Abstract
Aim:
The current study aimed to investigate the anti-hyperglycemic, anti-hyperlipidemic and insulin sensitizing effects of the cyanobacterium Spirulina versicolor extract in fructose-fed rats.
Materials and Methods:
Rats were fed 30% fructose solution in drinking water for 4 weeks. Animals exhibited hyperglycemia and hyperinsulinemia were selected for further investigations. Diabetic and control rats were orally supplemented with 50 mg/kg body weight S. versicolor extract for 4 weeks.
Results:
At the end of 8 weeks, fructose-fed rats showed a significant increase in serum glucose, insulin, cholesterol, triglycerides, cardiovascular risk indices and insulin resistance. Treatment of the fructose-fed rats with S. versicolor extract improved this metabolic profile. Fructose feeding produced a significant increase in serum tumor necrosis factor alpha and a decrease in adiponectin levels. In addition, fructose-fed rats exhibited a significant increase in liver, kidney and heart lipid peroxidation levels, and declined antioxidant defenses. Supplementation of the fructose-fed rats with S. versicolor extract reversed these alterations.
Conclusion:
S. versicolor attenuates hyperglycemia-mediated oxidative stress and inflammation, and is thus effective in improving insulin sensitivity in fructose-fed rats.
KEY WORDS: Diabetes, fructose, inflammation, insulin resistance, oxidative stress, Spirulina
INTRODUCTION
Type 2 diabetes mellitus is a metabolic disease characterized by the presence of chronic hyperglycemia that results from defective or deficient insulin [1,2]. It accounts for more than 90% of all diabetic patients [3]. According to the International Diabetes Federation, the number of patients with diabetes mellitus in 2015 was estimated to be 415 million, and is expected to increase to 642 million by 2040 [4]. Type 2 diabetes and its complications constitute a major public health problem [5]. Several lifestyle factors such as physical inactivity [6], sedentary lifestyle [7], alcohol consumption [8] and smoking [9] are of key importance to the development of Type 2 diabetes. In addition, diet is a modifiable risk factor for Type 2 diabetes. The consumption of fructose has been enormously increased in the last few centuries because of the high increase in using sucrose and high fructose syrup [10]. Previous studies have demonstrated that high fructose intake is hazardous for human beings and animals [11,12], and results in hyperlipidemia, fatty liver, and insulin resistance [13]. The metabolism of fructose in the liver increases de novo lipogenesis [14], and an increase in high fructose corn syrup consumption has been linked to a rise in obesity and metabolic disorders [11]. Fructose feeding has also been shown to provoke oxidative damage and exert disturbing effects by diminishing antioxidant defenses, and increasing generation of free radicals [15]. Thus, the use of antioxidants could offer protection against fructose-induced metabolic alterations.
Currently, there is growing interest in the usefulness of algae for the treatment of diabetes. The cyanobacterium Spirulina is gaining a more attention as a nutraceutical and as a source of potential pharmaceutical. Studies have revealed the potential properties of Spirulina including antigenotoxic, anti-carcinogenic, immunostimulants, anti-inflammatory, anti-hepatotoxic, anti-diabetic and anti-hypertensive. Spirulina is a well-known source of anti-oxidant and anti-inflammatory molecules [16] such as c-phycocyanin, vitamins, β-carotene, phenolic compounds γ-linolenic acid and minerals [17,18]. Spirulina maxima (Arthrospira maxima), Spirulina platensis (Arthrospira platensis) and Spirulina fusiformis (Arthrospira fusiformis) are the most intensively investigated species of Spirulina [17,19,20]. Recently, the preliminary anti-diabetic effect of Spirulina versicolor was reported in the study of AbouZid et al. [21] in streptozotocin/nicotinamide-induced diabetic mice. The authors reported that S. versicolor exerts anti-hyperglycemic effect, depending on assaying fasting and postprandial blood glucose levels in diabetic mice. To the best of our knowledge, nothing has yet been reported on the beneficial effects of S. versicolor in fructose-fed rats. Therefore, the current study was undertaken to investigate the anti-hyperglycemic, insulin sensitizing, anti-hyperlipidemic and antioxidant effects of S. versicolor in high fructose-fed rats. This investigation could provide an understanding of the anti-diabetic mechanism of S. versicolor.
METHODS
Preparation of S. versicolor extract
S. versicolor was purchased from Harraz medicinal plant company, Cairo, Egypt (www.harrazegypt.com). The algae was ground to a fine powder and extracted by 80% aqueous ethanol. Following filtration, the filtrate was concentrated under reduced pressure in a rotary evaporator and was stored at −20°C until use.
Experimental Animals
Male Wistar rats weighing 130-150 g, obtained from animal house of the National Research Centre (El-Giza, Egypt), were included in the present investigation. The animals were housed in plastic well-aerated cages at a normal atmospheric temperature (25±2°C) and normal 12 h light/dark cycle. Rats had free access to water and were supplied daily with laboratory standard diet of known composition. All animal procedures were undertaken with the approval of Institutional Animal Ethics Committee of Beni-Suef University (Egypt).
Experimental Design
About 24 rats were allocated into 4 groups, each consisting of six (n = 6) animals and were subjected to the following treatments:
Group 1 (Control): Received the vehicle 1% carboxymethylcellulose (CMC) and served as control rats.
Group 2 (Control + S. versicolor): Received 50 mg/kg b.wt. S. versicolor extract suspended in 1% CMC and served as drug control.
Group 3 (Diabetic): Received 30% fructose in tap water.
Group 4 (Diabetic + S. versicolor): Received 30% fructose in tap water and 50 mg/kg b.wt. S. versicolor extract suspended in 1% CMC.
Rats were fed 30% fructose solution in drinking water for 4 weeks, and biochemical parameters were estimated. Rats exhibited hyperglycemia and hyperinsulinemia were selected for further subsequent studies. S. versicolor extract has been administered by oral gavage for 4 weeks. The doses were balanced consistently as indicated by any change in body weight to keep up the comparable dosage for every kg body weight over the entire period of study.
Samples Preparation
By the end of the experiment, overnight fasted animals were sacrificed, and blood samples were collected, left to coagulate and centrifuged at 3000 rpm for 15 min to separate serum. Liver, kidney, and heart samples were immediately excised and perfused with ice-cold saline. Frozen samples (10% w/v) were homogenized in chilled saline, and the homogenates were centrifuged at 3000 rpm for 10 min. The clear homogenates were collected and used for subsequent assays.
Biochemical Study
Oral glucose tolerance test (OGTT)
On the day before sacrifice, OGTT was performed using blood samples obtained from lateral tail vein of rats deprived of food overnight. Successive blood samples were then taken at 30, 60, 90 and 120 min following the administration of glucose solution (3 g/kg b.wt.). Blood samples were left to coagulate, centrifuged, and clear sera were obtained for determination of glucose concentration according to the method of Trinder [22] using reagent kit purchased from Spinreact (Spain).
Determination of Serum Insulin, Adiponectin and Tumor Necrosis Factor Alpha (TNF-α)
Serum levels of insulin, adiponectin and TNF-α were determined using specific ELISA kits (R&D systems) following the manufacturer’s instructions. The concentrations of assayed parameters were measured specrophotometrically at 450 nm. Standard curves were constructed by using standard proteins and concentrations of the unknown samples were determined from the standard plots.
Determination of Homeostasis Model of Insulin Resistance (HOMA-IR)
The insulin resistance was evaluated by homeostasis model assessment estimate of insulin resistance (HOMA-IR) [23] as follows:

Determination of Lipid Profile and Cardiovascular Risk Indices
Serum total cholesterol [24], triglycerides [25] and high density lipoprotein (HDL)-cholesterol [26] were assayed using commercial diagnostic kits (Spinreact, Spain). Serum very low density lipoprotein (vLDL)-cholesterol concentration was calculated according to the following formula [27]: vLDL-cholesterol = triglycerides/5. Serum LDL-cholesterol level was calculated from the formula [28]: LDL-cholesterol = Total cholesterol - ([Triglycerides/5] + HDL-cholesterol]. Cardiovascular risk indices were calculated according to Ross [29] as follows: Cardiovascular risk index 1 = Total cholesterol/HDL-cholesterol and cardiovascular risk index 2 = LDL-cholesterol/HDL-cholesterol. Antiatherogenic index (AAI) was determined according to the following equation [30]: AAI = HDL-cholesterol × 100/Total cholesterol - HDL-cholesterol.
Assay of Serum Enzymes
Serum aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK-MB) activities were assayed using reagent kits purchased from Biosystems (Spain) following the methods of Schumann and Klauke [31], Teitz and Andresen [32] and Kachmar and Moss [33], respectively.
Assay of Lipid Peroxidation and Antioxidant Defenses
Lipid peroxidation levels in liver, kidney, and heart homogenates were assayed by measurement of malondialdehyde (MDA) formation according to the method of Preuss et al. [34]. Reduced glutathione (GSH) content and activity of the antioxidant enzymes superoxide dismutase (SOD) and GSH peroxidase (GPx) were measured according to the methods of Beutler et al. [35] Marklund and Marklund [36] and Matkovics et al. [37], respectively.
Statistical Analysis
Data were analyzed using Graph Pad Prism 5 software and all statistical comparisons were made by means of the one-way ANOVA test followed by Tukey’s test post hoc analysis. Results were articulated as mean ± standard error of the mean (SEM) and a P value < 0.05 was considered significant.
RESULTS
S. versicolor Represses Hyperglycemia and Insulin Resistance in Fructose-fed Rats
OGTT of the fructose-induced diabetic rats showed significantly (P < 0.001) elevated glucose levels and at all points of the OGTT when compared with the normal control rats [Figure 1a]. Oral supplementation of S. versicolor extract to fructose-fed rats significantly alleviated the blood glucose levels. The OGTT area under the curve (AUC) showed non-significant (P > 0.05) difference between the control and S. versicolor supplemented control rats. On the other hand, fructose-induced diabetic rats exhibited a significant (P < 0.01) increase in AUC when compared with the control rats. Treatment of the diabetic rats with S. versicolor markedly (P < 0.01) decreased OGTT AUC when compared with the diabetic control rats, as depicted in Figure 1b.
Figure 1.
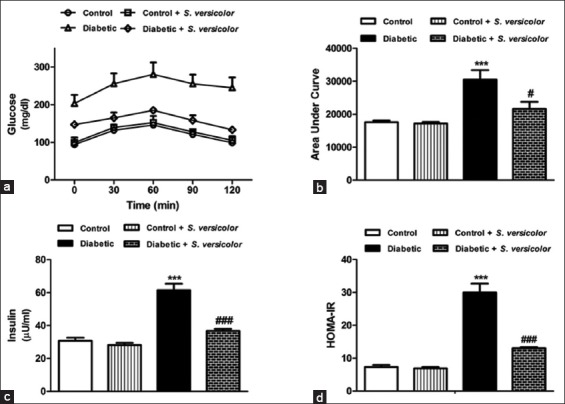
Effect of Spirulina versicolor on (a and b) glucose tolerance, (c) serum insulin and (d) homeostasis model of insulin resistance. Results are mean ± standard error of the mean (n = 6). ***P < 0.001 versus control, and #P < 0.05, and ###P < 0.001 versus diabetic group
Serum insulin level was significantly (P < 0.001) increased in fructose fed rats compared with the control group as depicted in Figure 1c. Oral treatment with S. versicolor markedly ameliorated serum insulin levels in the fructose-induced diabetic rats. Similarly, diabetic rats exhibited a significant (P < 0.001) increase in HOMA-IR, an effect that was reversed by oral administration of S. versicolor to fructose-induced diabetic rats [Figure 1d].
S. versicolor Exerts Anti-hyperlipidemic, Cardioprotective and Anti-atherogenic effects in Fructose-fed Rats
Data represented in Table 1 show the effect of S. versicolor on lipid profile, cardiovascular risk indices, heart marker enzymes and antiatherogenic index of control and diabetic rats. Compared with the control group, rats supplemented with S. versicolor exhibited non-significant (P > 0.05) changes in all lipid profile parameters. On the other hand, fructose-induced diabetic rats exhibited significant increase in serum total cholesterol (P < 0.001), triglycerides (P < 0.01), LDL-cholesterol (P < 0.001) and vLDL-cholesterol (P < 0.01) when compared with the control group. Serum levels of HDL-cholesterol showed a non-significant (P > 0.05) difference between all studied groups. In addition, diabetic rats showed significantly (P < 0.001) increased HDL-cholesterol/T. cholesterol and LDL-cholesterol/HDL-cholesterol. In addition, the antiatherogenic index was significantly (P < 0.05) declined in diabetic rats. By comparison, the oral supplementation of S. versicolor extract to diabetic rats potentially ameliorated the altered serum lipid profile as well as cardiovascular risk indices.
Table 1.
Effect of S. versicolor on serum lipid profile, cardiovascular risk indices and antiatherogenic index in control and fructose-fed rats
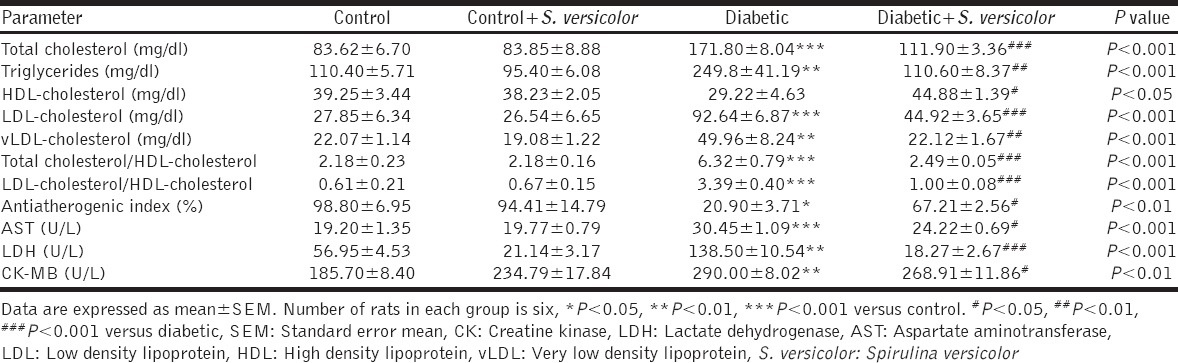
Serum AST, CK-MB and LDH activities were significantly increased in the fructose-induced diabetic rats when compared with the control group [Table 1]. Treatment of the diabetic rats with S. versicolor extract significantly ameliorated serum activities of AST (P < 0.05), CK-MB (P < 0.05) and LDH (P < 0.001).
S. versicolor Increases Circulating Adiponectin and Decreases TNF-α in Fructose-fed Rats
Fructose-fed rats exhibited markedly (P < 0.01) declined serum adiponectin levels when compared with the control group, as represented in Figure 2a. Treatment of the fructose-induced diabetic rats with S. versicolor extract significantly (P < 0.01) alleviated serum adiponectin levels. The effect of S. versicolor on serum levels of TNF-α in control and fructose-induced diabetic rats showed a significantly (P < 0.01) increased levels of TNF-α (P < 0.001) in diabetic rats and potential (P < 0.05) alleviation following treatment with S. versicolor extract [Figure 2b].
Figure 2.
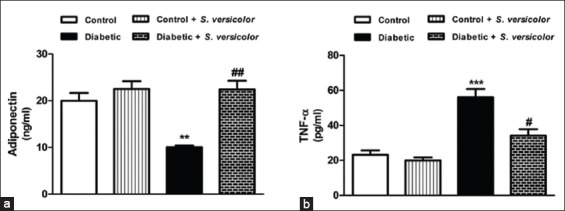
Effect of Spirulina versicolor on (a) serum adiponectin and (b) tumor necrosis factor alpha. Results are mean ± standard error of the mean (n = 6). **P < 0.01, and ***P < 0.001 versus control, and #P < 0.05, and ##P < 0.01 versus diabetic group
S. versicolor Attenuates Hyperglycemia-induced Oxidative Stress in Liver, Kidney and Heart of Fructose-fed Rats
Fructose-induced diabetic rats showed significantly increased MDA levels in liver (P < 0.001), kidney (P < 0.01) and heart (P < 0.01) when compared with the control rats [Figure 3a]. Treatment of the fructose-induced diabetic rats with S. versicolor extract significantly alleviated liver (P < 0.01), kidney (P < 0.001) and heart (P < 0.001) lipid peroxidation levels. Oral supplementation of S. versicolor to normal rats produced significant (P < 0.05) decrease in kidney MDA content, with no effect exerted on liver and heart.
Figure 3.
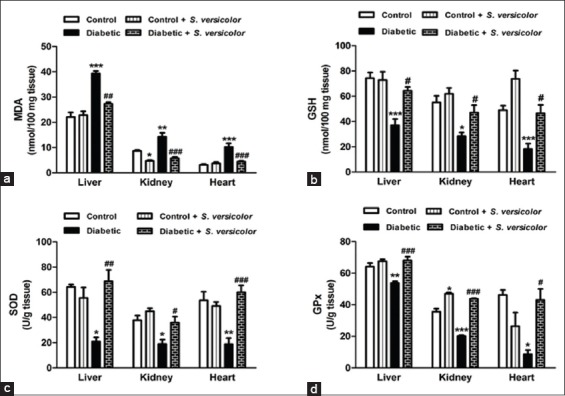
Effect of Spirulina versicolor on (a) lipid peroxidation, (b) reduced glutathione, (c) superoxide dismutase and (d) glutathione peroxidase in liver, kidney and heart. Results are mean ± standard error of the mean (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 versus control, and #P < 0.05, ##P < 0.01, and ###P < 0.001 versus diabetic group
On the contrary, fructose supplementation significantly decreased liver (P < 0.001), kidney (P < 0.05) and heart (P < 0.001) GSH content when compared with the control group, as represented in Figure 3b. Similarly, SOD activity was significantly decreased in the liver (P < 0.05), kidney (P < 0.05) and heart (P < 0.01) of fructose-induced diabetic rats when compared with the control group [Figure 3c]. GPx activity showed a similar pattern where it was significantly decreased in the liver (P < 0.01), kidney (P < 0.001) and heart (P < 0.05) of fructose-induced diabetic rats, as depicted in Figure 3d. On the other hand, treatment of the fructose-induced diabetic rats with S. versicolor extract potentially ameliorated GSH content as well as activities of SOD and GPx in the liver, kidney and heart.
DISCUSSION
Several studies have demonstrated the deleterious effects of fructose on insulin sensitivity and glucose metabolism [38]. In the present study, fructose-fed rats showed significantly impaired glucose tolerance accompanied with hyperinsulinemia and increased HOMA-IR. Therefore, it is suggested that insulin resistance has been developed in these animals. This would closely reflect the natural history and metabolic characteristics of human diabetes, and it is further sensitive to pharmacological testing [2]. Long term fructose feeding has been demonstrated to induce diabetes associated with insulin resistance in experimental animals [38-41]. The fructose-induced insulin resistance may be linked to alteration of insulin signaling. In this context, high fructose feeding has been reported to decrease insulin receptor substrate (IRS)-1 phosphorylation in rat skeletal muscles [42]. In addition, fructose-induced hyperlipidemia [43] and fat deposition [44] may generate lipid-derived metabolites which reduce insulin signaling via increasing serine/threonine phosphorylation of IRS-1 [45]. Oral supplementation of S. versicolor extract markedly reduced blood glucose and improved insulin sensitivity in fructose-fed rats. Although the anti-hyperglycemic effect of different Spirulina species has been previously reported, studies demonstrating the anti-diabetic efficacy of S. versicolor are scarce. In this context, Mani et al. [46] showed a significant decrease in the fasting blood sugar level of patients received 2 g/day Spirulina for 21 days, and Layam et al. [47] proved the same effect in diabetic rats treated with 15 mg/kg Spirulina for 45 days. The hypoglycemic effect of Spirulina could perhaps attributed to its high fiber content that diminish glucose absorption [48], or to the possible action of peptides generated by the digestion of Spirulina proteins [49].
Insulin resistance in Type 2 diabetes is also associated with hyperlipidemia and atherosclerosis [50]. Fructose-fed rats in the present investigation exhibited hypercholesterolemia and hypertriglyceridemia. The fructose-induced hyperlipidemia may be attributed to the increased de novo hepatic lipogenesis through providing large amounts of hepatic triose-phosphate for fatty acid synthesis [14]. In addition, fructose increases the expression of key lipogenic enzymes and induces the expression of sterol regulatory element binding protein-1c which is the principal inducer of hepatic lipogenesis [51,52]. Moreover, fructose has been demonstrated to activate carbohydrate-responsive element binding protein (ChREBP), leading to up-regulated expression of hepatic fatty acid synthase and acetyl-CoA carboxylase [53]. Activation of ChREBP may be attributed to the fructose-induced expression of glucose-6-phosphate dehydrogenase and intermediary substrates of the hexose-monophosphate shunt [54].
The elevated triglycerides and cholesterol levels in the fructose-induced diabetic rats represent atherogenic lipid profile. The recorded values of atherogenic indices in the present study showed the bad impact of fructose-induced dyslipidemia on the cardiovascular system. These findings were confirmed by the elevated serum levels of AST, CK-MB, and LDH. Treatment of the diabetic rats with S. versicolor extract significantly ameliorated the altered lipid profile and atherogenic indices. Reduction of these indices in treated fructose-fed rats strongly supported the notion that dietary supplementation with S. versicolor may reduce the risk of developing heart diseases. These findings were further confirmed by the significantly decreased serum activities of the cardiac markers, CK-MB, LDH and AST, in S. versicolor treated fructose-fed rats. The anti-hyperlipidemic effects of Spirulina sp. have been demonstrated in animal [55,56] and human studies [57-59].
The beneficial effects of S. versicolor extract in fructose-induced diabetic rats might be explained, at least in part, through its ability to increase serum adiponectin levels. Serum level of adiponectin is in agreement with insulin sensitivity and its reduced levels are associated with insulin resistance [60]. Adiponectin regulates glucose metabolism [61], increases muscle fat oxidation and glucose transport mediated [62], inhibits hepatic gluconeogenesis [63] and activates peroxisome proliferator activated receptor-a leading to decreased triglyceride content in skeletal muscles and liver [64]. We also assume that suppression of the release of TNF-α following S. versicolor administration could be a direct result of increased serum adiponectin levels. Adiponectin is well known to inhibit the expression of the pro-inflammatory cytokine TNF-α in various tissues [65]. TNF-α diminishes the ability of insulin to stimulate peripheral glucose uptake and to suppress hepatic glucose production [66], and increases circulating free fatty acids; thus contributes to the pathogenesis of insulin resistance [67]. In the present study, treatment of the fructose-induced diabetic rats with S. versicolor markedly decreased serum levels of TNF-α, confirming its anti-inflammatory efficacy.
Oxidative stress has been implicated in fructose-induced insulin resistance and Type 2 diabetes in rats [13]. Oxidative stress can cause oxidation and damage to many cellular components such as DNA, lipids and proteins [68]. Reactive oxygen species (ROS) in diabetes could react with polyunsaturated fatty acids leading to lipid peroxidation [69]. In addition, high levels of free radicals and the simultaneous decline in endogenous antioxidants can lead to damage of cellular organelles, and development of insulin resistance [70]. Hence, it was recommended by Mahmoud et al. [2] that therapy with antioxidants represents a useful pharmacologic overture to the management of diabetes. The present findings showed significant elevation in lipid peroxidation levels in liver, kidney and heart of fructose-administered rats. Treatment of the fructose-fed rats with S. versicolor extract significantly decreased lipid peroxidation levels, reflecting its radical scavenging property.
In contrary, GSH and the antioxidant enzymatic defenses showed a simultaneous decrease in the liver, kidney and heart of fructose-induced diabetic rats. Antioxidant defenses are known to decrease under hyperglycemia [71] and oxidative stress [72]. Treatment of diabetic rats with S. versicolor significantly increased levels of GSH and activity of the antioxidant enzymes SOD and GPx. GSH is an endogenous antioxidant that protects against oxidative stress-induced cellular damage by reacting with oxidants or as a substrate for GPx. SOD and GPx provide a defense system against ROS-induced cellular damage [73]. The antioxidant effect of Spirulina and their constituents has been previously demonstrated. Ahmed et al. [74] reported that S. versicolor extract protected against diethylnitrosamine-induced hepatocarcinogenesis through potentiating the antioxidant defense system.
Several studies have reported the in vitro and in vivo antioxidant and/or anti-inflammatory efficacies of Spirulina and its extracts, suggesting the beneficial effects of Spirulina in managing insulin resistance and diabetes. The antioxidant and anti-inflammatory effects of Spirulina Sp. could be attributed to its active constituents. Spirulina contains a relative high concentration of β-carotene, provitamin A, vitamin B, vitamin C, vitamin D, vitamin E, ω-3 and ω-6 polyunsaturated fatty acids, and phycocyanin [75]. Phycocyanin has the ability to scavenge free radicals, decrease nitrite production, suppress inducible nitric oxide synthase expression, and inhibit liver microsomal lipid peroxidation. In addition, phycocyanin has been reported to inhibit pro-inflammatory cytokine formation, suppress cyclooxygeanase-2 expression and decrease prostaglandin E2 production [76-78]. Another constituent, β-carotene, has been reported to have antioxidant and anti-inflammatory activities [79,80].
CONCLUSION
The current findings provide new information on the antidiabetic mechanism of S. versicolor in fructose-fed rats. High fructose feeding induces insulin resistance, inflammation and oxidative stress. Oral administration of S. versicolor ameliorates insulin sensitivity, increases serum adiponectin, and attenuates oxidative stress and inflammation in diabetic rats. Our findings suggest that S. versicolor extract could be used as a dietary supplement in diabetes management, pending further studies to trace out its exact mechanistic pathways.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced Type 2 diabetic rats. J Diabetes Complications. 2012;26:483–90. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi BK, Srivastava AK. Diabetes mellitus: Complications and therapeutics. Med Sci Monit. 2006;12:RA130–47. [PubMed] [Google Scholar]
- 4.International Diabetes Federation (IDF) IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. Available from: http://www.idf.org/diabetesatlas . [Google Scholar]
- 5.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to Type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of Type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 8.Cullmann M, Hilding A, Östenson CG. Alcohol consumption and risk of pre-diabetes and Type 2 diabetes development in a Swedish population. Diabet Med. 2012;29:441–52. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109:538–42. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 10.Regy J, Padmaja G. Comparative studies on the production of glucose and high fructose syrup from tuber starches. Int Res J Biol Sci. 2013;2:68–75. [Google Scholar]
- 11.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 13.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60:1259–70. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 15.Faure P, Rossini E, Lafond JL, Richard MJ, Favier A, Halimi S. Vitamin E improves the free radical defense system potential and insulin sensitivity of rats fed high fructose diets. J Nutr. 1997;127:103–7. doi: 10.1093/jn/127.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Chu WL, Lim YW, Radhakrishnan AK, Lim PE. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med. 2010;10:53. doi: 10.1186/1472-6882-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershwin ME, Belay AE. Spirulina in Human Nutrition and Health. Boca Raton, London, New York: CRC Press Taylor & Francis Group; 2008. [Google Scholar]
- 18.Kelly Moorhead K, Capelli B, Cysewski GR. Spirulina Nature’s Super Food. Hawaii: Cyanotech Corporation; 1993. pp. 1–63. [Google Scholar]
- 19.Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373–9. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- 20.Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: Evidence-based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. doi: 10.1093/ecam/nen058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AbouZid SF, Ahmed OM, Ahmed RR, Mahmoud A, Abdella E, Ashour MB. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J Med Food. 2014;17:400–6. doi: 10.1089/jmf.2013.0068. [DOI] [PubMed] [Google Scholar]
- 22.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 23.Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342:1040–2. doi: 10.1056/NEJM200004063421408. [DOI] [PubMed] [Google Scholar]
- 24.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 25.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- 26.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 27.Tietz NW. Clinical Guide to Laboratory Tests. Philadelphia: W. B. Saunders Co; 1995. [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis. In: Braunwald E, editor. Heart Disease: A Textbook of Cardiovascular Medicine. 4th ed. Philadelphia, PA: W. B. Saunders; 1992. pp. 1106–24. [Google Scholar]
- 30.Guido S, Joseph T. Effect of chemically different calcium antagonists on lipid profile in rats fed on a high fat diet. Indian J Exp Biol. 1992;30:292–4. [PubMed] [Google Scholar]
- 31.Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta. 2003;327:69–79. doi: 10.1016/s0009-8981(02)00341-8. [DOI] [PubMed] [Google Scholar]
- 32.Tietz NW, Andresen BD. Textbook of Clinical Chemistry. Philadelphia: W. B. Saunders; 1986. [Google Scholar]
- 33.Kachmar JF, Moss DW. Fali naslov poglavlja. In: Tietz NW, editor. Fundamentals of Clinical Chemistry. 2nd ed. Philadelphia, PA: W. B. Saunders Co; 1976. p. 682. [Google Scholar]
- 34.Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 1998;17:116–23. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- 35.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 36.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 37.Matkovics B, Szabo L, Varga IS. Determination of enzyme activities in lipid peroxidation and glutathione pathways (in Hungarian) Lab Diagn. 1998;15:248–9. [Google Scholar]
- 38.Mahmoud AM, Hozayen WG, Soliman HA, Mostafa SR. Enteromorpha flexuosa improves insulin sensitivity and metabolic control in fructose-induced diabetic rats. J Endocrinol Diabetes Obes. 3(2):1072. [Google Scholar]
- 39.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 40.Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Dodonaea viscosa (L). Lacq. aerial parts in high fructose-fed insulin resistant rats: A mechanism based study. Indian J Exp Biol. 2010;48:800–10. [PubMed] [Google Scholar]
- 41.Padiya R, Khatua TN, Bagul PK, Kuncha M, Banerjee SK. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab (Lond) 2011;8:53. doi: 10.1186/1743-7075-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eiffert KC, McDonald RB, Stern JS. High sucrose diet and exercise: Effects on insulin-receptor function of 12- and 24-mo-old Sprague-Dawley rats. J Nutr. 1991;121:1081–9. doi: 10.1093/jn/121.7.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bantle JP, Laine DC, Thomas JW. Metabolic effects of dietary fructose and sucrose in Types 1 and 2 diabetic subjects. JAMA. 1986;256:3241–6. [PubMed] [Google Scholar]
- 44.Crapo PA, Kolterman OG. The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr. 1984;39:525–34. doi: 10.1093/ajcn/39.4.525. [DOI] [PubMed] [Google Scholar]
- 45.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani S, Iyer UM, Subramanian S. Studies on the effect of Spirulina supplementation in control of diabetes mellitus. In: Subramanian G, editor. Cyanobacterial Biotechnology. USA: Science Publishers Inc; 1998. pp. 301–4. [Google Scholar]
- 47.Layam A, Chandra L, Reddy K. Antidiabetic property of Spirulina. Diabetol Croat. 2006;35:29–33. [Google Scholar]
- 48.Samuels R, Mani UV, Iyer UM, Nayak US. Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food. 2002;5:91–6. doi: 10.1089/109662002760178177. [DOI] [PubMed] [Google Scholar]
- 49.Mani UV, Desai S, Iyer UM. Studies on long term effect of Spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J Nutraceut. 2000;2:25–32. [Google Scholar]
- 50.Prashantha Kumar BR, Praveen TK, Nanjan MJ, Karvekar MD, Suresh B. Serum glucose and triglycerides lowering activity of some novel glitazones against dexamethasone-induced hyperlipidemia and insulin resistance. Indian J Pharmacol. 2007;39:299–302. [Google Scholar]
- 51.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–32. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H, Tamura Y, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–9. doi: 10.2337/diabetes.53.3.560. [DOI] [PubMed] [Google Scholar]
- 53.Denechaud PD, Dentin R, Girard J, Postic C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett. 2008;582:68–73. doi: 10.1016/j.febslet.2007.07.084. [DOI] [PubMed] [Google Scholar]
- 54.Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta. 2008;1782:341–8. doi: 10.1016/j.bbadis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Iwata K, Inayama T, Kato T. Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J Nutr Sci Vitaminol (Tokyo) 1990;36:165–71. doi: 10.3177/jnsv.36.165. [DOI] [PubMed] [Google Scholar]
- 56.Torres-Durán PV, Miranda-Zamora R, Paredes-Carbajal MC, Mascher D, Blé-Castillo J, Díaz-Zagoya JC, et al. Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethnopharmacol. 1999;64:141–7. doi: 10.1016/s0378-8741(98)00120-2. [DOI] [PubMed] [Google Scholar]
- 57.Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in Type 2 diabetes mellitus. J Med Food. 2001;4:193–199. doi: 10.1089/10966200152744463. [DOI] [PubMed] [Google Scholar]
- 58.Lee EH, Park JE, Choi YJ, Huh KB, Kim WY. A randomized study to establish the effects of Spirulina in Type 2 diabetes mellitus patients. Nutr Res Pract. 2008;2:295–300. doi: 10.4162/nrp.2008.2.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamalpreet K, Rajbir S, Kiran G. Effect of supplementation of Spirulina on blood glucose and lipid profile of the non-insulin dependent diabetic male subjects. J Dairy Foods Home Sci. 2008;27:3–4. [Google Scholar]
- 60.Statnick MA, Beavers LS, Conner LJ, Corominola H, Johnson D, Hammond CD, et al. Decreased expression of apM1 in Omental and subcutaneous adipose tissue of humans with Type 2 diabetes. Int J Exp Diabetes Res. 2000;1:81–8. doi: 10.1155/EDR.2000.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 62.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CC, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–61. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–63. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 65.Bastard JP, Piéroni L, Hainque B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes Metab Res Rev. 2000;16:192–201. doi: 10.1002/1520-7560(200005/06)16:3<192::aid-dmrr114>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 66.Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- 67.Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, et al. Mapping of early signaling events in tumor necrosis factor-alpha -mediated lipolysis in human fat cells. J Biol Chem. 2002;277:1085–91. doi: 10.1074/jbc.M109498200. [DOI] [PubMed] [Google Scholar]
- 68.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–68. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karthikesan K, Pari L, Menon VP. Combined treatment of tetrahydrocurcumin and chlorogenic acid exerts potential antihyperglycemic effect on streptozotocin-nicotinamide-induced diabetic rats. Gen Physiol Biophys. 2010;29:23–30. doi: 10.4149/gpb_2010_01_23. [DOI] [PubMed] [Google Scholar]
- 70.Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–6. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 71.Aragno M, Mastrocola R, Catalano MG, Brignardello E, Danni O, Boccuzzi G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53:1082–8. doi: 10.2337/diabetes.53.4.1082. [DOI] [PubMed] [Google Scholar]
- 72.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 73.Viswanatha Swamy AH, Kulkarni RV, Thippeswamy AH, Koti BC, Gore A. Evaluation of hepatoprotective activity of Cissus quadrangularis stem extract against isoniazid-induced liver damage in rats. Indian J Pharmacol. 2010;42:397–400. doi: 10.4103/0253-7613.71920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed OM, Ashour BM, Fahim HE, Mahmoud AM, Ahmed NA. Preventive effect of Spirulina versicolor and Enteromorpha flexuosa ethanolic extracts against diethylnitrosamine/benzo(a)pyrene-induced hepatocarcinogenecity in rats. J Int Acad Res Multidiscipl. 2014;2:633–50. [Google Scholar]
- 75.Huang Z, Guo BJ, Wong RN, Jiang Y. Characterization and antioxidant activity of selenium containing phycocyanin isolated from Spirulina platensis. Food Chem. 2007;100:1137–43. [Google Scholar]
- 76.Patel A, Mishra S, Ghosh PK. Antioxidant potential of C-phycocyanin isolated from cyanobacterial species Lyngbya, Phormidium and Spirulina spp. Indian J Biochem Biophys. 2006;43:25–31. [PubMed] [Google Scholar]
- 77.Riss J, Décordé K, Sutra T, Delage M, Baccou JC, Jouy N, et al. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem. 2007;55:7962–7. doi: 10.1021/jf070529g. [DOI] [PubMed] [Google Scholar]
- 78.Cherng SC, Cheng SN, Tarn A, Chou TC. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007;81:1431–5. doi: 10.1016/j.lfs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, et al. Beta-carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med. 2005;37:323–34. doi: 10.1038/emm.2005.42. [DOI] [PubMed] [Google Scholar]
- 80.Katsuura S, Imamura T, Bando N, Yamanishi R. beta-Carotene and beta-cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol Nutr Food Res. 2009;53:1396–405. doi: 10.1002/mnfr.200800566. [DOI] [PubMed] [Google Scholar]


