Abstract
Background
Extracorporeal shock wave therapy (ESWT) can modulate cell behavior through mechanical information transduction. Human periodontal ligament fibroblasts (hPDLF) are sensible to mechanical stimulus and can express pro-inflammatory molecules in response. The aim of this study was to evaluate the impacts of shock waves on interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP-1), and tumor necrosis factor-alpha (TNF-α) expression by hPDLF.
Material/Methods
After being treated by shock waves with different parameters (100–500 times, 0.05–0.19 mJ/mm2), cell viability was tested using CCK-8. IL-6, IL-8, MCP-1, and TNF-α gene expression was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) and IL-6 and IL-8 protein was measured by enzyme-linked immunosorbent assay (ELISA) at different time points.
Results
Shock waves with the parameters used in this study had no significant effects on the viability of hPDLF. A statistical inhibition of IL-6, IL-8, MCP-1, and TNF-α expression during the first few hours was observed (P<0.05). Expression of IL-8 was significantly elevated in the group receiving the most pulses of shock wave (500 times) after 4 h (P<0.05). At 8 h and 24 h, all treated groups demonstrated significantly enhanced IL-6 expression (P<0.05). TNF-α expression in the groups receiving more shock pulses (300, 500 times) or the highest energy shock treatment (0.19 mJ/mm2) was statistically decreased (P<0.05) at 24 h.
Conclusions
Under the condition of this study, a shock wave with energy density no higher than 0.19 mJ/mm2 and pulses no more than 500 times elicited no negative effects on cell viability of hPDLF. After a uniform initial inhibition impact on expression of inflammatory mediators, a shock wave could cause dose-related up-regulation of IL-6 and IL-8 and down-regulation of TNF-α.
MeSH Keywords: Chemotactic Factors, High-Energy Shock Waves, Interleukin-6, Interleukin-8, Periodontal Ligament, Tumor Necrosis Factor-alpha
Background
There has been a paradigm shift of application of shock waves in medicine in recent decades. Initially, high-energy focused extracorporeal shock wave therapy (ESWT) was used to disintegrate renal stones by its physically destructive property [1]. Nowadays, owing to its regenerative potential, ESWT is regarded as a form of mechanotherapy in regenerative medicine. ESWT has been effectively used to treat various musculoskeletal disorders [2], chronic soft tissue wounds [3], neurological pathologies [4], andrologic disturbances [5], and ischemia heart disease [6,7]. It is proposed that shock waves could promote tissue regeneration through mechanotransduction, in which target cells can sense and adapt their biological behavior to extracellular physical signals of shock waves [8].
Although just being introduced into dentistry, ESWT has already demonstrated its potential in regeneration of alveolar bone [9], removal of tooth biofilm [10], and eradication of periodontal pathogens [11]. Recently, in a clinical trial to assess the influence of ESWT on tooth stability after active orthodontic movement, shock wave treatment achieved faster reduction of tooth mobility [12]. These findings suggest that ESWT might be a promising noninvasive adjunctive therapy for periodontal and orthodontic treatment.
In most cases of periodontal and orthodontic treatment in dentistry, multiple teeth are involved. It is preferred to choose soft-focused or non-focused shock wave applicators to cover a large area of the target field. However, due to the anatomical restriction, it is inevitable to have neighboring teeth included in the target area. Even if the focused shock wave application is used, it is also impossible to precisely adjust the cigar-like focus to exclude any influence on the normal teeth nearby. Thus, periodontal ligaments (PDL) of neighboring teeth also withstand shock wave treatment. However, until now, no study has reported on the direct impacts of shock wave on cells derived from the periodontal ligament. Accumulating evidence has demonstrated that biological response induced by shock wave treatment is dose-dependent and cell-specific [13,14]. Therefore, from the clinical point of view, it is imperative to determine the safety threshold and investigate the effects of shock wave treatment on the biological behavior of periodontal ligament fibroblasts (PDLF).
Being a critical role player in maintaining homeostasis and remodeling of periodontal tissue, PDLF not only possess fibrogenic and osteogenic properties, but also take part in immune reaction [15]. Stimulated by biological promoters like lipopolysaccharide (LPS), PDLF can express pro-inflammatory molecules such as IL-6 [16,17], IL-8 [18,19], MCP-1 [20,21], and TNF-α [17], participating in development of periodontal diseases. Moreover, in response to extracellular mechanical forces [22,23] during orthodontic treatment, PDLF could also release inflammatory cytokines, including IL-6 [23,24], IL-8 [25], MCP-1 [25], and TNF-α [22,24]. Although the underlying mechanism remains unclear, these findings indicate that mechanical stimulus can trigger inflammatory biological response of PDLF. As shock wave treatment also represents transduction of mechanical information to cells, it is rational to speculate that ESWT might induce the inflammatory reaction of PDLF as well.
Therefore, we hypothesize that shock waves could promote the expression of inflammatory mediators by PDLF. To test this, the expression levels of IL-6, IL-8, MCP-1, and TNF-α by hPDLF (human periodontal ligament fibroblasts) receiving shock wave treatment with different parameters were measured in the present study.
Material and Methods
Cell culture
The hPDLF isolated from 16-year-old male were purchased from Lonza Group, Ltd (Basel, Switzerland). The cells were cultured and expanded in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Cells from passage levels 4–5 were used in the present study. The cell culture medium was refreshed every 3 days.
Shock wave device
In this study, shock wave pulses were generated by use of a DermaGold®100 unfocused electrohydraulic shock wave device. An OP155 applicator (Tissue Regeneration Technologies, LLC, manufactured by MTS Europe GmbH) was used to apply shockwave treatment on hPDLF. As described previously [26], a water bath set-up [27] connected to the applicator was used to guarantee unhampered physical propagation and reproducible application of shockwaves to the sample in vitro.
Shock wave treatment
Cell suspension was collected in a 15-ml polypropylene centrifuge tube by Accutase™ (PAA, Austria) in 1ml medium, and the concentration was adjusted to 1×106/ml. Polypropylene tubes containing the cell suspension were exposed to shock wave pulses under identical and reproducible treatment conditions in terms of temperature (37°C) and distance (4 cm) to the shockwave applicator. ESWT at frequency of 3 Hz with pre-set parameters was applied on cell suspensions accordingly. Every tube was put into the water bath set-up and kept for an identical time period of 3 minutes regardless of the treatment it received. After shock wave treatment, the hPDLF were reseeded on 24-well plates to continue cultivation separately.
Cell viability/proliferation test
To investigate possible impacts of shock wave treatment on the viability/proliferation of PDLF, the effects of shock waves with different energy density (0.05, 0.10, and 0.19 mJ/mm2) and different impulses (100, 300, 500 times) were analyzed using a cell counting kit-8 (CCK-8) (Dojindo laboratories, Japan). After being treated with a shock wave, 5×103 cells were seeded into 96-well plates to grow for 24, 48, and 72 hours. We then added 30 μl of CCK-8 reagent to each well and culture plates were incubated at 37°C for 4 hours. The absorbance was measured photometrically at 450 nm (Spectramax Plus 384, Molecular Devices, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR) measurements
To assess the impacts of impulse numbers and energy densities of shock waves on gene expression by hPDLF, the cells to be treated were divided into 5 groups receiving ESWT of the same energy density with various impulses (0.05 mJ/mm2, 100, 300, and 500 times) or the same impulse numbers with different energy densities (0.05, 0.10, 0.19 mJ/mm2, and 100 times). Untreated hPDLF served as control. Thus, there were 6 subgroups in total. After shock wave treatment, concentration of cell suspension in each 15-ml polypropylene centrifuge tube was adjusted to 1×105/ml by adding DMEM with 10% FBS, then the hPDLF suspension was seeded into 24-well plates with total volume of 500μl in each well. Consequently, the cells were grown in a humidified atmosphere of 5% CO2 at 37°C. At 1, 2, 4, 8, and 24 hours after treatment, cells were detached from the plates with Accutase™ and harvested in centrifuge tubes. After the cells were washed twice with phosphate-buffered saline, total RNA was extracted and complementary DNA (cDNA) was reversely transcribed using the TaqMan® GeneExpression Cells-to-CT™ Kit according to the manufacturer’s instructions. qRT-PCR was conducted with an ABI Prism SDS 7000 detection system (Applied Biosystems) through the TaqMan Gene Expression Assay. The primer ID numbers were: IL-6, Hs00985639_m1; IL-8, Hs00174103_m1; MCP-1, Hs00234140_m1; TNF-α, Hs00174128_m1; β-actin: Hs99999903_m1. β-actin was used as the housekeeping gene. Real-time PCR reactions were done in triplicate under the following thermocycling conditions: 95°C for 10 minutes; 50 cycles of 15 seconds for 95°C and 60°C for 1 minute. The point at which the PCR product was first detected above a value of cycle threshold (Ct), was determined for each sample. Relative mRNA expression levels were calculated using the 2(−ΔΔCt) method based on the Ct value of each PCR product and normalized to a housekeeping gene (β-actin) with the comparative Ct method, where ΔΔCt=(Cttarget – Ctβ-actin)sample – (Cttarget – Ctβ-actin)control, taking untreated group as a control.
Enzyme-linked immunosorbent assay (ELISA)
The cell culture medium was collected at different time points (1, 2, 4, 8, and 24 hours) for the ELISA. The levels of IL-6 and IL-8 released into the culture supernatant were measured using human IL-6 and IL-8 ELISA kits (Human IL-6 ELISA Ready-SET-Go, Human IL-8 ELISA Ready-SET-Go, eBioscience, USA) respectively, according to the manufacturer’s protocol.
Statistical analysis
All experiments were performed in triplicate and repeated 3 times. Quantitative data are expressed as mean ± standard deviation. Statistical analysis for CCK-8 and ELISA results was adequately performed by one-way analysis of variance (ANOVA) followed by the post hoc LSD-t multiple comparison procedure. Statistically significant differences among mean levels of qRT-PCR results were determined by ANOVA followed by the post hoc t-test. Statistical analysis was performed using SPSS for Windows ver.19.0 (SPSS, Inc, Chicago, IL). A probability value of P<0.05 was considered statistically significant.
Results
Cell viability/proliferation test
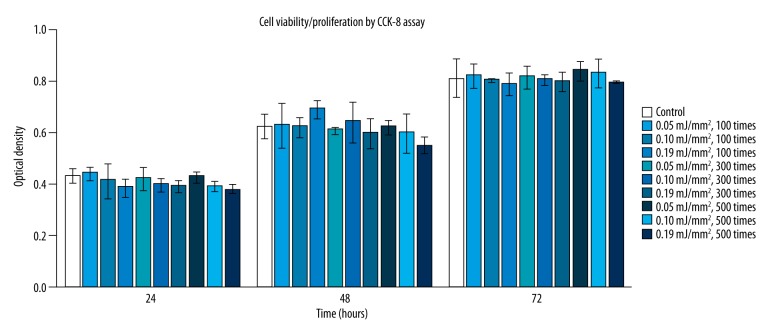
Results of hPDLF viability/proliferation assay using CCK-8 are shown (Figure 1). No significant difference in cell viability/proliferation was found between treated groups and the control group at each time point (24, 48, and 72 hours) (P>0.05). In addition, there was also no significant difference in the viability/proliferation of hPDLF among groups receiving shock wave treatment with different energy intensities and pulses (P>0.05).
Figure 1.
Cell viability/proliferation of hPDLF at different time points (24, 48, and 72 hours) in response to shock wave treatment at different energy densities (0.05, 0.10, and 0.19 mJ/mm2) with different pulses (100, 300, and 500 times). Viability/proliferation of cells were measured using CCK-8 assay. * Means significant difference from the control group (P<0.05).
Gene expression of IL-6, IL-8, MCP-1, and TNF-α
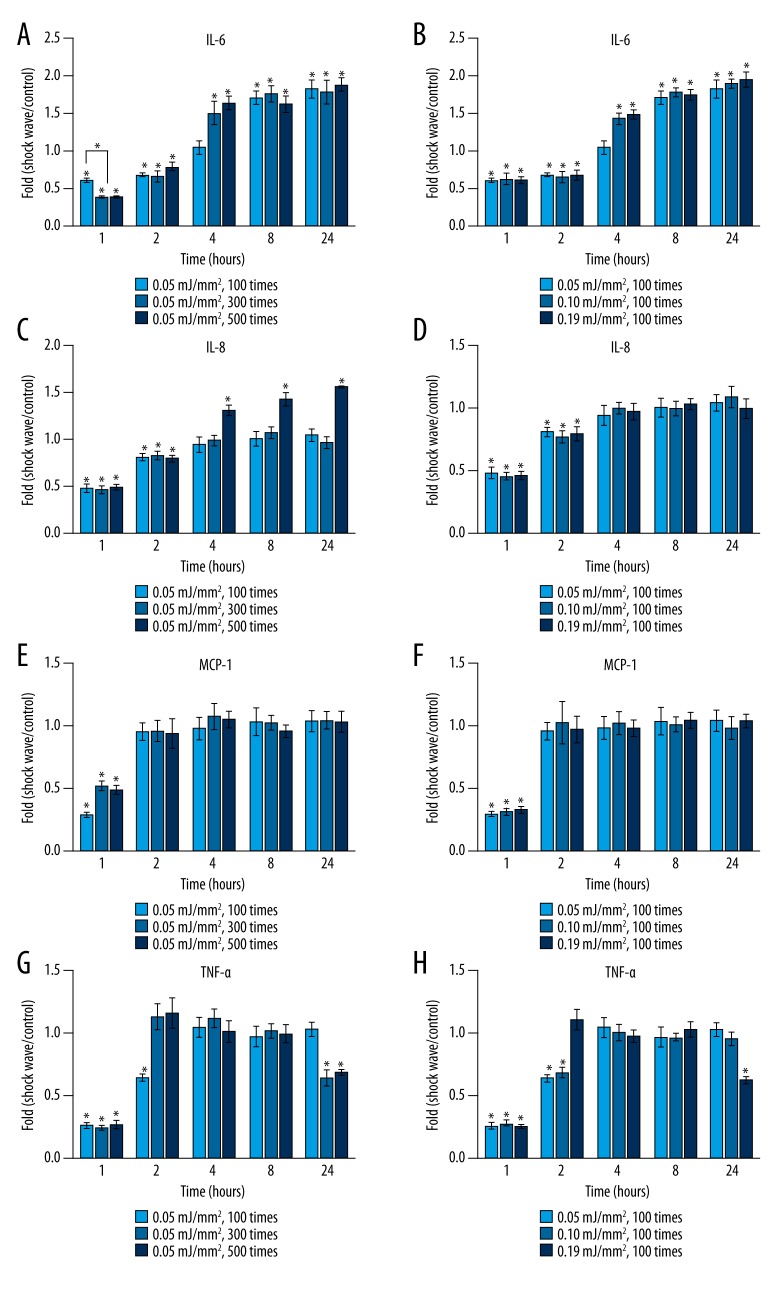
The effects of shock wave pulses or energy intensities on IL-6 expression are illustrated in Figure 2A and 2B, respectively. During the first 2 hours, IL-6 levels in all treated groups dropped significantly compared to that in the untreated group (P<0.05) (Figure 2A, 2B). Moreover, concentrations of IL-6 in the groups receiving more shock pulses (300 and 500 times) were significantly lower than that in the group receiving fewer shock pulses (100 times) at the 1-hour time point (P<0.05) (Figure 2A). After 4 hours, IL-6 levels in the groups receiving shock wave treatment with more pulses (300 and 500 times) (Figure 2A) or higher energy intensities (0.10 mJ/mm2 and 0.19 mJ/mm2) (Figure 2B) were significantly higher than that in the control group (P<0.05). At 8 and 24 hours, all treated groups demonstrated statistically higher IL-6 expression than the untreated group (P<0.05). In addition, no significant difference in IL-6 mRNA concentration was detected among all treated groups after 8 hours (P>0.05) (Figure 2A, 2B).
Figure 2.
Gene expression levels of IL-6 (A), IL-8 (C), MCP-1 (E), and TNF-α (G) in hPDLF at different time points (1, 2, 4, 8, and 24 hours) in response to shock wave treatment at energy intensity of 0.05 mJ/mm2 with different pulses (100, 300, and 500 times). Gene expression levels of IL-6 (B), IL-8 (D), MCP-1 (F), and TNF-α (H) in hPDLF at different time points (1, 2, 4, 8, and 24 hours) after being treated by shock waves at different energy intensities (0.05, 0.10, and 0.19 mJ/mm2) with 100 pulses. Y-axis represents the n-fold expression levels of the target gene in relation to untreated cells (control). * Means significant difference from the control group (2(−ΔΔCt)=1) (P<0.05).
The impacts of shock wave pulses or energy intensities on IL-8 expression are shown in Figure 2C and 2D, respectively. During the first 2 hours, expression of IL-8 was significantly inhibited in all treated groups (P<0.05). No significant difference in IL-8 levels was observed among these treated groups (P>0.05) (Figure 2C, 2D). Four hours after ESWT, expression levels of IL-8 mRNA in all treated groups went back to normal (P>0.05) except for those in the groups receiving the most shock pulses (500 times), which were significantly elevated (P<0.05) (Figure 2C).
Figure 2E and 2F depict the effects of shock wave pulses or energy intensities on MCP-1 expression, respectively. Significant decreases of MCP-1 expression were revealed in all treated groups at 1 hour after ESWT (P<0.05). After 2 hours, no significant difference in MCP-1 expression was detected in any of the treated groups compared with the control. Furthermore, no significant difference was shown between MCP-1 levels of treated groups at any time point observed (Figure 2E, 2F).
Figure 2G and 2H illustrate the effects of shock wave pulses or energy intensities, respectively, on TNF-α expression. Significant decreases in TNF-α expression by hPDLF were detected in all groups at 1 hour after ESWT (P<0.05) (Figure 2G, 2H). At 2 hours, TNF-α levels in the groups receiving shock wave treatment with more pulses (300 and 500 times) (Figure 2G) or at the highest energy intensity (Figure 2H) (0.19 mJ/mm2) rose back to normal. At 4 and 8 hours, no significant difference in TNF-α expression in the treatment groups was revealed compared with that in the untreated group. However, TNF-α mRNA expression in the groups receiving more shock wave pulses (300 and 500 times) or the highest energy shock wave treatment (0.19 mJ/mm2) were statistically lower than that in the control (P<0.05) at 24 hours.
Protein expression of IL-6 and IL-8 by hPDLF
The effects of shock waves on IL-6 protein expression are demonstrated in Figure 3A. No significant difference in IL-6 protein concentration was detected between the treated groups and the control during the first 4 hours. After 8 hours, IL-6 expression levels in all the treated groups were significantly elevated (P<0.05). There was no significant difference in IL-6 expression among these treated groups at each time point.
Figure 3.
Effect of shock wave treatment at different time points (1, 2, 4, 8, and 24 hours) in response to shock wave treatment with different parameters. The levels of IL-6 (A) and IL-8 (B) were measured in cell supernatants using ELISA. * Means significant difference (P<0.05).
The effects of ESWT on IL-8 production by hPDLF are shown in Figure 3B. No significant difference in IL-8 protein concentration was detected among the groups during the first 2 hours after ESWT. IL-8 expression levels in the group receiving the most shock pulses (500 times) were significantly higher than those in other treatment groups and the control group at 4, 8, and 24 hours (P<0.05).
Discussion
Our results show that shock wave treatment with the parameters used in this study did not cause detrimental effects on the viability of hPDLF. Moreover, after a uniform initial inhibition effect on expression of all inflammatory mediators, shock waves caused dose-related up-regulation of IL-6 and IL-8 and down-regulation of TNF-α. To the best of our knowledge, this is the first in vitro study confirming the modulating potential of shock waves on inflammatory cytokine and chemokine expression by hPDLF.
It has been documented that there is an energy safety threshold above which shock wave treatment could induce destructive sequelae on cell biological behavior. Martini et al. demonstrated that shock waves at energy density of 0.15 mJ/mm2 performed better in promoting osteogenic differentiation than higher-energy shock waves (0.31 mJ/mm2, 0.40 mJ/mm2) [14]. It has been reported that 500 shock impulses had the best result in stimulating growth of bone marrow stromal cells, whereas more shock impulses could induce a suppression effect [13]. Obviously, ESWT at high energy can suppress cell growth, while lower-energy shock waves might enhance cell proliferation. It is also noticeable that this safety threshold differs according to cell type. Therefore, it is necessary to determine the safe dosage and optimize the parameters of shock waves before clinical use. In vivo experiments have shown the promise of ESWT in clinical dentistry to facilitate orthodontic and periodontal treatment. Shock wave treatment has even been used in clinical pilot studies. However, to date, no data are available on its safety threshold for PDLF, the most important cell line in periodontal tissue inflammation and remodeling. The results of our study confirmed that no negative effects on cell viability/proliferation could be elicited when hPDLF were treated by shock waves with energy density no higher than 0.19 mJ/mm2 and pulses no more than 500 times, providing useful information for future clinical application of ESWT in dentistry.
It was found that shock waves can elicit an early transient inhibition of IL-6, IL-8, MCP-1, and TNF-α expression during the first few hours. Moosavi-Nejad et al. investigated the impacts of shock waves on the morphology and cytoskeleton of a human renal carcinoma cell line (ACHN). It was demonstrated that shock wave treatment can cause temporary morphological deformation of the cytoskeletal filament, which is associated with disorganization of some intracellular cytoskeletal protein, including actin and tubulin, but not vimentin. The deformed cells can reorganize their cytoskeletal network within 3 hours, with a pattern similar to the untreated cells [28]. This transient shock wave-induced cytoskeletal damage might explain the early suppression of inflammatory mediator expression by hPDLF. Significant inhibition of IL-6 and IL-8 mRNA expressed by hPDLF was observed immediately after ESWT, but no change in protein concentration was detected in the supernatant during this period. The discrepancy between the IL-6 and IL-8 mRNA and protein expression during the early phase might be caused by the following: (1) Proteins in cell culture medium accumulate over time and transient suppression of IL-6 by hPDLF might not give rise to significant changes of its level in the supernatant; (2) There is a lapse between mRNA and protein expression; and (3) Before production and secretion of protein products, mRNA may undergo complicated intracellular procedures, including translation and modification.
In spite of the initial inhibition, enhancement of IL-6 expression was observed in treatment groups later. This finding was in line with most of the previous research [29,30]. In the study conducted by Clark et al., shock wave treatment significantly elevated IL-6 level of renal medulla in a pig model [29]. In a report by Tepekoylu et al., ESWT with parameters (0.10 mJ/mm2, 500 impulses) within the range of our experiment increased mRNA levels of IL-6 in subcutaneously implanted grafts [30]. In an in vitro study, Holfeld et al. applied ESWT to human umbilical vein endothelial cells (HUVECs) with 0.08 mJ/mm2 and 250 impulses. The results showed that shock waves can induce IL-6 expression by HUVECs [27]; however, unlike IL-6, TNF-α expression was suppressed again at 24 hours in the groups that received more pulses (300 and 500 times) or the highest-energy (0.19 mJ/mm2) treatment. This dose-dependent inhibition effect on TNF-α expression at the late phase is inconsistent with previous reports [29,31]. In vivo studies with animal models using rats [31] and pigs [29] both showed enhanced TNF-α mRNA expression in the urinary system by shock wave treatment. It was also reported that shock waves can elevate TNF-α level in decellularized aortic xenografts in mice [30]. The difference between results of the present study and those of previous studies are likely due to the cell-specific effects of ESWT.
Chemokine is a family of chemoattractant proteins with similar molecular structures. According to the position and number of the first conserved cysteine residues, chemokine can be classified into 4 subgroups: CXC, CC, XC, and CX3C. Among these, interleukin-8 (IL-8) from CXC and monocyte chemotactic protein-1 (MCP-1) from CC subfamilies possess potent chemotactic properties to neutrophils and monocytes, respectively. These chemokines are essential signals in periodontal disease progression [32] and root resorption in orthodontic treatment [25]. There is little evidence of the effects of ESWT on cell chemokine expression. In an in vivo study conducted in an animal model using rats, shock waves dramatically enhanced MCP-1 expression in renal tissue [31]. By contrast, in our study, despite a transient suppression, ESWT elicited no alteration of MCP-1 concentration. In another clinical study [33], IL-8 concentration in peri-tendon tissue was elevated immediately and remained at high level for 4 hours after ESWT. This finding is inconsistent with ours, which demonstrated an immediate inhibition followed by a dose-related increase in IL-8 expression by hPDLF. The discrepancy between the results of previous reports and the present study might be caused by differences in the cell types and the parameters at which the shock waves were applied to the cells. Nevertheless, for the first time, our in vitro study confirmed the potential of shock waves in directly modulating chemokine expression by hPDLF.
Overexpression of IL-6, IL-8, and TNF-α is correlated with development of periodontitis [18,34]. Inhibition of the expression of these inflammatory molecules can contribute to regenerative processes of periodontal tissue. However, our results demonstrate somewhat contrary effects of shock wave treatment on expression of IL-6, IL-8, and TNF-α by hPDLF. Therefore, it is difficult to determine whether ESWT could have positive or negative effects on periodontal tissue regeneration at this stage. Further in vivo studies are needed to evaluate the combined effects of these regulated inflammatory mediators on PDL inflammation. Before the clinical application of ESWT in orthodontic and periodontitis treatment, experimental studies need to be performed to resolve the following issues: (1) Long-term effects of shock waves on inflammatory reaction of hPDLF should be evaluated; (2) Effects of ESWT on LPS pre-treated hPDLF mimicking periodontitis need to be assessed; and (3) Other cell behaviors of hPDLF, including proliferation, adhesion, migration, and differentiation, should also be investigated.
It should be pointed out that commercially available cells were used in this study. The advantage is that these experiments might be reproduced in other laboratories, but the major disadvantage is that the results might be affected by individual differences. It also should be mentioned that the present experiment was an in vitro study. Although this study design exclusively evaluated the biological reaction of hPDLF to shock wave treatment, it could not reflect the complexity of the oral environment and the shielding effect of surrounding tissue. Thus, the parameters obtained from our study should be optimized when they are applied in the clinical situation.
Conclusions
Under the conditions of our experiment, shock waves were shown to cause an initial inhibition of IL-6, IL-8, MCP-1, and TNF-α expression, followed by a dose-dependent enhancement in IL-6 and IL-8 and suppression in TNF-α expression by hPDLF. This is the first study that exclusively and systematically evaluated the cytobiological impacts of shock waves with different parameters on inflammatory mediator expression by PDLF. We hope that our results will provide the basis for future application of ESWT in dentistry.
Acknowledgements
We thank Mrs. Nguyen Phuong Quynh and Mrs. Hedwig Rutschek of the Competence Centre of Periodontal Research, Bernhard Gottlieb School of Dentistry, Medical University of Vienna, Austria and Mrs. Anna M. Weihs from the Department of Biochemical Engineering, University of Applied Sciences Technikum Wien, Austria for their help in preparing and performing the experiments.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: This work is supported by the Key Specialist Facility of Fujian Province (20121589)
References
- 1.Graff J, Pastor J, Funke PJ, et al. Extracorporeal shock wave lithotripsy for ureteral stones: a retrospective analysis of 417 cases. J Urol. 1988;139:513–16. doi: 10.1016/s0022-5347(17)42507-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003;26:220–32. [PubMed] [Google Scholar]
- 3.Mittermayr R, Antonic V, Hartinger J, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20:456–65. doi: 10.1111/j.1524-475X.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 4.Manganotti P, Amelio E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke. 2005;36:1967–71. doi: 10.1161/01.STR.0000177880.06663.5c. [DOI] [PubMed] [Google Scholar]
- 5.Chung E, Cartmill R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: An Australian first open-label single-arm prospective clinical trial. BJU Int. 2015;115(Suppl 5):46–49. doi: 10.1111/bju.13035. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Guo T, Cai HY, et al. Cardiac shock wave therapy reduces angina and improves myocardial function in patients with refractory coronary artery disease. Clin Cardiol. 2010;33:693–99. doi: 10.1002/clc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Ito K, Shiroto T, et al. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia-reperfusion injury in pigs in vivo. Coron Artery Dis. 2010;21:304–11. doi: 10.1097/mca.0b013e32833aec62. [DOI] [PubMed] [Google Scholar]
- 8.d’Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24:147–53. doi: 10.1016/j.ijsu.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Sathishkumar S, Meka A, Dawson D, et al. Extracorporeal shock wave therapy induces alveolar bone regeneration. J Dent Res. 2008;87:687–91. doi: 10.1177/154405910808700703. [DOI] [PubMed] [Google Scholar]
- 10.Muller P, Guggenheim B, Attin T, et al. Potential of shock waves to remove calculus and biofilm. Clin Oral Investig. 2011;15:959–65. doi: 10.1007/s00784-010-0462-2. [DOI] [PubMed] [Google Scholar]
- 11.Novak KF, Govindaswami M, Ebersole JL, et al. Effects of low-energy shock waves on oral bacteria. J Dent Res. 2008;87:928–31. doi: 10.1177/154405910808701009. [DOI] [PubMed] [Google Scholar]
- 12.Falkensammer F, Rausch-Fan X, Schaden W, et al. Impact of extracorporeal shockwave therapy on tooth mobility in adult orthodontic patients: A randomized single-center placebo-controlled clinical trial. J Clin Periodontol. 2015;42:294–301. doi: 10.1111/jcpe.12373. [DOI] [PubMed] [Google Scholar]
- 13.Wang FS, Wang CJ, Huang HJ, et al. Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochem Biophys Res Commun. 2001;287:648–55. doi: 10.1006/bbrc.2001.5654. [DOI] [PubMed] [Google Scholar]
- 14.Martini L, Giavaresi G, Fini M, et al. Effect of extracorporeal shock wave therapy on osteoblastlike cells. Clin Orthop Relat Res. 2003;(413):269–80. doi: 10.1097/01.blo.0000073344.50837.cd. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: A fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011;46:153–57. doi: 10.1111/j.1600-0765.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogura N, Shibata Y, Kamino Y, et al. Stimulation of interleukin-6 production of periodontal ligament cells by Porphyromonas endodontalis lipopolysaccharide. Biochem Med Metab Biol. 1994;53:130–36. doi: 10.1006/bmmb.1994.1068. [DOI] [PubMed] [Google Scholar]
- 17.Shu L, Guan SM, Fu SM, et al. Estrogen modulates cytokine expression in human periodontal ligament cells. J Dent Res. 2008;87:142–47. doi: 10.1177/154405910808700214. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Kita M, Oseko F, et al. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 2006;41:554–59. doi: 10.1111/j.1600-0765.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Bak EJ, Kim M, et al. Induction of IL-8 in periodontal ligament cells by H(2)O (2) J Microbiol. 2008;46:579–84. doi: 10.1007/s12275-008-0182-3. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson D, Nebel D, Bratthall G, Nilsson BO. LPS-induced MCP-1 and IL-6 production is not reversed by oestrogen in human periodontal ligament cells. Arch Oral Biol. 2008;53:896–902. doi: 10.1016/j.archoralbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki K, Hanazawa S, Takeshita A, et al. Interleukin-1 beta and tumor necrosis factor-alpha stimulate synergistically the expression of monocyte chemoattractant protein-1 in fibroblastic cells derived from human periodontal ligament. Oral Microbiol Immunol. 1996;11:109–14. doi: 10.1111/j.1399-302x.1996.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 22.Bletsa A, Berggreen E, Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci. 2006;114:423–29. doi: 10.1111/j.1600-0722.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 23.Kikuta J, Yamaguchi M, Shimizu M, et al. Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells. J Dent Res. 2015;94:140–47. doi: 10.1177/0022034514555364. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–67. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 25.Asano M, Yamaguchi M, Nakajima R, et al. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis. 2011;17:489–98. doi: 10.1111/j.1601-0825.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 26.Weihs AM, Fuchs C, Teuschl AH, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289:27090–104. doi: 10.1074/jbc.M114.580936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holfeld J, Tepeköylü C, Kozaryn R, et al. Shockwave therapy differentially stimulates endothelial cells: implications on the control of inflammation via toll-like receptor 3. Inflammation. 2014;37:65–70. doi: 10.1007/s10753-013-9712-1. [DOI] [PubMed] [Google Scholar]
- 28.Moosavi-Nejad SF, Hosseini SH, Satoh M, Takayama K. Shock wave induced cytoskeletal and morphological deformations in a human renal carcinoma cell line. Cancer Sci. 2006;97:296–304. doi: 10.1111/j.1349-7006.2006.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark DL, Connors BA, Evan AP, et al. Effect of shock wave number on renal oxidative stress and inflammation. BJU Int. 2011;107:318–22. doi: 10.1111/j.1464-410X.2010.09311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tepeköylü C, Lobenwein D, Blunder S, et al. Alteration of inflammatory response by shock wave therapy leads to reduced calcification of decellularized aortic xenografts in micedagger. Eur J Cardiothorac Surg. 2015;47:e80–90. doi: 10.1093/ejcts/ezu428. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Long Q, Cheng X, He D. Shock wave induces biological renal damage by activating excessive inflammatory responses in rat model. Inflammation. 2014;37:1317–25. doi: 10.1007/s10753-014-9859-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Tang X, Li C, et al. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Arch Oral Biol. 2015;60:1153–61. doi: 10.1016/j.archoralbio.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Waugh CM, Morrissey D, Jones E, et al. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy. Eur Cell Mater. 2015;29:268–80. doi: 10.22203/ecm.v029a20. discussion 280. [DOI] [PubMed] [Google Scholar]
- 34.Ebersole JL, Kirakodu S, Novak MJ, et al. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J Clin Periodontol. 2014;41:853–61. doi: 10.1111/jcpe.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]