Abstract
Background:
Natural products with therapeutic properties such as plants, minerals, and animal products, for many years, were the main sources of drugs for the treatment of numerous diseases; hence selection of Lawsonia inermis L. (Henna) to study its hepatoprotective activity was considered.
Objectives:
This was an attempt to evaluate the hepatoprotective effect of L. inermis leaves’ methanolic extract on carbon tetrachloride (CCl4)-induced hepatotoxicity in rats.
Materials and Methods:
The L. inermis leaves’ methanolic extract, which obtained by maceration, was orally administered in doses of 100 mg/kg and 200 mg/kg to the tested animals to assess its effects on serum levels of hepatotoxicity parameters, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, and total proteins along with histopathological liver sections examination, while silymarin (25 mg/kg), a potent hepatoprotective drug, was used as standard control.
Results:
The two doses of the plant extract showed dose-dependent hepatoprotective effect, as evident by the significant reduction (P < 0.05) in serum levels of AST, ALT, ALP, and bilirubin along with the improvement in histopathological liver sections compared to CCl4-only treated animals.
Conclusion:
As experimentally evident, it could be concluded that this plant material could provide a hepatoprotective effect that could be attributed to its antioxidant properties.
KEY WORDS: Carbon tetrachloride hepatotoxicity, hepatoprotective, Lawsonia inermis leaves, liver enzymes
INTRODUCTION
Because liver plays important functions in the maintenance, performance, and regulating homeostasis of the body, maintenance of a healthy liver is essential for the overall well-being of an individual [1]. In spite of the tremendous advances in modern medicine, there were few drugs available that offer protection to the liver from damage and help to regenerate hepatic cells [2]. Therefore, it is necessary to search for new drugs for the treatment of liver diseases to supplement/replace the currently used ones. Fortunately, the plant kingdom is a valuable source of new medicinal agents, and it has been reported that approximately 25% of modern medications have been derived from plant materials [3,4]. Hepatoprotective plants contain a variety of chemical constituents such as phenols, coumarins, lignans, essential oil, monoterpenes, carotinoids, glycosides, flavanoids, organic acids, lipids, alkaloids, and xanthenes [3]. However, among numerous plant materials used for liver protection, a considerable number of them lack the scientific prove for these claims [5]. Therefore, it is important to follow systematic research methodology to scientifically evaluate the plant materials that claimed to possess hepatoprotective activity.
Lawsonia inermis (Henna) is a shrub or small tree cultivated in many regions as an ornamental and commercial dye crop [6]. It is mostly found in the tropic, sub-tropic, and semi-arid zones of Africa (tropical Savannah and tropical arid zones), south Asia, and north Australia [7]. As reported by Varghese et al., (2010) [8], wide range of chemical constituents have been isolated from Henna which includes naphthoquinone derivatives (lawsone which is the chief ingredient and the coloring matter in the leaves), phenolic derivatives, coumarins, xanthones, tannins, flavonoids, aliphatic components, triterpenes, sterols and other chemical constituents such as glucose, gallic acid, amino acids, mannitol, trace elements and minerals.
As a medicinal plant, Henna has been used in folk remedy as astringent, hypotensive, sedative, and against a headache, jaundice, and leprosy [9]. Leaves were also used for skin diseases, venereal diseases, smallpox, and spermatorrhea. Powered seeds were effective against dysentery and liver disorders. The bark used in a variety conditions, such as burns, jaundice, spleen enlargement, calculus, leprosy, and skin disorders. Root was considered as a potent medicine for gonorrhea, herpes infection, sore eyes, as an abortifacient, and in the treatment of some nervous disorders [10]. Because, seeds were able to alleviate liver disorders, a question was raised about the protective effects of other plants’ parts.
Therefore, this work was aimed to study the hepatoprotective effect of L. inermis leaves’ methanolic extract on carbon tetrachloride (CCl4)-induced hepatotoxicity in rats as an initial step that can aid further elucidation of the therapeutic potential of this plant product.
MATERIALS AND METHODS
Plants Materials
Fresh leaves of L. inermis were obtained from the local market, Wad Medani, Sudan, which was further identified at the herbarium of the phytochemistry and taxonomy Department, Medicinal and Aromatic Plants Institute, National Center for Research, Khartoum, Sudan, and a voucher specimen was placed for future referencing.
Extraction of Plants Materials
The leaves of L. inermis were washed with distilled water; shade-dried at room temperature for 72 h, then grinded into powder by glass mortar and pestle. A 300 g of dry powder were extracted by maceration using 1.5 liters of methanol (99%) as a solvent system in conical flasks for 72 h, with intermittent shaking, and then filtered under vacuum using Buchner funnel. The filtrate was allowed to evaporate at room temperature, in dark, for 7 days, and the greenish-black semisolid extract was collected and stored in an amber glass container in a refrigerator until used for biological testing.
Experimental Animals
Albino rats weighing about 130-200 g (12-15 weeks old) males and females obtained from the animal house, Faculty of Pharmacy, University of Gezira. They were housed in polyacrylic cages and acclimatized to laboratory condition for 14 days before commencement of the experiment (temperature 25 ± 2°C with dark and light cycle 14/10 h). The animals were allowed free access to diet and water. They were divided into five groups as follow:
Group I (normal control, n = 5) received distilled water
Group II (negative control, n = 5) received CCl4 only
Group III (standard control, n = 5) received silymarin (25 mg/kg)
Groups IV and V (test control, n = 10) received L. inermis leaves extract 100 mg/kg and 200 mg/kg, respectively.
Water suspensions of silymarin and L. inermis extract were daily orally administered to the animal groups (III, IV, and V), respectively. Carbon tetrachloride (CCl4) was administered every 72 h with intrapretonial injection in a dose of 2 ml/kg [11] to all rats except the rats of Group I.
This study was ethically approved by the Faculty of Pharmacy, University of Gezira ethical committee, and in compliance with the Faculty of Pharmacy Guiding Principles in the Use of Animals in Toxicology 2006.
Determination of Hepatoprotective Effect
Hepatoprotective effect of L. inermis extract on CCl4-induced hepatotoxicity in rats were carried out for 21 days based on the methods described by Sapakal et al., [12] and Azeem et al., [13].
Blood samples were collected at day 7, day 14, and day 21, allowed to clot for 45 min at room temperature, and serum was separated by centrifugation at 3500 rpm for 15 min at 4°C then used for measurement of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and total proteins levels using an auto-analyzer (Cobas Integra 400, Roche Diagnostics, Switzerland).
Histopathological Studies
Slices of liver from each animal in all groups were preserved in 10% buffered neutral formalin, the tissues were mounted in the laboratory by embedding paraffin sections, then stained with hemotoxyline and eosin and subjected to histopathological examination at the Histopathology Department - Medical Laboratory - University of Gezira.
Data Analysis
The biochemical data were statistically analyzed using paired t-test and expressed as mean ± standard error of mean. For comparisons with the CCl4-only treated group (Group II), differences were considered significant if P < 0.05. The percentages of hepatoprotection (H) were calculated as described by Singh et al., [14], using the mean value of each group by the following equation:

RESULTS
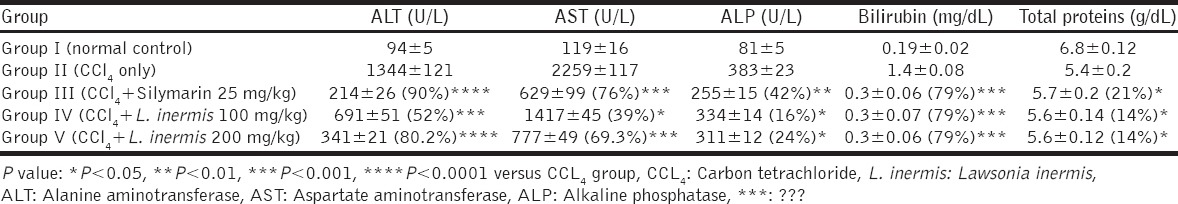
The obtained biochemical data showed that administration of CCl4 to rats significantly elevated the serum levels of ALT and AST compared to the normal group (P < 0.01) which indicated acute hepatocellular damage. A significant reduction (P < 0.001) in ALT and AST levels were recorded in Silymarin treated group (Group III) compared to CCl4-alone treated group (Group II) at day 7 and day 14 of the experimental course. However, at day 21, all treatment groups (Groups III, IV, and V) showed a significant reduction (P < 0.01) in ALT and AST levels with the greatest reduction recorded in Group V [Table 1].
Table 1.
Effects of methanolic extract of L. inermis leaves and Silymarin on serum levels of ALT and AST in CCl4-induced hepatotoxicity
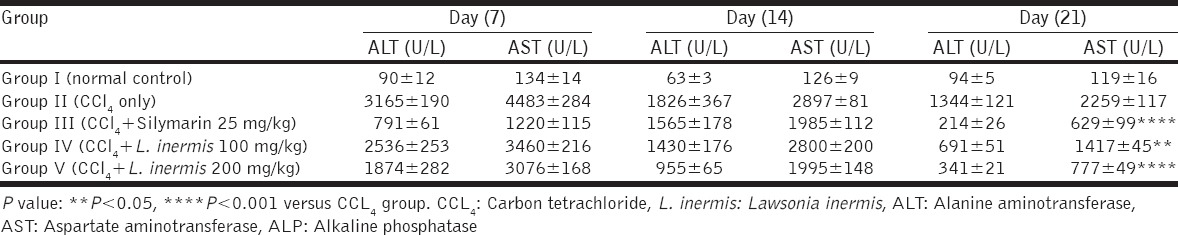
The serum levels of ALT, AST, ALP, total bilirubin, and total proteins that obtained from the tested animals at day 21, along with the percentage of hepatoprotection for each biochemical parameter, showed that both plant extract and silymarin decreased the levels of liver enzymes that elevated by CCl4 administration when compared to CCl4-only treated group. The two different concentrations that used in this study for the plant extract showed a concentration-dependent effect [Table 2].
Table 2.
Hepatoprotective percentage of L. inermis leaves methanolic extract and Silymarin on CCl4-induced hepatotoxicity at day 21
In the histopathological analysis, the liver section of Group I (normal control) exhibited normal hepatic cells with distinct hepatic cells, sinusoidal spaces, and central vein [Figure 1], whereas that of CCl4-only intoxicated animals showed necrosis of hepatic architecture with vacuolization and congestion of sinusoids [Figure 2]. Treatment with silymarin, and the methanolic extract of L. inermis leaves, showed minimal necrosis and regeneration of hepatocytes compared to the CCl4-only intoxicated group which provided supportive evidence for the biochemical analysis [Figures 3 and 4].
Figure 1.
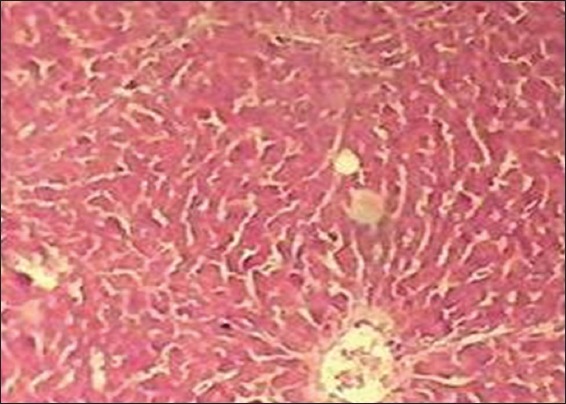
Histopathological liver section of normal control group (H&E, ×10)
Figure 2.
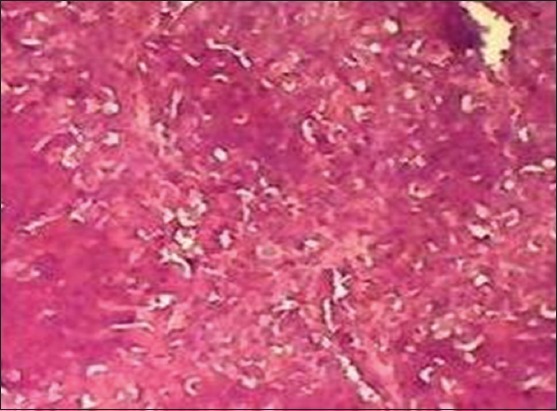
Histopathological liver section of carbon tetrachloride-only intoxicated group (H&E, ×10)
Figure 3.
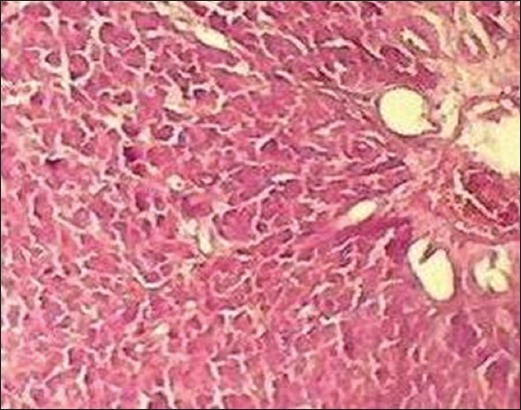
Histopathological liver section of Silymarin treated group (H&E, ×10)
Figure 4.
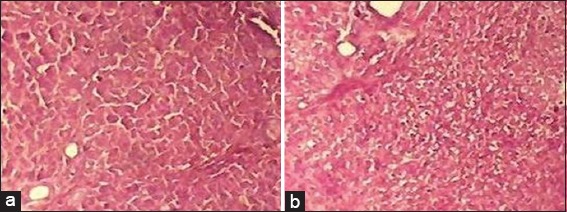
(a and b) Histopathological liver sections of Lawsonia inermis treated groups (H&E, ×10)
DISCUSSION
In animal model studies, induction of liver injury by CCl4 is commonly used to evaluate the of hepatoprotective agents, because CCl4 administration significantly elevated the serum levels of ALT, AST, ALP, and bilirubin [15] that considered as mutual signs for liver injury. CCl4 bio-transformed by cytochrome P450 system in the endoplasmic reticulum to produce trichloromethyl free radical which combined with cellular lipids and proteins in the presence of oxygen form trichloromethyl peroxyl radical, and may attack lipids on the membrane of endoplasmic reticulum faster than trichloromethyl free radical [16]. The intraperitoneal route was found to be the best route for CCl4-induced hepatotoxicity in rats, and the optimum dose was found to be 2 ml/kg (dissolved in an equal volume of olive oil), and this significantly increased the level of serum enzymes, without causing the death of the animals [11].
The serum levels of aminotransferases were markedly elevated by hepatocytes damage due to toxins, drugs, or viruses [17], and estimating the activities of the serum marker enzymes could made a useful quantitative biomarker of the extent and type of hepatocellular damage, and the tendency of these enzymes to return to near normal levels considered as a clear manifestation of antihepatotoxic effects of the administered agent [18].
Hepatoprotective agents exert their action against CCl4-induced liver injury by impairment of CCl4-mediated lipid peroxidation, either through decreased production of free radical derivatives or due to the antioxidant activity of the protective agent itself [19]. It has been reported that L. inermis is a rich plant in phenolic compounds such as phenolic acids, flavonoids, tannins, lignin, and others that possess antioxidant, anticarcinogenic, and antimutagenic effects as well as antiproliferative potentials [20].
The obtained results indicated that the tested plant material could protect the liver against CCl4 toxicity in a dose-dependent manner as evident from the protection provided when compared to the enzyme levels in CCl4-only treated rats along with histopathological observations. It has been reported that the plant extract affords hepatoprotective activity due to its antioxidant property attributed to the flavonoids content that are effective scavengers of superoxide anions, peroxynitrite, peroxyl and hydroxyl radicals [21].
CONCLUSION
It could be concluded that biochemical and histopathological alterations induced by CCl4 administration were improved under the effect of the plant extract. The concentration of 200 mg/kg was found to be more effective than 100 mg/kg. The present study also suggested that the traditional medicine could have the potential to be transposed successfully in the context of modern medical interventions, but additional researches are necessary to characterize the active constituents and to assess their pharmacological properties.
ACKNOWLEDGMENTS
This work was partially supported by Department of Pharmacology, Faculty of Pharmacy, University of Gezira.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vishnu Priya V, Niveda S, Pratiksha G, Gayathri R. A review of hepatoprotective natural products. Rec Res Sci Technol. 2010;2:49–52. [Google Scholar]
- 2.Adewusi E, Afolayan A. A review of natural products with hepatoprotective activity. J Med Plant Res. 2010;4:1318–4. [Google Scholar]
- 3.Sharma B, Upendra KS. Hepatoprotective activity of some indigenous plants. Int J Pharmtech Res. 2009;1:1330–4. [Google Scholar]
- 4.Rahmatullah M, Mukti IJ, Fahmidul Haque AK, Mollik AH, Parvin K, Jahan R, et al. An ethnobotanical survey and pharmacological evaluation of medicinal plants used by the Garo tribal community living in Netrakona district, Bangladesh. Adv Nat Appl Sci. 2009;3:402–18. [Google Scholar]
- 5.Alqasomi SI, Al-Howiriny TA, Abdel-Kader MS. Evaluation of hepatoprotective effect of Aloe vera, Clematis hirsute, Cucumis prophetarum and bee propolis against experimentally induced liver injury in rats. Int J Pharmacol. 2008;4:213–7. [Google Scholar]
- 6.Muthumani P, Meera R, Sundaraganapathy Devi P, Mohamed Sheik Arabath S, Cholarja K. Biological evaluation of dried fruits of Lawsonia inermis. J Pharm Biomed Sci. 2010;1:1–5. [Google Scholar]
- 7.Donkor Sarkodie C, Quainoo AK, Gustav M. Propagation of Henna (Lawsonia inermis) cuttings using nathelene acetic acid, indole-3-butyric acid and wood ash. JPBAS. 2013;1:115–23. [Google Scholar]
- 8.Varghese JK, Silvipriya K, Resmi S, Jolly C. Lawsonia Inermis (Henna): A natural dye of various therapeutic uses - A review. Inventi Rapid: Cosmeceuticals. 2010;1:1–5. [Google Scholar]
- 9.Abdulmoneim Saadabi M. Evaluation of Lawsonia inermis Linn. (Sudanese Henna) leaf extracts as an antimicrobial agent. Res J Biol Sci. 2007;2:419–23. [Google Scholar]
- 10.Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int J Pharm Sci Drug Res. 2010;2:91–8. [Google Scholar]
- 11.Janakat S, Al-Merie H. Optimization of the dose and route of injection, and characterisation of the time course of carbon tetrachloride-induced hepatotoxicity in the rat. J Pharmacol Toxicol Methods. 2002;48:41–4. doi: 10.1016/S1056-8719(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 12.Sapakal V, Chadge R, Adnaik R, Naikwade N, Magdum C. Comparative hepatoprotective activity of Liv-52 and Livomyn against carbon tetrachloride – Induced hepatic injury in rats. Int J Green Pharm. 2008;43:79–82. [Google Scholar]
- 13.Azeem A Khalaf, Mohy Eldin M Mekawy, Mohamed S Moawad, Amal M Ahmed. Comparative study on the protective effect of some antioxidants against CCl4 hepatotoxicity in rats. Egypt J Nat Toxins. 2009;6:59–82. [Google Scholar]
- 14.Singh B, Saxena AK, Chandan BK, Agarwal SG, Anand KK. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian J Physiol Pharmacol. 2001;45:435–41. [PubMed] [Google Scholar]
- 15.Solomon R, Rao G, Latha M, Srinivas K. Antihepatotoxic activity of Smilax china roots on CCl4 induced hepatic damage in rats. Int J Pharm PharmSci. 2012;4:494–6. [Google Scholar]
- 16.Mayuren C, Reddy VV, Priya SV, Devi VA. Protective effect of Livactine against CCl4 and paracetamol induced hepatotoxicity in adult Wistar rats. N Am J Med Sci. 2010;2:491–5. doi: 10.4297/najms.2010.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habermann TM, Ghosh AK. Mayo Clinic Internal Medicine Concise Textbook. Ch. 7. USA: Mayo Clinic Scientific Press and Information Healthcare Inc; 2008. pp. 217–304. [Google Scholar]
- 18.Rajendran R, Hemalatha S, Akasakalai K, MadhuKrishna C, Vittal BS, Meenakshi Sundaram R. Hepatoprotective activity of Mimosa pudica leaves against carbon tetrachloride induced toxicity. J Nat Prod. 2009;2:116–22. [Google Scholar]
- 19.Ahmed S, Rahman A, Alam A, Saleem M, Athar M, Sultana S. Evaluation of the efficacy of Lawsonia alba in the alleviation of carbon tetrachloride-induced oxidative stress. J Ethnopharmacol. 2000;69:157–64. doi: 10.1016/s0378-8741(99)00091-4. [DOI] [PubMed] [Google Scholar]
- 20.Uma D, Aida W. Optimization of extraction parameters of total phenolic compounds from Henna (Lawsonia inermis) leaves. Sains Malays. 2010;39:119–28. [Google Scholar]
- 21.Sanni S, Thilza B, Ahmed M, Sanni F, Talle M, Okwor G. The effect of aqueous leaves extract of henna (Lawsonia inermis) in carbon tetrachloride induced hepatotoxicity in Swiss albino mice. Acad Arena. 2010;2:87–9. [Google Scholar]


