Abstract
Background
We hypothesized that phosphorus has an effect on anemia in both normal kidney function and early chronic kidney disease (CKD). We sought to determine whether higher phosphorus levels are associated with anemia in a large diverse population without CKD and early CKD.
Methods
This study is a historical population-based study within the Kaiser Permanente Southern California health system (1 January 1998 to 31 December 2013) among individuals aged 18 years and older with estimated glomerular filtration rate >30 mL/min/1.73 m2 and measurements of serum phosphorus, creatinine and hemoglobin. Individuals were excluded if they had secondary causes of anemia. Odds ratio (OR) estimated for moderate anemia defined as hemoglobin <11 g/dL for both sexes. Mild anemia was defined as <12 g/dL (females) and <13 g/dL (males).
Results
Among 155 974 individuals, 4.1% had moderate anemia and 12.9% had mild anemia. Serum phosphorus levels ≥3.5 mg/dL were associated with both mild and moderate anemia. Moderate anemia OR (95% confidence interval) was 1.16 (1.04–1.29) for every 0.5 mg/dL phosphorus increase and 1.26 (1.07–1.48) in the highest versus middle phosphorus tertile. Additional independent anemia risk factors, including female sex, Asian race, diabetes, low albumin and low iron saturation, were observed, but did not alter the anemia–phosphorus association.
Conclusions
Higher phosphorus levels were associated with a greater likelihood for anemia in a population with early CKD and normal kidney function. Phosphorus may be a biomarker for anemia and may affect aspects of hematopoiesis.
Keywords: anemia risk, epidemiology, serum phosphorus
INTRODUCTION
Phosphorus plays a major role in physiological functioning, including energy production, cellular replication and bone mineral metabolism. The level of phosphorus is tightly regulated by three main hormones [parathyroid hormone (PTH), vitamin D and fibroblast growth factor-23 (FGF-23)], which affect the intestinal absorption and renal excretion of phosphorus and bone mineral metabolism [1]. Dysregulation of these processes resulting in chronically low or high serum phosphorus has been associated with adverse outcomes [2]. Although chronic kidney disease (CKD) often leads to hyperphosphatemia, abnormalities in phosphorus levels have been observed in populations with and without kidney disease.
Hyperphosphatemia is associated with inflammation and may affect normal cellular physiology including erythropoiesis [3, 4]. Phosphorus alone has been implicated in inhibiting red blood cell production in uremic patients [5]. In dialysis and kidney transplant patients, hyperphosphatemia is associated with anemia independent of other mineral bone disease components [6, 7]. Along with anemia, hyperphosphatemia has been linked with lower bone mineral density in the peritoneal dialysis population [5]. It is also associated with high levels of PTH, which has been shown to inhibit erythropoiesis, induce hemolysis and cause bone marrow fibrosis in CKD states [8–12]. In addition, FGF-23 and its interplay with klotho, vitamin D and iron have been implicated with anemia [13–15]. In murine models that lack FGF-23 or klotho, the resultant hyperphosphatemia is associated with cell toxicity, premature aging and vascular calcifications [16].
Determining a relationship between phosphorus levels and prevalence of anemia in the non-CKD population may have important clinical implications. A study evaluating the National Health and Nutrition Examination Survey population showed that serum phosphorus levels >4.4 mg/dL were associated with mild anemia among individuals with normal kidney function [estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2] [17]. Another study of kidney transplant patients showed that a one standard deviation higher of serum phosphorous level (0.8 mg/dL) was associated with 77% greater odds for anemia [7]. These few studies that evaluated the relationship between serum phosphorus and anemia have been limited in various ways, including smaller population size, lack of heterogeneity and non-consideration of secondary causes of anemia [7, 17].
Given the biological importance of phosphorus, a better understanding of its levels in the body and association with pathophysiological processes such as anemia would provide invaluable insights. The objective of our study was to determine whether higher phosphorus levels were associated with anemia in a large diverse population with and without early CKD. We hypothesized that higher phosphorus levels increase the likelihood for anemia in people with both normal kidney function and mild CKD.
MATERIALS AND METHODS
Study design and setting
A historical population cohort study of Kaiser Permanente Southern California (KPSC) members was performed in the period of 1 January 1998 through 31 December 2013. The KPSC health system is an integrated healthcare system comprising 14 medical centers and over 200 satellite clinics, geographically spanning from Bakersfield to San Diego, CA, USA. As of 31 December 2013, there were over 3.6 million members of whom more than 2.4 million were adults. The membership population is racially and ethnically diverse reflective of the practicing area and the state of California [18]. All members have similar access to healthcare facilities, procedures and referrals. All information collected during clinical care is captured in a common electronic health record system. The study was approved by the regional Institutional Review Board and exempted from informed consent.
Inclusion and exclusion criteria
The study population included individuals aged 18 years and older with continuous membership within KPSC for at least 6 months to ensure documentation of comorbidities. Individuals must have had a minimum of one serum phosphorus, serum creatinine and hemoglobin measurement within 120 days of phosphorus measurement. The first available serum phosphorous value was used as the index date, and individuals with an eGFR >30 mL/min/1.73 m2 within 120 days of phosphorus measurement were included in the study.
Individuals were excluded if they had serum phosphorus levels at the highest 0.5% or lowest 0.5% to eliminate outliers and potential assay interactions. To eliminate confounders for anemia, individuals were excluded if they were receiving hemodialysis, erythrocyte-stimulating agents or blood transfusions or had other secondary causes of anemia including any organ transplant, malignancy, hemorrhage, hematological disorder, pregnancy, inflammatory or autoimmune disorder or diseases of the liver, lung or spleen. The secondary causes of anemia were determined by inpatient and outpatient International Classification of Diseases (ICD-9 ninth revision) diagnoses coding (Supplementary data, Table S1).
Data sources and laboratory measurements
Data on age, sex, race/ethnicity, laboratory values and comorbidities were extracted from the electronic health records. Race/ethnicity was categorized as white, black, Hispanic, Asian or other. Individuals were categorized as other if no race data were available or if they were not classified as any of the other races. Comorbidities, including diabetes mellitus, were assessed on the basis of inpatient and outpatient ICD-9 coding. The earliest serum creatinine within 120 days after baseline phosphorus measurement was used to establish eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [19]. Serum phosphorus level was measured using a standard colorimetric method with normal reference values of 2.7–4.5 mg/dL (Roche Diagnostics, Alameda, California). When available, laboratory values on serum albumin, ferritin, iron saturation and total iron binding capacity were extracted. All laboratory measurements were performed and reported from an American College of Pathology/Clinical Laboratory Improvement Act-certified laboratory.
Statistical methods
The primary outcome evaluated was moderate anemia defined by the World Health Organization (WHO) as hemoglobin <11.0 g/dL for both sex groups [20]. Secondary outcome was mild anemia defined by the WHO as hemoglobin <12.0 g/dL for females and <13.0 g/dL for males. Individuals were categorized into population tertiles on the basis of the distribution of serum phosphorus levels, with the middle tertile serving as the reference group (Supplementary data, Figure S1).
Each individual contributed only one cross-sectional phosphorus and hemoglobin combination. The distributions of categorical variables were assessed via χ2 or Fisher's exact test and continuous variables via independent samples t-test or non-parametric Kruskal–Wallis test, as appropriate. Univariate and multivariable logistic regression analyses were used to estimate the odds ratio (OR) for mild and moderate anemia across population-based phosphorus tertiles and per 0.5 mg/dL increments of phosphorus. Multivariable models included phosphorus as the exposure and potential confounders that included age, sex, race/ethnicity, diabetes mellitus, albumin, iron saturation and ferritin. Missing variable data were not included in the analyses. Restricted cubic spline modeling was employed to estimate continuous ORs across differing serum phosphorus levels. Pearson's correlation coefficients were estimated for hemoglobin and phosphorus measurements within the entire study population and by sex and race/ethnicity.
RESULTS
Cohort characteristics
A total of 360 379 individuals were identified with outpatient phosphorus and hemoglobin measurements. Of these, 204 405 individuals were not eligible on the basis of the exclusion criteria (Figure 1). The study cohort comprised 155 974 individuals. Baseline characteristics of the cohort revealed a mean age of 52 years with 61.6% females, 38.1% whites, 13.6% blacks, 24.6% Hispanics and 6.9% Asians. Diabetes mellitus was present in 15.8% of the population (Table 1).
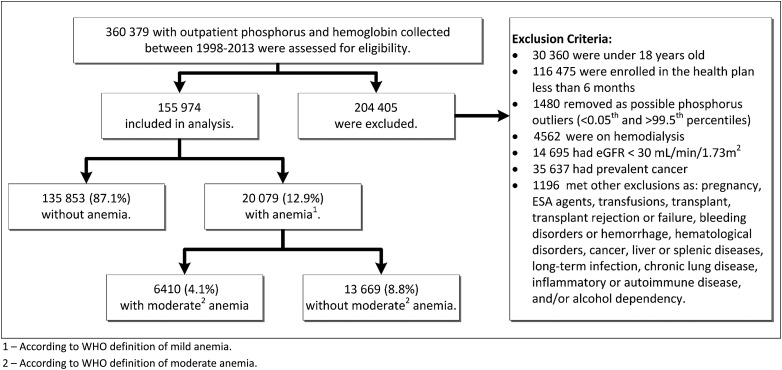
FIGURE 1:
Study cohort flow chart. A total of 360 379 adult KPSC members were identified with outpatient phosphorus and hemoglobin (Hgb) measurements; 204 405 individuals were not eligible on the basis of the exclusion criteria and 155 974 individuals were included in the analysis. Mild anemia, defined as Hgb <12 g/dL for females and <13 g/dL for males, was identified in 12.9% of the cohort (N = 20 079). Moderate anemia, defined as Hgb <11 g/dL for both sex, was identified in 4.1% of the cohort (N = 6410).
Table 1.
Characteristics of study population by moderate anemia status
| No moderate anemia | Moderate anemia | Total | P-value | |
|---|---|---|---|---|
| Hgb ≥ 11 g/dL | Hgb < 11 g/dL | |||
| N = 149 564 | N = 6410 | N = 155 974 | ||
| Demographic variables | ||||
| Age (years) | 52.2 (15.8) | 54.3 (17.3) | 52.3 (15.8) | <0.001 |
| Female sex (%) | 60.9 | 78.8 | 61.6 | <0.001 |
| Asian race (%) | 6.9 | 7.2 | 6.9 | <0.001 |
| Black race (%) | 13.0 | 25.9 | 13.6 | <0.001 |
| Hispanic race (%) | 24.5 | 27.0 | 24.6 | <0.001 |
| Other (%) | 17.2 | 7.8 | 16.8 | <0.001 |
| White (%) | 38.3 | 32.2 | 38.1 | <0.001 |
| History of diabetes (%) | 15.3 | 26.5 | 15.8 | <0.001 |
| Laboratory parameters | ||||
| Serum phosphorus (mg/dL) | ||||
| Mean (SD) | 3.5 (0.6) | 3.5 (0.7) | 3.5 (0.6) | <0.001 |
| Median (IQR) | 3.5 (3.0, 3.9) | 3.5 (3.0, 4.0) | 3.5 (3.0, 3.9) | |
| Hemoglobin (g/dL) | ||||
| Mean (SD) | 13.9 (1.3) | 9.9 (0.9) | 13.7 (1.6) | <0.001 |
| Median (IQR) | 13.8 (12.9, 14.8) | 10.2 (9.5, 10.6) | 13.7 (12.7, 14.8) | |
| eGFR (mL/min/1.73 m2) | ||||
| Mean (SD) | 85.0 (23.1) | 80.2 (29.4) | 84.8 (23.4) | <0.001 |
| Median (IQR) | 86.5 (69.0, 102.1) | 82.4 (53.9, 105.4) | 86.2 (68.6, 10.5) | |
| Serum albumin (g/dL) | ||||
| <4.0 | 53.7% | 85.7% | 56.1% | <0.001 |
| ≥4.0 | 46.3% | 14.3% | 43.9% | |
| Ferritin (ng/mL) | ||||
| <100 | 49.8% | 59.2% | 51.2% | <0.001 |
| ≥100 | 50.2% | 40.8% | 48.8% | |
| Iron saturation (%) | ||||
| <20 | 34.1 | 64.5 | 37.8 | <0.001 |
| ≥20 | 65.9 | 35.5 | 62.2 | |
Values are mean (SD) when appropriate. Categorical variables were analyzed using χ2 or Fisher's exact test, continuous variables using independent samples t-test, Kruskal–Wallis or Mann–Whitney U-test, as appropriate. Higher percentages of females (78.8%), blacks (25.9%), diabetics (26.5%) and individuals with low albumin (85.7%) and low iron saturation (64.5%) were present in the moderate anemia group compared with the non-anemia group.
Anemia outcomes
Moderate anemia
A total of 6410 (4.1%) individuals had moderate anemia (Figure 1). The baseline characteristics for individuals with moderate anemia revealed higher percentages of females, blacks, diabetics, low albumin and low iron saturation compared with those without anemia (Table 1). Serum phosphorus levels ranged from 1.5 to 6.2 mg/dL, with mean phosphorus 3.5 mg/dL in both moderate anemia and non-anemia groups. Mean hemoglobin was 9.9 g/dL and mean eGFR was 80.2 mL/min/1.73 m2 in the moderate anemia group, compared with hemoglobin 13.9 g/dL and eGFR 85.0 mL/min/1.73 m2 in the non-anemia group (Table 1). A comparison of different race/ethnic groups showed that blacks had the lowest mean phosphorus (3.5 mg/dL), hemoglobin (13.0 g/dL) and eGFR 77.5 mL/min/1.73 m2, compared with all other race/ethnic groups (Table 2). Blacks also had the highest percentage of moderate anemia (7.9%).
Table 2.
Characteristics of study population categorized by race/ethnicity
| Asian | Black | Hispanic | Other | White | Total | P-value | |
|---|---|---|---|---|---|---|---|
| n = 10 829 (6.9) | n = 21 159 (13.6) | n = 38 363 (24.6) | n = 26 247 (16.8) | n = 59 376 (38.1) | n = 155 974 | ||
| Demographic variables | |||||||
| Moderate anemia (%) | 4.2 | 7.9 | 4.5 | 1.9 | 3.5 | 4.1 | <0.0001 |
| Age (years) | 54.1 (14.76) | 52.3 (15.49) | 48.1 (15.10) | 49.1 (15.11) | 55.9 (16.05) | 52.3 (15.85) | <0.0001 |
| History of diabetes (%) | 18.9 | 19.6 | 18.9 | 10.5 | 14.2 | 15.8 | <0.0001 |
| Laboratory parameters | |||||||
| Serum phosphorus (mg/dL) | |||||||
| Mean (SD) | 3.5 (0.63) | 3.4 (0.63) | 3.5 (0.64) | 3.5 (0.61) | 3.5 (0.63) | 3.5 (0.63) | <0.0001 |
| Median (IQR) | 3.5 (3.1, 3.9) | 3.4 (3.0, 3.8) | 3.5 (3.1, 3.9) | 3.5 (3.1, 3.9) | 3.5 (3.1, 3.9) | 3.5 (3.0, 3.9) | |
| Hemoglobin (g/dL) | |||||||
| Mean (SD) | 13.6 (1.54) | 13.0 (1.56) | 13.7 (1.58) | 14.1 (1.46) | 13.8 (1.51) | 13.7 (1.56) | <0.0001 |
| Median (IQR) | 13.7 (12.7, 14.7) | 13 (12.1, 14.0) | 13.7 (12.7, 14.8) | 14.1 (13.1, 15.1) | 13.8 (12.9, 14.8) | 13.7 (12.7, 14.8) | |
| CKD-EPI eGFR (mL/min/1.73 m2) | |||||||
| Mean (SD) | 86.0 (23.67) | 77.5 (23.28) | 93.1 (23.17) | 87.0 (22.21) | 80.8 (22.36) | 84.8 (23.43) | <0.0001 |
| Median (IQR) | 89.2 (70.3, 103.3) | 77.3 (60.5, 94.2) | 96.5 (79.0, 109.9) | 88.4 (72.1, 103.3) | 81.8 (65.2, 97.1) | 86.2 (68.6, 102.5) | |
| Serum albumin (g/dL) | |||||||
| <4.0 | 51% | 67.8% | 62.8% | 41.5% | 55.1% | 56.1% | <0.0001 |
| ≥4.0 | 49% | 32.2% | 37.2% | 58.5% | 44.9% | 43.9% | |
| Ferritin (ng/mL) | |||||||
| <100 | 39.3% | 48.8% | 56.5% | 50.6% | 51.2% | 51.2% | <0.0001 |
| ≥100 | 60.7% | 51.2% | 43.5% | 49.4% | 48.8% | 48.8% | |
| Iron saturation (%) | |||||||
| <20 | 31.7 | 41.1 | 40 | 33.3 | 37.6 | 37.8 | <0.0001 |
| ≥20 | 68.3 | 58.9 | 60 | 66.7 | 62.4 | 62.2 | |
Mean and median values of phosphorus, hemoglobin and eGFR are shown. Blacks had the lowest mean phosphorus levels with lower hemoglobin levels and the greatest rates of moderate anemia. Values are mean (SD) when appropriate. Categorical variables were analyzed using χ2 or Fisher's exact test and continuous variables using independent samples t-test, Kruskal–Wallis or Mann–Whitney U-test, as appropriate.
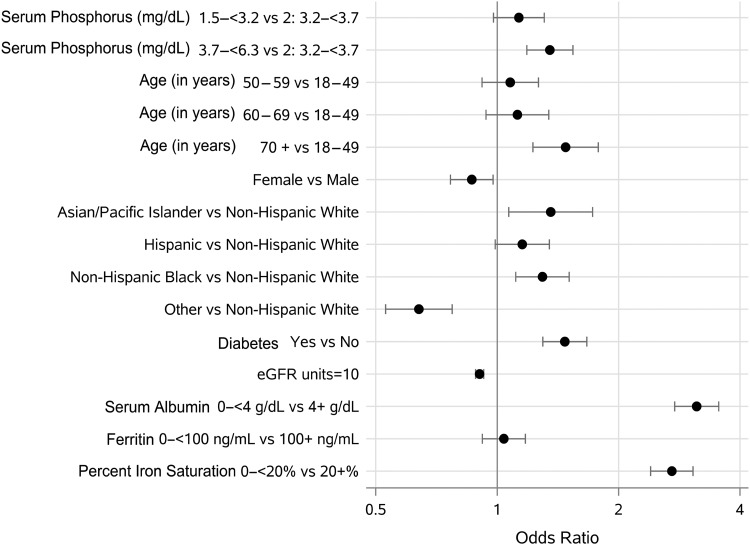
The population-based phosphorus tertiles ranged as follows: 1.5–3.1 mg/dL (Q1), 3.2–3.6 mg/dL (Q2) and 3.7–6.2 mg/dL (Q3). Individuals in the third tertile had the highest percentage of moderate anemia (Figure 2). Compared with the second tertile, individuals in both the lowest and highest tertiles had a higher OR for moderate anemia in unadjusted models (Table 3). In fully adjusted models, the associations with moderate anemia were attenuated in the lowest tertile, but remained robust in the third tertile with a 26% higher likelihood [OR (95% confidence interval (CI)) 1.26 (1.07–1.48)]. When modeled continuously in a fully adjusted analysis, each 0.5 mg/dL phosphorus elevation was associated with a 16% increased likelihood for moderate anemia [OR (95% CI) 1.16 (1.04–1.29)] (Table 3). In addition, this multivariable adjusted analysis revealed that Asian race 1.53 (1.15–2.01), diabetes 1.17 (1.00–1.37), female sex 1.76 (1.50–2.06) and individuals with low iron saturation 2.57 (2.23–2.98) and low albumin 3.21 (2.67–3.87) were associated with higher likelihood for moderate anemia, whereas each 10 U increase in eGFR was associated with lower moderate anemia likelihood [OR (95% CI) 0.94 (0.91–0.97)] (Table 3 and Figure 3).
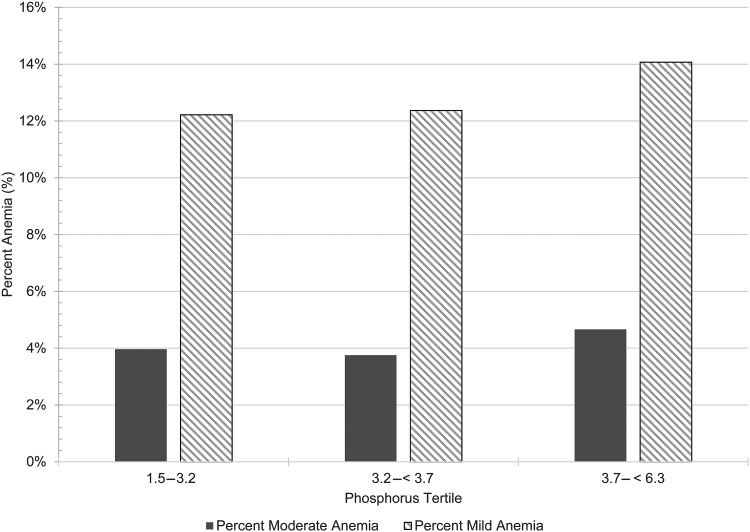
FIGURE 2:
Percentage of anemia across phosphorus tertiles. Phosphorus tertiles were categorized as: first (1.5 to <3.2 mg/dL), second (3.2 to <3.7 mg/dL) and third (3.7 to <6.3 mg/dL). The highest percentages of mild and moderate anemia were noted in the third tertile.
Table 3.
Multivariable adjusted OR for moderate anemia
| Unadjusted |
Demographics, eGFR- adjusted |
Fully adjusteda |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Phosphorus (mg/dL)b | |||||||||
| 1.5 to <3.2 | 1.06 | 1.00–1.13 | <0.01 | — | — | — | 1.01 | 0.84–1.21 | 0.93 |
| 3.2 to <3.7 | 1.00 | Reference | — | — | — | — | 1.00 | Reference | — |
| 3.7 to <6.3 | 1.26 | 1.18–1.33 | <0.01 | — | — | — | 1.26 | 1.07–1.48 | <0.01 |
| Every 0.5 mg/dL increase | 1.14 | 1.09–1.18 | <0.01 | 1.09 | 1.05–1.13 | <0.01 | 1.16 | 1.04–1.29 | <0.01 |
| Age (years) | |||||||||
| 18–49 | 1.00 | Reference | — | 1.00 | Reference | — | 1.00 | Reference | — |
| 50–59 | 0.79 | 0.74–0.84 | <0.01 | 0.65 | 0.60–0.70 | <0.01 | 0.98 | 0.80–1.21 | 0.86 |
| 60–69 | 0.87 | 0.80–0.93 | <0.01 | 0.62 | 0.57–0.68 | <0.01 | 1.01 | 0.81–1.27 | 0.91 |
| ≥70 | 1.49 | 1.40–1.59 | <0.01 | 0.98 | 0.90–1.06 | <0.01 | 1.15 | 0.92–1.45 | 0.22 |
| Sex | |||||||||
| Male | 1.00 | Reference | — | 1.00 | Reference | 1.00 | Reference | — | |
| Female | 2.39 | 2.25–2.54 | <0.01 | 2.37 | 2.23–2.52 | <0.01 | 1.76 | 1.50–2.06 | <0.01 |
| Race | |||||||||
| White | 1.00 | Reference | — | 1.00 | Reference | — | 1.00 | Reference | — |
| Black | 2.37 | 2.22–2.53 | <0.01 | 2.14 | 2.00–2.29 | <0.01 | 1.13 | 0.94–1.36 | 0.19 |
| Hispanic | 1.31 | 1.23–1.40 | <0.01 | 1.36 | 1.27–1.46 | <0.01 | 1.11 | 0.91–1.34 | 0.31 |
| Asian/PIc | 1.23 | 1.11–1.37 | <0.01 | 1.26 | 1.13–1.40 | <0.01 | 1.53 | 1.15–2.01 | <0.01 |
| Other | 0.54 | 0.49–0.60 | <0.01 | 0.62 | 0.56–0.69 | <0.01 | 0.53 | 0.40–0.70 | <0.01 |
| Diabetes | |||||||||
| No | 1.00 | Reference | — | 1.00 | Reference | 1.00 | Reference | — | |
| Yes | 2.00 | 1.89–2.12 | <0.01 | 1.87 | 1.76–1.98 | <0.01 | 1.17 | 1.00–1.37 | 0.05 |
| Ferritin (ng/ mL) | |||||||||
| <100 | 1.46 | 1.35–1.58 | <0.01 | — | — | — | 0.97 | 0.83–1.13 | 0.66 |
| ≥100 | 1.00 | Reference | — | — | — | — | 1.00 | Reference | — |
| Iron saturation (%) | |||||||||
| <20 | 3.52 | 3.26–3.80 | <0.01 | — | — | — | 2.57 | 2.23–2.98 | <0.01 |
| ≥20 | 1.00 | Reference | — | — | — | — | 1.00 | Reference | — |
| Albumin (g/dL) | |||||||||
| <4.0 | 5.18 | 4.57–5.88 | <0.01 | — | — | — | 3.21 | 2.67–3.87 | <0.01 |
| ≥4.0 | 1.00 | Reference | — | — | — | — | 1.00 | Reference | — |
| eGFRd (×10 U) | 0.92 | 0.91–0.93 | <0.01 | 0.92 | 0.90–0.93 | <0.01 | 0.94 | 0.91–0.97 | <0.01 |
In the fully adjusted model, OR was adjusted for age, sex, race, history of diabetes, ferritin, iron saturation, albumin and renal function. OR was the highest in the third phosphorus tertile and significant in females, Asians, diabetics and individuals with low iron saturation and low albumin.
aIn the fully adjusted model, ORs have been adjusted for demographics, eGFR, serum albumin, ferritin and iron saturation.
bPhosphorus tertiles were modeled separately; other covariate estimates are reported for the model with continuous phosphorus.
cAsian/PI, Asian/Pacific Islander race.
deGFR (per 10 mL/min/1.73 m2).
FIGURE 3:
Forest plot of fully adjusted OR for moderate anemia. OR was adjusted for demographics, eGFR, serum albumin, ferritin and iron saturation. A significant likelihood of moderate anemia was detected in the third phosphorus tertile, females, Asians, diabetics and individuals with low albumin and low iron saturation. Other race and every 10 U increase in eGFR were associated with decreased anemia.
A comparison of moderate anemia across various age groups and sex revealed that compared with males aged 18–49, females in all age groups (particularly ages 18–49 and 70+ years) had a higher likelihood for moderate anemia in fully adjusted models. Males in any other age group had no significant increase when compared with the reference group (Supplementary data, Table S3).
Mild anemia
A total of 20 079 (12.9%) individuals had mild anemia (Figure 1). The baseline characteristics of individuals with mild anemia were similar to those with moderate anemia: higher percentages of females, blacks, diabetics, low albumin and low iron saturation (Supplementary data, Table S2). For the mild anemia group, mean serum phosphorus was 3.5 mg/dL, mean hemoglobin was 11.2 g/dL and mean eGFR was 78.8 mL/min/1.73 m2 (Supplementary data, Table S2).
A higher percentage of mild anemia was noted in the third tertile (Figure 2). Unadjusted OR for mild anemia demonstrated the highest likelihood in the third tertile and a modestly high likelihood in the lowest tertile when compared with the second tertile (Supplementary data, Table S4). Even after adjusting for demographics, eGFR, albumin, iron saturation and ferritin, the OR for mild anemia was highest in the third tertile 1.35 (1.18–1.55), but no longer significant in the lowest tertile. Moreover, in fully adjusted models of continuous phosphorous levels, each 0.5 mg/dL phosphorus elevation was associated with a 17% higher likelihood for mild anemia [OR (95% CI) 1.17 (1.07–1.28)]. Similar to the moderate anemia group, the multivariable adjusted analysis revealed the following risk factors associated with mild anemia: Asian race 1.35 (1.06–1.72), diabetes 1.47 (1.29–1.67), low iron saturation 2.71 (2.40–3.07) and low albumin 3.12 (2.76–3.57) (Supplementary data, Figure S2). However, in contrast to the moderate anemia group, black race 1.30 (1.11–1.51) and age >70 years 1.48 (1.21–1.77) were significantly associated with higher mild anemia likelihood, and female sex had lower mild anemia OR 0.87 (0.76–0.98). Furthermore, each 10 U (1 U = 1 mL/min/1.73m2) increase in eGFR was associated with a 9% lower likelihood for mild anemia [OR (95% CI) 0.91 (0.88–0.93)] (Supplementary data, Table S4).
When comparing the prevalence of mild anemia by age group and sex, a higher percentage of anemia was noted in females aged 18–49 years (Supplementary data, Figure S3). However, after adjusting for demographics, eGFR, serum albumin, ferritin and iron saturation, compared with males aged 18–49 years, the odds of mild anemia were significantly higher in females aged 18–49 and 70+ years, as well as in males aged 50 + years, but highest in males aged 70+ years (Supplementary data, Table S3).
Secondary analyses
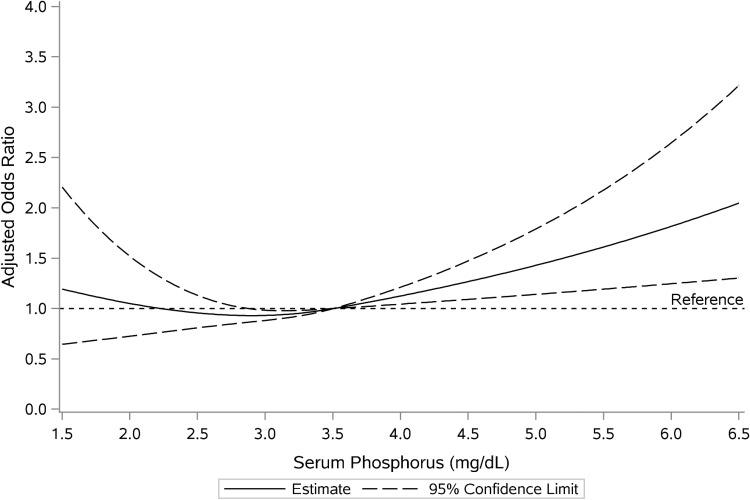
Restricted cubic spline modeling of multivariable adjusted OR across various ranges of serum phosphorus revealed a higher likelihood for moderate anemia with phosphorus levels >3.5 and <2.0 mg/dL (Figure 4). For mild anemia, phosphorus levels >3.5 and <2.5 mg/dL were associated with higher likelihood (Supplementary data, Figure S4).
FIGURE 4:
Cubic spline plot of serum phosphorus and moderate anemia likelihood. OR was adjusted for age, sex, race, history of diabetes, renal function, serum albumin, ferritin and iron saturation. There was a significant likelihood of moderate anemia with phosphorus levels >3.5 mg/dL and a trend toward higher anemia likelihood with phosphorus levels <2.0 mg/dL.
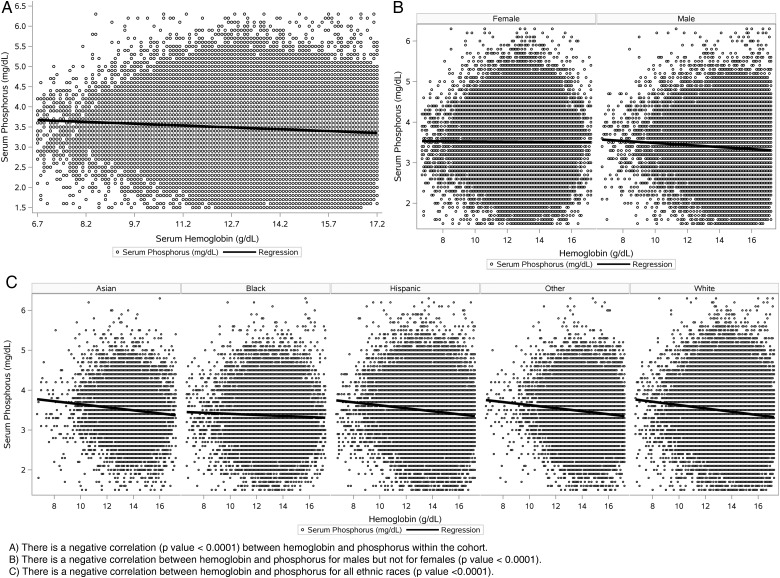
Correlation analyses demonstrated a significant negative correlation between phosphorus and hemoglobin for the entire cohort, among males but not among females, and across all racial/ethnic groups (P < 0.001) (Figure 5).
FIGURE 5:
Correlation analyses between hemoglobin and phosphorus. There was a significant negative correlation detected between phosphorus (mg/dL) and hemoglobin (g/dL) for (A) the entire cohort population, (B) males but not females and (C) all ethnic race groups including Asians, blacks, Hispanics, whites and other.
DISCUSSION
Higher serum phosphorus levels within the upper limits of normal have been associated with morbidities as well as adverse outcomes, including cardiovascular disease, progression to end stage renal disease (ESRD) and mortality even among those with normal kidney function [21–31]. Hyperphosphatemia and its association with anemia have been described in dialysis and kidney transplant populations [9, 11, 12]. A recent study suggested that individuals with normal kidney function who have higher phosphorus levels but within the normal range were associated with a higher risk of anemia [17].
Main findings
We found an association between serum phosphorus levels and both mild and moderate anemia within a large diverse population without CKD and early CKD. This association remained after adjustments for demographics, renal function, serum albumin and iron status. A dose-dependent effect was seen across ranges of serum phosphorus, with both risk of anemia and absolute hemoglobin levels. This was demonstrated by the correlation plots of phosphorus and hemoglobin as well as by linear regression modeling for anemia risk. Every 0.5 mg/dL phosphorus level increase demonstrated a 16% greater likelihood for moderate anemia. Although serum phosphorus levels >3.5 mg/dL were associated with a greater likelihood for moderate anemia, levels <2.0 mg/dL also showed a trend toward anemia, suggesting a curvilinear relationship across ranges of serum phosphorus. Every 10 U increase in eGFR was associated with a 6% decrease in moderate anemia risk.
Additional risk factors for anemia included female sex, Asian race, diabetes, low albumin and low iron saturation. We found a greater likelihood for anemia in all females compared with males, but highest in females aged 18–49 and 70 years and older. Our study also showed that anemia risk increases with low phosphorus levels, low albumin and older age (70+ years in females and 50+ years in males), which may be explained by malnutrition and poor nutrient intake. Overall, our findings were drawn from one of the largest and ethnically diverse populations to date evaluating the relationship between phosphorus and anemia among a non-dialysis population within a real world clinical environment.
Mechanism
Several mechanisms for hyperphosphatemia and anemia have been proposed for CKD and ESRD populations. Erythropoietin deficiency, inflammation and oxidative stress have been implicated as potential factors linking hyperphosphatemia and anemia [7]. Kovesdy et al. [7] suggested that high serum phosphorus may lead to higher polyamines production, which can function as uremic toxins inhibiting erythropoiesis. In the presence of high serum phosphorus, vascular calcification can form including within the renal arteries, which may eventually result in erythropoietin deficiency and anemia [3, 10]. Hyperphosphatemia in CKD can also cause a decrease in vitamin D synthesis, resulting in hypocalcemia and elevated PTH [2]. Elevated PTH, in turn, has been shown to directly inhibit erythropoiesis, induce hemolysis and cause bone marrow fibrosis in CKD [8–12]. The mechanisms underlying serum phosphorus and anemia in those with early CKD and normal kidney function are less certain.
FGF-23 may play a role in the mechanism underlying the association between hyperphosphatemia and anemia. High serum phosphorus itself has been shown to increase FGF-23 levels, which in turn is associated with suppression of klotho [32, 33]. In murine models, low klotho levels are associated with poor outcomes including early senescence, cell toxicity, premature aging and vascular calcifications [34]. Low klotho also causes a deficiency in activated vitamin D [15]. Vitamin D deficiency in itself has been implicated with anemia [35, 36].
Limitations and strengths
Our study has several potential limitations. The information available and presented in this study was drawn from experiences of a real-world clinical practice environment. Thus, there is a bias toward those individuals who had indications for concurrent hemoglobin, creatinine and phosphorus values to be drawn. This cohort may represent a sicker population with unhealthy lifestyles who sought medical care. In addition, certain information that would provide insight and contribute to confounding was not available for all individuals. For instance, experimental laboratory measurements such as FGF-23, klotho, hepcidin or erythropoietin levels were never measured for our study cohort. We had data on a very small portion of the study cohort with regard to serum PTH, vitamin D, calcium and other components of bone mineral disorders. Elevated PTH levels, in particular, may have contributed to residual confounding, especially because PTH has been associated with erythropoiesis in CKD [8–12]. We also determined a priori that the first phosphorus value available for each individual would be used for our cross-sectional analysis. Phosphorus measurements were drawn and performed throughout different working times of the day in which circadian variations of phosphorus occur. Serum phosphorus values are lowest in the morning and higher later in the day [37]. Given the large size of our study cohort, the variations in phosphorus measurements are likely minimized.
Our study lacked information on medication use among the study cohort. The use of medications such as phosphorus binders, erythropoietin, steroids or iron salts was not ascertained. Among CKD patients, certain phosphorus binders have been associated with improved anemia parameters [38–40]. Nutritional supplements and dietary phosphorus intake were also not available to evaluate their effects on serum phosphorus levels. We could not definitively exclude renal function as a causative mechanism for anemia and hyperphosphatemia as we included those with eGFR > 30 mL/min/1.73 m2, though we did attempt to adjust for renal function in our multivariable regression models. Finally, correlation does not indicate causation, and the results of our cross-sectional analyses should not be interpreted as causal association between phosphorus and anemia.
One strength of our study is that it is one of the largest non-dialysis populations to date (N = 155 974), evaluating the association between phosphorus and anemia. Our cohort was a racially and ethnically diverse, nationally representative population observed within a real world clinical environment. Compared with previous studies, we had comprehensive information on comorbidities and the laboratory, pharmacy and health utilization of our study population. This enabled us to adjust for potential confounders and to exclude many secondary causes of anemia.
Implications/conclusion
Higher phosphorus levels (>3.5 mg/dL) were associated with a greater likelihood for moderate anemia in individuals with early CKD stages and those with relatively intact kidney function. We also observed a trend for lower phosphorus levels (<2.0 mg/dL). Our study suggests that phosphorus may be a biomarker associated with anemia and/or play a role in hematopoiesis. Understanding the impact of phosphorus on hemoglobin levels may have implications on the prevention of anemia in individuals with non-CKD or early stage CKD. Theoretically, a ‘treatment’ effect of moving individuals with phosphorus levels of 4.0 mg/dL or higher to levels of 3.5 mg/dL or lower may lead to possible prevention of moderate anemia. Given our findings, 87.1 (95% CI 70.7–112.8) individuals would need to have this 0.5 mg/dL lowering of phosphorus to prevent one excess case of moderate anemia [34]. Within our population, a 0.5 mg/dL lower shift in the distribution of phosphorus could have correlated with prevention of anemia in hundreds of individuals. Anemia has already been associated with adverse outcomes, including higher incidences of coronary events, stroke, progression to ESRD and death. Our study cohort established using an electronic health-record-based approach has the potential to describe many clinical outcomes related to CKD-bone mineral disorders across different ranges of renal function.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
AUTHORS’ CONTRIBUTIONS
J.J.S. and M.B. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis ; study concept and design: J.J.S., L.T. and M.B.; acquisition, analysis or interpretation of data: J.J.S., L.T. and M.B.; drafting of the manuscript: L.T. and J.J.S.; critical revision of the manuscript for important intellectual content: C.M.R., E.S., K.K.-Z. and S.J.J.; statistical analysis: M.B.; administrative, technical or material support: S.J.J. and study supervision: J.J.S. and S.J.J.
CONFLICT OF INTEREST STATEMENT
J.J.S. has research grant funding from Keryx Pharmaceuticals and Sanofi-Aventis Pharmaceuticals.
Supplementary Material
ACKNOWLEDGEMENTS
This study was partially funded by investigator-initiated research grants (J.J.S. PI) from Keryx Pharmaceuticals and Sanofi-Aventis Pharmaceuticals. Support was also provided by a research grant from the National Institutes of Health grant K24-DK091419 to K.K.-Z.
REFERENCES
- 1.Kuro OM. A phosphate-centric paradigm for pathophysiology and therapy of chronic kidney disease. Kidney Int Suppl (2011) 2013; 3: 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015; 10: 1257–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroo M. [New developments in CKD-MBD. Why is phosphate overload harmful?]. Clin Calcium 2014; 24: 1785–1792 [PubMed] [Google Scholar]
- 4.Smith ER, Ford ML, Tomlinson LA et al. . Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 2012; 27: 1957–1966 [DOI] [PubMed] [Google Scholar]
- 5.Lichtman MA, Miller DR. Erythrocyte glycolysis, 2,3-diphosphoglycerate and adenosine triphosphate concentration in uremic subjects: relationship to extracellular phosphate concentration. J Lab Clin Med 1970; 76: 267–279 [PubMed] [Google Scholar]
- 6.Grzegorzewska AE, Mlot-Michalska M. Bone mineral density, its predictors, and outcomes in peritoneal dialysis patients. Adv Perit Dial 2011; 27: 140–145 [PubMed] [Google Scholar]
- 7.Kovesdy CP, Mucsi I, Czira ME et al. . Association of serum phosphorus level with anemia in kidney transplant recipients. Transplantation 2011; 91: 875–882 [DOI] [PubMed] [Google Scholar]
- 8.Bogin E, Massry SG, Levi J et al. . Effect of parathyroid hormone on osmotic fragility of human erythrocytes. J Clin Invest 1982; 69: 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Lee GH, Miller JE et al. . Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis 2009; 53: 823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meytes D, Bogin E, Ma A et al. . Effect of parathyroid hormone on erythropoiesis. J Clin Invest 1981; 67: 1263–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao DS, Shih M-S, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 1993; 328: 171–175 [DOI] [PubMed] [Google Scholar]
- 12.Zingraff J, Drüeke T, Marie P et al. . Anemia and secondary hyperparathyroidism. Arch Int Med 1978; 138: 1650–1652 [PubMed] [Google Scholar]
- 13.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 2013; 28: 1793–1803 [DOI] [PubMed] [Google Scholar]
- 14.Braithwaite V, Jarjou LM, Goldberg GR et al. . Iron status and fibroblast growth factor-23 in Gambian children. Bone 2012; 50: 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez O, Isakova T, Rhee E et al. . Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 2005; 16: 2205–2215 [DOI] [PubMed] [Google Scholar]
- 16.Morishita K, Shirai A, Kubota M et al. . The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr 2001; 131: 3182–3188 [DOI] [PubMed] [Google Scholar]
- 17.Wojcicki JM. Hyperphosphatemia is associated with anemia in adults without chronic kidney disease: results from the National Health and Nutrition Examination Survey (NHANES): 2005–2010. BMC Nephrol 2013; 14: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koebnick C, Langer-Gould AM, Gould MK et al. . Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012; 16: 37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; http://www.who.int/vmnis/indicators/haemoglobin (2 February 2015, date last accessed). [Google Scholar]
- 21.Aronson D, Kapeliovich M, Hammerman H et al. . The relation between serum phosphorus levels and clinical outcomes after acute myocardial infarction. PLoS ONE 2013; 8: e58348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chonchol M, Dale R, Schrier RW et al. . Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122: 380–386 [DOI] [PubMed] [Google Scholar]
- 23.Dhingra R, Gona P, Benjamin EJ et al. . Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail 2010; 12: 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra R, Sullivan LM, Fox CS et al. . Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167: 879–885 [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Collins AJ, Herzog CA et al. . Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 2009; 20: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley RN, Collins AJ, Ishani A et al. . Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156: 556–563 [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, De Boer IH, Peralta CA et al. . Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 2009; 4: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendrick J, Ix JH, Targher G et al. . Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 2010; 106: 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onufrak SJ, Bellasi A, Shaw LJ et al. . Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 2008; 199: 424–431 [DOI] [PubMed] [Google Scholar]
- 30.Sim JJ, Bhandari SK, Smith N et al. . Phosphorus and risk of renal failure in subjects with normal renal function. Am J Med 2013; 126: 311–318 [DOI] [PubMed] [Google Scholar]
- 31.Tonelli M, Sacks F, Pfeffer M et al. . Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112: 2627–2633 [DOI] [PubMed] [Google Scholar]
- 32.Krajisnik T, Olauson H, Mirza MA et al. . Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int 2010; 78: 1024–1032 [DOI] [PubMed] [Google Scholar]
- 33.Komaba H, Goto S, Fujii H et al. . Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 2010; 77: 232–238 [DOI] [PubMed] [Google Scholar]
- 34.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials 2001; 22: 102–110 [DOI] [PubMed] [Google Scholar]
- 35.Lac PT, Choi K, Liu IA et al. . The effects of changing vitamin D levels on anemia in chronic kidney disease patients: a retrospective cohort review. Clin Nephrol 2010; 74: 25–32 [DOI] [PubMed] [Google Scholar]
- 36.Sim JJ, Lac PT, Liu IL et al. . Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol 2010; 89: 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jubiz W, Canterbury JM, Reiss E et al. . Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest 1972; 51: 2040–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikee R, Tsunoda M, Sasaki N et al. . Potential influence of sevelamer hydrochloride on responsiveness to erythropoiesis-stimulating agents in haemodialysis patients. Nephrology (Carlton) 2012; 17: 225–229 [DOI] [PubMed] [Google Scholar]
- 39.Lewis JB, Sika M, Koury MJ et al. . Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 2015; 26: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama K, Hirakata H, Akiba T et al. . Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 2014; 9: 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.