cingulate cortex (Cg2), nucleus accumbens shell (NAcS), nucleus accumbens core (NAcC) and dorsal caudate/putamen (dCPu). The sections were taken such that the rostral-caudal extent of each region of interest was sampled (340 μm). Fos immunoreactivity was counted and identified by a blue-black oval-shaped nucleus (Fig. 6). Each region of interest was analyzed using both hemispheres from three tissue sections from each animal. The area of each sample measure was 0.26 mm2, but two samples were taken from each hemisphere for the Orb and dCPu. The counts from all the sample areas from a given region were averaged and scaled to provide a mean number of Fos-positive cells per mm2.
Figure 5.
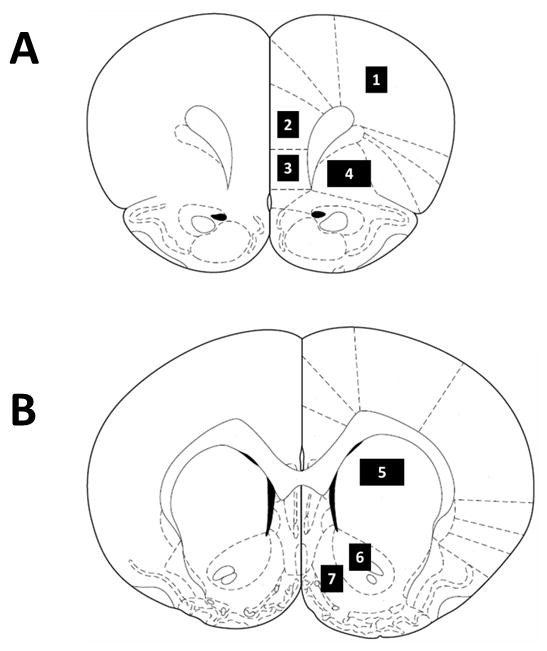
Schematic representation of coronal sections of the rat brain taken at (A) +3.2 and (B) +1.6 mm from Bregma (Paxinos and Watson, 1998) illustrating the regions in this study as follows: (1) M1 region of the motor cortex (M1); (2) prelimbic cortex (PrL); (3) infralimbic cortex (IL); (4) orbital cortex (Orb); (5) dorsal caudate/putamen (dCPu); (6) nucleus accumbens core (NAcC); (7) nucleus accumbens shell (NAcS). All sample areas were 0.26 mm2, except for Orb and dCPu, which were sampled with 0.52 mm2 areas.
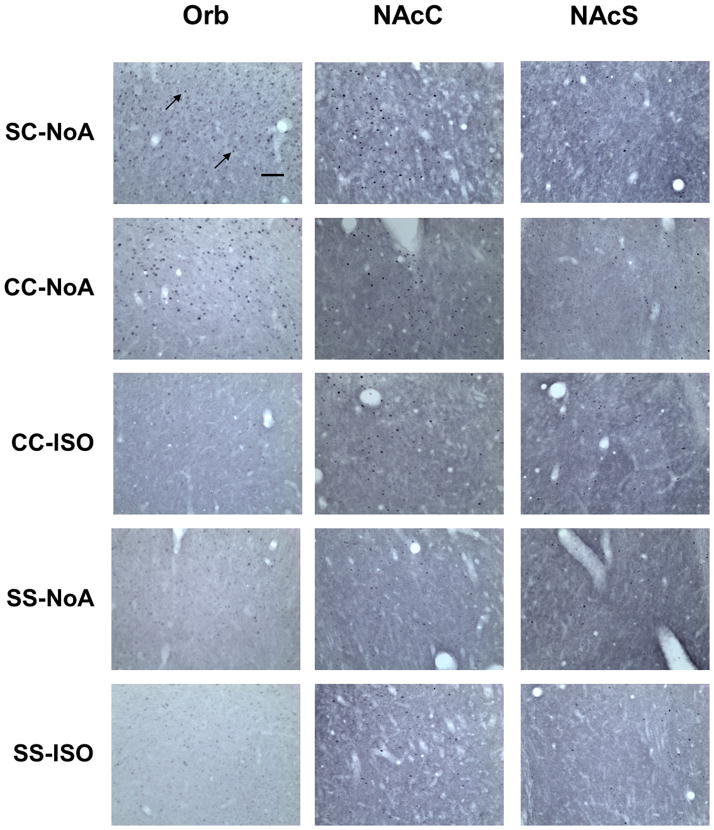
Figure 6.
Representative photomicrographs of Fos protein expression in the Orb, NAcC and NAcS of rats from all experimental groups at 20× magnification, where Fos protein expression was visible as dark ovals (highlighted by arrows). Scale bar is equal to 100 μm.
4.10. Statistical analysis
To examine the effects of repeated administration of cocaine or saline on locomotor activity, crossovers and rearing behavior accumulated over the first 20 min of the Day 1 and Day 7 sessions were analyzed with 2 × 2 factorial ANOVAs, with day (Day 1 or Day 7) as a within-subjects factor and repeated treatment (cocaine or saline) as a between-subjects factor. Significant interactions were followed by post hoc Newman-Keuls tests between groups. The effects of the challenge injection on locomotor activity in non-anesthetized animals, crossovers and rearing behavior over the first 20 min of the test session were assessed with 3 × 1 ANOVAs, with repeated/challenge treatment as a between-subjects factor (i.e., the SC-NoA, SS-NoA and CC-NoA groups).
Statistical evaluations of Fos protein expression were executed independently for each region of interest. First, the possible presence of tolerance effects on cocaine-induced Fos was tested by planned t-tests between SC-NoA and CC-NoA groups. This was followed by analyses of the effects of anesthesia and challenge injection of Fos expression by 2 × 2 factorial ANOVAs, where anesthesia (none or isoflurane) and challenge (cocaine or saline) were between-subjects factors (i.e., the CC-ISO, SS-ISO, CC-NoA and SS-NoA groups). Significant interactions were followed by post hoc Newman-Keuls tests. Finally, the presence of cocaine-induced Fos in non-anesthetized rats were evaluated by planned t-tests between SC-NoA and SS-NoA groups, and between CC-NoA and SS-NoA groups. Effects were considered significant when p < 0.05.
Acknowledgments
The authors thank Valeria Routt, Mike Painter, Lara A. Pockros and Kenneth J. Thiel for help with surgeries, and Lauren E. Hood and Emile Saad for other technical contributions.
Grant Support: R01 DA11064, F32 DA021485
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest.
References
- Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. The influence of environment on the induction of sensitization to the psychomotor activating effects of intravenous cocaine in rats is dose-dependent. Psychopharmacology (Berl) 1998;137:90–8. doi: 10.1007/s002130050597. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–62. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti M, Geso M, Williams J. The effects of anaesthesia on cortical stimulation in rats: a functional MRI study. Australas Phys Eng Sci Med. 2005;28:21–5. doi: 10.1007/BF03178860. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–31. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139:167–76. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30:936–43. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L, Turrini G, Crestan V, Bertani S, Cristofori P, Bifone A, Gozzi A. A robust experimental protocol for pharmacological fMRI in rats and mice. J Neurosci Methods. 2012;204:9–18. doi: 10.1016/j.jneumeth.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Shou M, Samaha AN, Watson CJ, Kennedy RT, Robinson TE. The rate of intravenous cocaine administration alters c-fos mRNA expression and the temporal dynamics of dopamine, but not glutamate, overflow in the striatum. Brain Res. 2008;1209:151–6. doi: 10.1016/j.brainres.2008.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanusch C, Hoeger S, Beck GC. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43:68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–67. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–99. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–8. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci. 2006;24:867–75. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Ishizawa Y. Mechanisms of anesthetic actions and the brain. J Anesth. 2007;21:187–99. doi: 10.1007/s00540-006-0482-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreuter JD, Mattson BJ, Wang B, You ZB, Hope BT. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience. 2004;127:233–42. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Pentkowski NS, Heintzelman K, Neisewander JL. Cocaine-induced Fos expression is detectable in the frontal cortex and striatum of rats under isoflurane but not α-chloralose anesthesia: implications for FMRI. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, Bascaramurty S, Jordan LM, Malisza KL. Correlation of functional activation in the rat spinal cord with neuronal activation detected by immunohistochemistry. Neuroimage. 2004;22:1802–7. doi: 10.1016/j.neuroimage.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–67. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, O’Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–33. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–85. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasik VM, Gillies RJ. Animal anaesthesia for in vivo magnetic resonance. NMR Biomed. 2003;16:459–67. doi: 10.1002/nbm.836. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane–anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–50. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–12. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–56. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–7. [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–80. [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology (Berl) 1996;123:144–53. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. European Journal of Neuroscience. 2003;17:429–35. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Orona RA, Mayfield RD, Cline EJ, Zahniser NR. Repeated intravenous cocaine administration to rats produces behavioral sensitization without changing brain cocaine levels. Neurosci Lett. 1994;167:121–4. doi: 10.1016/0304-3940(94)91042-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Persico AM, Schindler CW, O’Hara BF, Brannock MT, Uhl GR. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res Mol Brain Res. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Persico AM, Schindler CW, Zaczek R, Brannock MT, Uhl GR. Brain transcription factor gene expression, neurotransmitter levels, and novelty response behaviors: alterations during rat amphetamine withdrawal and following chronic injection stress. Synapse. 1995;19:212–27. doi: 10.1002/syn.890190309. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–6. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2008;200:347–55. doi: 10.1007/s00213-008-1210-z. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–2. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Crabbe JC. Gene expression induced by drugs of abuse. Current Opinion in Pharmacology. 2005;5:26–33. doi: 10.1016/j.coph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE. ITF mapping after drugs of abuse: pharmacological versus perceptional effects. Acta Neurobiol Exp (Wars) 2000;60:547–55. doi: 10.55782/ane-2000-1375. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Bachtell RK, Kinney AE, Grubb MC, Mark GP. Cocaine- and alcohol-mediated expression of inducible transcription factors is blocked by pentobarbital anesthesia. Brain Res. 2000;877:251–61. doi: 10.1016/s0006-8993(00)02681-0. [DOI] [PubMed] [Google Scholar]
- Salmeron BJ, Stein EA. Pharmacological applications of magnetic resonance imaging. Psychopharmacol Bull. 2002;36:102–29. [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–50. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–70. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Stein EA, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–86. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–85. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–90. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–81. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–81. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology (Berl) 2005;180:687–704. doi: 10.1007/s00213-005-2213-7. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Mihalakopoulos A, Stellar JR. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–9. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists. Brain Res Bull. 1993;30:173–6. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C, Weiss F. A ketamine mixture anesthetic inhibits neuroendocrine and behavioral consequences of cocaine administration. Brain Res. 1994;656:33–42. doi: 10.1016/0006-8993(94)91363-3. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Mactutus CF, Booze RM. Repeated intravenous cocaine administration: locomotor activity and dopamine D2/D3 receptors. Synapse. 1996;23:152–63. doi: 10.1002/(SICI)1098-2396(199607)23:3<152::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Kwak NB, Daniels K, Hemmings HC., Jr Regional differences in the effects of isoflurane on neurotransmitter release. Neuropharmacology. 2011;61:699–706. doi: 10.1016/j.neuropharm.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Desai KM, Hemmings HC., Jr Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth. 2013;110:592–9. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–53. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991;88:1291–5. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology. 2010;35:445–63. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]