Abstract
Background/Aim:
Wounds are the outcome of injuries to the skin that interrupt the soft tissue. Healing of a wound is a complex and long-drawn-out process of tissue repair and remodeling in response to injury. A large number of plants are used by folklore traditions for the treatment of cuts, wounds and burns. Moringa oleifera (MO) is an herb used as a traditional folk medicine for the treatment of various skin wounds and associated diseases. The underlying mechanisms of wound healing activity of ethyl acetate fraction of MO leaves extract are completely unknown.
Materials and Methods:
In the current study, ethyl acetate fraction of MO leaves was investigated for its efficacy on cell viability, proliferation and migration (wound closure rate) in human normal dermal fibroblast cells.
Results:
Results revealed that lower concentration (12.5 µg/ml, 25 µg/ml, and 50 µg/ml) of ethyl acetate fraction of MO leaves showed remarkable proliferative and migratory effect on normal human dermal fibroblasts.
Conclusion:
This study suggested that ethyl acetate fraction of MO leaves might be a potential therapeutic agent for skin wound healing by promoting fibroblast proliferation and migration through increasing the wound closure rate corroborating its traditional use.
KEY WORDS: Active fractions, cell proliferation, migration, natural products, scratch assay
INTRODUCTION
Skin plays a crucial role in the sustenance of life by acting as a barrier to external noxious agents. When this barrier is disrupted, skin may not be able to adequately perform its crucial function; therefore, it is vital to restore its integrity forthwith. A normal wound healing necessitates a series of dynamic and overlapping process. A traumatized skin, expose underlying tissue to outside environment, provide an open access to infection and often results in the development of unpleasant exudates and toxins, which is associated with concomitant killing of regenerating cells [1,2]. Thus, interplay between cellular and extracellular components is required to restore tissue integrity. Modulation of diverse growth factors in tissue repair influence the cellular proliferation, migration and other cellular metabolic activity of the skin. The culmination of these biological processes results in the replacement of normal skin structures [3]. Though the healing process takes place naturally, wounds may enter a state of pathologic inflammation due to a postponed, incomplete, or uncoordinated healing process that exhibits impaired or delayed acute wounds and chronic wounds. Besides health status, age factors, body built, nutritional status, and physiological stress are speculated to be the result of impaired wound healing [4-6].
Wound healing is categorized into four classic stages and that are hemostasis, inflammation, fibroplasia, and maturation [7]. Hemostasis takes place immediately after injury and its form of protection to vascular system and bridges invading cells required for the following healing phases. The platelet aggregation and clot formation in hemostasis event recruit growth factors and cytokines such as transforming growth factors-β (TGF-β), platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF). These molecules act as promoters to following inflammatory phase in wound healing cascade. An influx of inflammatory cells including neutrophils, monocytes, and macrophages in the fibrin scaffold [8] enhances further tissue debridement and recruitment of additional growth factors vital for wound healing. Epithelialization, angiogenesis, granulation tissue formation, and collagen deposition, characterize the proliferative phase. The sequential events of epithelialization encompass cell detachment, proliferation migration, and differentiation, which are facilitated by TGF-β, VEGF, and multiple cytokines. Angiogenic response is critical as newly developed cell and tissue requires formation of new blood vessels to provide nutrients needed for homeostasis. The hypoxic condition allows proliferation of endothelial cells under influence of angiogenic factors. Fibroblasts multiply and increase collagen production to produce greater tensile strength about 80% of the original or uninjured tissue. Concurrently, cell and capillary density decrease in the final phase of wound healing which involves maturation or remodeling [9].
Achievement in developing the new-fangled information of unique biological markers linked with normal and pathological wound healing responses aids in discovery of new natural therapeutic agent to replacing those existing costly drugs such as silver sulfadiazine, vasolex and santyl accompanied by like skin irritation, rashes blood dyscrasias and life-threatening cutaneous reactions [10]. Many medicinal plants have a very significant role in the course of wound healing [11]. Plant-based therapy not only accelerate healing process but also maintains the aesthetics in a natural way [12].
At present, there has been growing interest on Moringa oleifera (MO) from biomedical researcher due to high potential and nutritive value. MO tree is native to the sub-Himalayan regions of Northwest India and cultivated throughout tropical and sub-tropical areas of the world including India, Pakistan, Malaysia, and Sri Lanka [13]. The pharmacological attributes of MO leaves mainly consist of glycosides, β-sitosterol, α-tocopherol, pyridoxine, ascorbic acids, lysine methionine and proteins [14]. This compound has been shown to be very rare in nature and exhibited anti-hyperthyroidism, antitumor, antispasmodic, antioxidant, hepatoprotective and antimicrobial activities [15-18]. The current work was aimed to investigate the in vitro wound healing potential of ethyl acetate fraction of MO leaves on normal human dermal fibroblast (HDF-N) cells and these activities were compared with positive control drug, Allantoin. Allantoin holds numerous therapeutic activities including wound healing, remover of necrotic tissue and promoter of epithelial stimulation and it has been used in pharmaceutical preparations for more than 70 years [19]. Besides neutrophils, endothelial cells, and keratinocytes, fibroblasts holds an important role in cutaneous wound repair and remodeling. They proliferate to expand, migrate into the wound bed and synthesize new extracellular matrix [20]. Understanding the mechanisms that regulate the cell proliferation and migration of fibroblasts cells by bioactive compound could be favorable in developing novel therapies to improve the wound healing process.
MATERIALS AND METHODS
Plant Collection, Extract Preparation and Isolation of Ethyl Acetate Fraction
The MO leaves were collected from Garden No.2 at Universiti Putra Malaysia (UPM), Malaysia with the voucher specimen (SK 1561/08) and deposited in the IBS Herbarium unit. The powdered leaves were taken and extracted with 90% ethanol using maceration technique in room temperature. The filtrate were collected and allowed for drying through rotary evaporator at 25°C (Virtis Bench Top K, United States. Further, by using solvent-solvent partition technique, ethyl acetate fraction (EtOAc) was isolated from leaves crude extract, and this was stored at −20°C until further use.
In-vitro Wound Healing Study
Cell culture maintenance
HDF-N cells were acquired from American Type Culture Collection (ATCC, Manassas, VA, USA, CRL-2301) and thawed as well as maintained according to the ATCC protocol. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) premixed with 5% fetal bovine serum, growth supplements, and antibiotics consisting of L-glutamine 15 mmol/L, streptomycin 100 µg/mL, and penicillin 100 U/Ml and incubated in 5% CO2 and 37°C. Cell passages between 12 and 15 at 70-80% confluence were used for seeding and treatment throughout the experiment.
3-(4, 5-DImethylthiazol-2-yl)-2, 5-diphenyl Tetrazolium Bromide [MTT]) Assays
HDF-N cells passage numbers between 12 to 15, were seeded into 96-well plate at a density of 1 × 105 (in 100 µL DMEM medium) per well and grown for 24 h. The medium was replaced with serial dilutions of EtOAc MO leaves concentration of 15.62, 31.25, 62.5, 125, 250 and 500 µg/ml and plates were incubated for 24 h. 10 µL of 5 mg/mL MTT reagent was then added to each of the wells and incubated for another 4 h. The purple formazan formed was solubilized by adding 100 µL dimethyl sulfoxide to all the wells including control (without any treatment), then swirled gently to mix well and this was then kept in the dark place at room temperature for about 30 min. Microplate reader was used to read absorbance at 570 nm with reference of 630 nm. Graph of absorbance against number of cells was plotted to determine the HDF-N cells viability as per the standard methods [21]. Experiments were performed in triplicate and the data were recorded and analyzed statistically using SPSS.
Cell Proliferation Assay
HDF-N cells were seeded on 96 well plates at a density of 1 × 105 (in 100 µL medium) per well and incubated at 37°C until confluent, and medium was replaced with serial dilutions of EtOAc MO leaves (15.62, 31.25, 62.5, 125, 250 and 500 µg/ml) and incubated for 24 h. 10 µL of cell counting kit-8 (CCK-8) was then added to each of the wells and incubated for another 4 h according to the manufacturer’s instructions (Dojindo Lab, Japan). Microplate reader was used to read absorbance at 450 nm with reference of 630 nm. Graph of absorbance against number of cells was plotted to determine the HDF cells proliferation according to the kit manufactures instructions. Experiments were performed in triplicate and the data were recorded and analyzed statistically using SPSS.
Wound Scratch Assay
The migration of HDF-N was examined using the scratch assay method [18]. HDF-N (2 × 105 cells) were seeded into each well of a 24-well plate and incubated with complete medium at 37°C and 5% CO2. After 24 h of incubation, the cells were treated with EtOAc fraction of MO leaves with varying concentration (12.5 µg/mL, 25 µg/mL and 50 µg/mL). Confluent cells were scrapped horizontally with a P200 pipette tips. The medium was replaced with fresh medium and wound closure were monitored and photographed by phase contrast microscopy using ×4 magnification at 0 h. After 24 h of incubation, the second set of images was photographed. To determine the migration rate, the images were analyzed using image-j software and percentage of the closed area was measured and compared with the value obtained before treatment. An increase of the percentage of closed area indicated the migration of cells. Experiments were performed in triplicate and the data were recorded and analyzed statistically using SPSS.
Statistical Analysis
Data are given as the mean ± standard deviation (SD) and statistical analyses were performed using one-way ANOVA (ANOVA) SPSS version 21.0 software (SPSS, USA). Results were obtained at the end of the experiment and it was compared with control and treated groups using Student’s t-test. Differences were considered as statistically significant at *P < 0.05, **P < 0.01, ###P < 0.001 versus control group.
RESULTS
Effect of MO on Cell Viability
The cytotoxic effect of MO EtOAc fraction was determined by MTT assay in HDF-N cells treated with different gradient concentration (15.62, 31.25, 62.5, 125, 250, and 500 µg/mL concentration). Cell viability scrutiny showed that MO ethyl acetate fraction had no toxic effect on HDF-N cells even at higher concentration [Figure 1]. By increasing the concentration of fraction, cell viabilities were not significantly different in percentage therefore, we have selected a lower concentration (12.5, 25 and 50 µg/mL) of ethyl acetate fraction for further wound healing studies.
Figure 1.
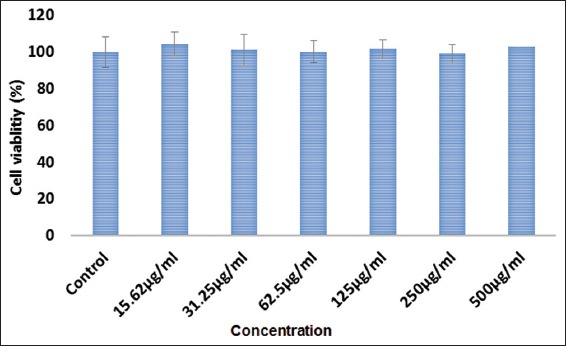
Cyto-toxicity effects of Moringa oleifera (MO) EtOAc fraction treatment in human dermal fibroblast - normal cells. At 24 h of treatment, effects of MO ethyl acetate fraction against the viability of treated cells were evaluated through mitochondrial activity using the MTT assay and calculated by comparing the values from the MO ethyl acetate fraction treatment group with the control group. Values are presented as the mean percentage ± standard deviation (n = 3) from three individual experiments.
Effect of M. oleifera on Cell Proliferation
The cell proliferation as represented in Figure 2 demonstrate significant increase in the rate of cell proliferation on HDF-N cells on treatment with MO EtOAc fraction using concentrations up to 62.5 µg/ml. However, concentration from 125 µg/ml and higher of MO EtOAc fraction reduced the HDF-N cells proliferative rate. Accordingly, the proliferative investigations, the concentration at 12.5 µg/mL, 25 µg/mL and 50 µg/mL were chosen for further wound healing experiments.
Figure 2.
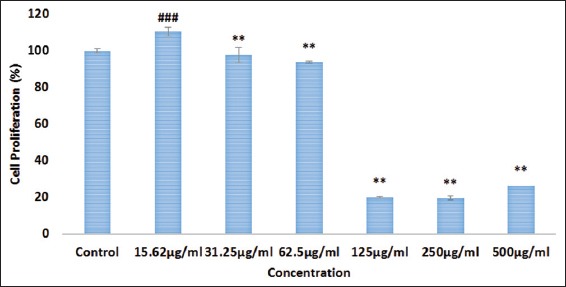
The effect of Moringa oleifera (MO) EtOAc fraction treatment on the proliferation rate of human dermal fibroblast-normal cells. The proliferation effect was estimated by cell counting kit-8 assay and calculated by comparing the values from the M. oleifera ethyl acetate fraction treatment group with the control group. Data are expressed as mean ± standard deviation from three individual experiments. **P < 0.01, ###P < 0.001 versus control group
Effect of MO on Wound Healing Activity
Based on cytotoxicity and proliferation experiment, an optimized concentration (non-toxic) of MO EtOAc fraction was assessed in scratch assay [Figures 3 and 4] to determine its effects on spreading and migration activities of HDF-N cells. Though, there is slight migration of cells in control, MO EtOAc treated HDF-N cells were found to migrate faster following a 24 h incubation period. The results revealed that MO EtOAc fraction administration created ± 9% difference in wound closure rate between treated and the positive control. It was also noticed that lower concentration of fraction (12.5 µg/ml) proliferate and migrate more rapidly than higher concentration (50 µg/ml). Despite the increased migration rate of HDF-N cells at 50 µg/ml, the morphology in terms of size and shape (fusiform) were altered, showing evidence of toxicity.
Figure 3.
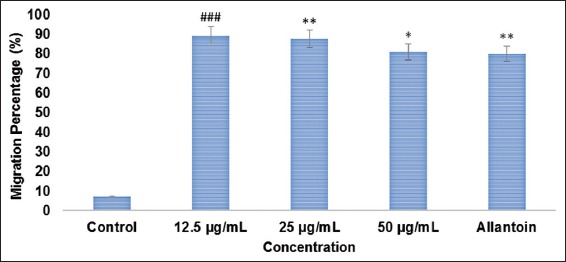
The migration rate in percentage for normal human dermal fibroblast after treatment with Moringa oleifera (MO) EtOAc fraction for 24 h. Quantitative analysis of the migration rate was analyzed with the use of Image-J software in MO ethyl acetate fraction-treated normal human dermal fibroblast. Data are expressed as mean ± standard deviation from three individual experiments. *P < 0.05, **P < 0.01, ###P < 0.001 versus control group
Figure 4.
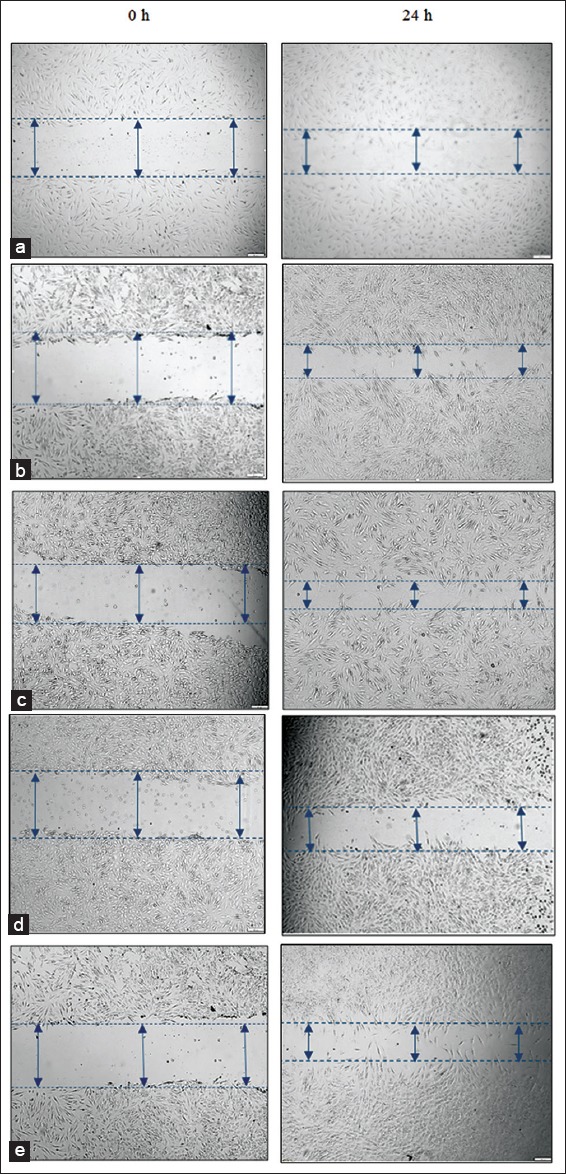
Effect of Moringa oleifera (MO) EtOAc fraction on migration rate (wound scratch assay) in normal human dermal fibroblast. The migration rate was quantified by the Image-J software and data’s are expressed as mean ± standard deviation from three individual experiments for migration assay (wound scratch assay) (a) Control, (b) MO EtOAc fraction 12.5 µg/mL, (c) MO EtOAc fraction 25 µg/mL, (d) MO EtOAc fraction 50 µg/mL, (e) Allantoin
DISCUSSION
Impaired wounds healing may occur in any individual but are more frequent in the elderly and chronically ill people. With an ageing population and a dramatically increasing prevalence of chronic diseases like cancer, diabetes, wound care will certainly become an even more noteworthy issue for health systems [22,23]. Delayed wound healing is signified with alteration in physical properties of collagen, flattening of the dermo-epidermal junctions, nutritional depletion and cellular immunity leading to abnormal changes in pro-inflammatory and anti-inflammatory cytokines [24]. A number of evidence has been collected to show immense potential of medicinal plants used in various traditional systems. Certain plants pose a serious risk of toxicity from a human health standpoint such as cycads in contempt of its rich source of alkaloids [25]. This encourage the healthcare team to shorten the time required for healing and to minimize the undesired consequences such as skin inflammation, allergy as plants are more potent healers because they promote the repair mechanisms without adverse effects. These efforts ensure the patient is given holistic wound care and offer wounds the best chance to heal [26].
Traditional healers claim that the leaves of the MO are used as antiviral, anti-inflammatory, and analgesic. Collateral to that, the discovery of scientific evidence of phytochemical compounds from MO leaves which consist of various clinically important constituents such as 4-[(4’-O-acetyl-alpha-L-rhamnosloxy)benzyl] isothiocyanate,4-[(3’-O-acetyl-alpha-i-rhamnosyloxy)benzyl]isothiocyanate and S-methyl-N-{4-[(alpha-I-rhamnosyloxy)benzyl]}thiocarbamate, which is an anti-inflammatory phenolic glycosides [27]. With this background, the present study objective was undertaken to emphasize the effect of ethyl acetate fraction of MO leaves on in-vitro wound healing properties. The study clearly submits that MO EtOAc fraction at the concentration of 12.5 and 25 µg/mL enhances the cell proliferation and migration of HDF. Cell proliferation and cell migration are two important events necessary for wound healing and an essential event during re-epithelialization, so proliferating fibroblasts at the wound site ensure an adequate supply of cells to migrate and cover the wound surface [28,29]. Both criteria were witnessed in in-vitro studies of cell proliferation and scratch assay [Figures 2-4]. In addition, increased response with concentration in the migration of fibroblast cell in scratch assay indicates EtOAc fraction of MO leaves was shown to be potent in promoting angiogenesis [Figure 3]. However at higher concentration, EtOAc fraction of MO leaves confer a strong anti-proliferative activity, possibly due to the strong accumulation of phenolic compounds which may be linked to activation of caspases and inducing apoptosis [30].
The results discussed above established the scientific basis of a traditional claim for the use of MO leaves as a wound healing agent. In the literature, the clinical importance of MO leaves including wound healing effect has not been fully studied with systematic manner up to now. This study confirms that ethyl acetate fraction of MO leaves might have wound healing effects based on the data of in-vitro assays on normal human fibroblast cells (Figure 5). Future studies will be centered on the identification and purification of the active component(s) of EtOAc fraction of MO leaves responding to the underlying wound healing mechanisms for novel cost-effective drug lead. In addition, in-vivo wound healing studies are still lacking to show the pharmacological activity of EtOAc fraction on the mammalian system to support the specific mechanism tangled in regulating the anti-inflammatory activity in wound healing.
Figure 5.
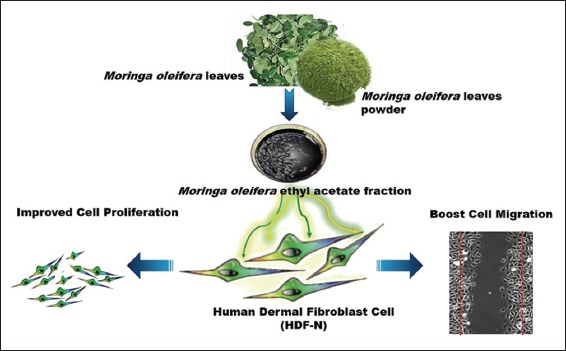
The potential role of ethyl acetate fraction of Moringa oleifera leaves in in-vitro wound healing model
CONCLUSION
This study demonstrated that EtOAc fraction of MO leaves was effective in promoting and accelerating wound closure process in HDF-N cells. This natural agent is rich target for development as alternative therapeutic wound healing agent, though, there is a need for scientific validation and safety evaluation before this plant could be commercialized for alternative wound treatment.
ACKNOWLEDGMENTS
This research work was supported by a grant (Project No: GP-1/2014/9443700) from the Research Management Centre, Universiti Putra Malaysia (UPM), Malaysia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Flanagan M. Wound measurement: Can it help us to monitor progression to healing? J Wound Care. 2003;12:189–94. doi: 10.12968/jowc.2003.12.5.26493. [DOI] [PubMed] [Google Scholar]
- 2.Jalalpure SS, Agrawal N, Patil MB, Chimkode R, Tripathi A. Antimicrobial and wound healing activities of leaves of Alternanthera sessilis Linn. Int J Green Pharm. 2008;2:141–4. [Google Scholar]
- 3.Mathieu D, Linke JC, Wattel F. Non-healing wounds. Handbook on Hyperbaric Medicine. Vol. 5. Netherlands: Springer; 2006. pp. 401–27. [Google Scholar]
- 4.Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):42S–58. doi: 10.1097/01.prs.0000225432.17501.6c. [DOI] [PubMed] [Google Scholar]
- 5.Vileikyte L. Stress and wound healing. Clin Dermatol. 2007;25:49–55. doi: 10.1016/j.clindermatol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Patil DN, Kulkarni AR, Shahapurkar AA, Hatappakki BC. Natural cumin seeds for wound healing activity in albino rats. Int J Biochem. 2009;3:148–52. [Google Scholar]
- 7.Whitney JD. Overview: Acute and chronic wounds. Nurs Clin North Am. 2005;40:191–205. doi: 10.1016/j.cnur.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 9.Robson MC, Steed DL, Franz MG. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 10.Caffee HH, Bingham HG. Leukopenia and silver sulfadiazine. J Trauma. 1982;22:586–7. doi: 10.1097/00005373-198207000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complement Alternat Med. 2011;2011:438056. doi: 10.1155/2011/438056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitra MB, Patil R. Preliminary phytochemical investigation and wound healing activity of Allium. Cepalin (Liliaceae) Int J Pharm Pharm Sci. 2009;2:7–9. [Google Scholar]
- 13.Mughal MH, Ali G, Srivastava PS, Iqbal M. Improvement of drumstick (Moringa pterygosperma Gaertn.) – A unique source of food and medicine through tissue culture. Hamdard Med. 1999;42:37–42. [Google Scholar]
- 14.Farooq F, Meenu R, Avinash T, Abdul A, Sahila F. Medicinal properties of Moringa oleifera : An overview of promising healer. J Med Plants Res. 2012;6:4368–74. [Google Scholar]
- 15.Dillard CJ, German JB. Phytochemicals: Nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–56. [Google Scholar]
- 16.Estrella MP, Mantaring JV, David GZ. A double blind, randomised controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breastmilk among non-nursing mothers of preterm infants. J Pediatr. 2000;49:3–6. [Google Scholar]
- 17.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–55. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 18.Atsukwei D, Eze ED, Moses DM, Adinoyi SS, Upkabi CN. Hypolipidaemic effect of ethanol leaf extract of Moringa Oleifera Lam. in experimentally induced hypercholesterolemic Wistar rats. Int J Nutr Food Sci. 2014;3:355–60. [Google Scholar]
- 19.Shestopalov AV, Shkurat TP, Mikashinovich ZI, Kryzhanovskaia IO, Bogacheva MA, Lomteva SV, et al. Biological functions of allantoin. Izv Akad Nauk Ser Biol. 2006:541–5. [PubMed] [Google Scholar]
- 20.D’Souza KM, Malhotra R, Philip JL. G protein-coupled receptor kinase-2 is a novel regulator of collagen synthesis in adult human cardiac fibroblasts. J Biol Chem. 2011;17:15507–16. doi: 10.1074/jbc.M111.218263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Tan WS, Arulselvan P, Karthivashan G, Fakurazi S. Moringa oleifera flower extract suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 Macrophages via NF-? B pathway. Mediators Inflamm. 2015;2015:720171. doi: 10.1155/2015/720171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int. 2013;2013:974580. doi: 10.1155/2013/974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurd TA. Nutrition and wound-care management/prevention. Clin Pract. 2004;2:20–8. [Google Scholar]
- 24.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Chitra S, Patil MB, Ravi K. Wound healing activity of Hyptis suaveolens (L) poit (Laminiaceae) Int J Pharm Technol. 2009;1:737–44. [Google Scholar]
- 27.Cheenpracha S, Park EJ, Yoshida WY, Barit C, Wall M, Pezzuto JM, et al. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg Med Chem. 2010;18:6598–602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 28.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 29.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–10. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]


