Abstract
Aim:
In the present study, we report a cost-effective, eco-friendly, and an efficient alternative method for large scale production of silver nanoparticles (AgNPs) from Adansonia digitata fruit pulp extract. The study mainly focused on the synthesis, characterization, and antimicrobial properties of AgNPs.
Materials and Methods:
Synthesis of AgNPs with the help of standard protocol and characterized by ultraviolet (UV)-vis spectrophotometry, Fourier transform infra-red (FTIR), X-ray diffractometer (XRD), atomic force microscopy (AFM), scanning electron microscopy (SEM) with EDAX, transmission electron microscopy (TEM) and explore their potential growth inhibitory effect on 07 bacterial and 05 fungal pathogens.
Results:
The synthesized AgNPs are characterized by UV-vis spectrophotometry shows a broad peak at 434 nm. The FTIR spectroscopic analysis clearly reveals phenols and proteins are main responsible for reduction and stabilization of nanoparticles. XRD studies show the nanoparticles are crystalline in nature owing 44 nm in size. EDAX spectrum shows a 33.28 weight percentage of Ag metal in the reaction medium confirms the purity of AgNPs. High resolution and magnification studies with AFM, SEM, and TEM reveal the nanoparticles are polydispersed, spherical in shape, having the size range from 3 to 57 nm without any agglomeration between the particles. Further, the antimicrobial studies reveal the potentiality of nanoparticles against different microbial pathogens.
Conclusion:
The present study is mainly focused on the synthesis of AgNPs from A. digitata fruit pulp extract. Here, we succeed to synthesize a narrow range of particles and validate its potential antimicrobial activity on different microorganisms. Based on this, we conclude that A. digitata pulp extract is a good source toward the reduction of AgNPs and acts as environment benign antimicrobial agents.
KEY WORDS: Adansonia digitata, antimicrobial activity, characterization, fruit pulp, silver nanoparticles
INTRODUCTION
Synthesis of metal nanoparticles with the help of plant extracts is an emerging field of nanotechnology, due to their novel properties, terrific applications in biomedicine and its eco-friendly nature [1]. In recent past great efforts were made for synthesis of environment benign and eco-friendly nanoparticle from plants such as iron oxide nanoparticles from Medicago sativa [2], copper nanoparticles from Magnolia kobus [3], calcium nanoparticles from Boswellia ovalifoliolata [4], gold nanoparticles from Avena sativa [5], zinc oxide nanoparticles from Catharanthus roseus [6], and silver nanoparticles (AgNPs) from Syzygium alternifolium [7]. Silver is one among the metal nanoparticles focused much interest due to its wide variety of applications [8]. It has different biological activities such as antimicrobial [9], anthelmintic [10], antilarvicidic [11], antioxidant [12], anticancer [13], anti-inflammatory [14], hepatoprotective [15], and wound healing activity [10]. Conventional methods for synthesizing AgNPs are mainly by chemical, physical, and microbe-mediated synthesis. In these chemical and physical methods, usage of hazardous chemicals, high energy requirements, difficult and wasteful materials generate potential and biological hazards to the environment [16]. Whereas in the case of microbe-mediated synthesis is not feasible industrially due its lab maintenance. Therefore biological synthesis of AgNPs by using plant materials is easy, efficient, and eco-friendliness in comparison to chemical mediated or microbe-mediated synthesis [17] and they possess secondary metabolites having the redox capacity to reduce metal nanoparticles in an easiest way [18].
Adansonia digitata L. belongs to the family Malvaceae is a large tree indigenous to Africa and found in many countries also. Traditionally, the fruit pulp dissolved in water or milk is used as a drink or sauce for food in Africa. Fruit pulp and powdered seeds are used for the treatment of diarrhea and dysentery in India [19]. The high content of Vitamin C in fruit pulp is recommended to pregnant women’s for daily intake [20]. In recent times, different scientists prove different activities of pulp such as hepatoprotective [21], antimicrobial [22], antiviral [23], antioxidant [24], antidiarrheal [25], hypoglycemic [26], anti-inflammatory, and antioxidant [27] activities. Due to high medicinal values and mythological significance of this plant is known as “Kalpavriksha” (a tree which fulfill all desires) in India [28]. In our previous studies, synthesis of AgNPs from stem bark and leaf extract of A. digitata acts as excellent reducing agents and show potential antimicrobial activity against different microorganisms [29,30]. However, the potentiality of fruit pulp mediated synthesis of AgNPs is not carried out so far. Hence, the present study is aimed to synthesize AgNPs from A. digitata fruit pulp extract, characterize and to know the potentiality of AgNPs against different microbial pathogens.
MATERIALS AND METHODS
Collection of Plant Material
2 to 4 kg weight mature fruits possess a great amount of pulp is collected from Acharya Ranga Agricultural University, Tirupati and cross checked by herbarium (Voucher no: SVU362) deposited in Department of Botany, Sri Venkateswara University, Tirupati. Cut open the fruit and separate the seeds adhesive to pulp. Grounded the collected pulp with the help of mortar and pestle, sieved the content for the synthesis of nanoparticles.
Extract Preparation and Synthesis of AgNPs
For synthesis, 25 g of fruit pulp is extracted with 100 ml of distilled water on boiling water bath for 30 min, filter the content with Whatman No. 1 filter paper and stored at room temperature. From this, 5 ml of the extract is taken into 250 ml of Erlenmeyer conical flask and titrated against with the solution of 1 mM AgNO3 at a 60-80°C temperature. The contents are cooled and centrifuged at 10,000 rpm for 20 min to avoid the presence of any biological impurities, and it is used for further characterization and antimicrobial studies.
Characterization of AgNPs
Confirmation of synthesized nanoparticles is AgNPs by ultraviolet (UV)-vis spectrophotometry absorption spectra using a spectro UV 2080 double beam, between the wavelength scan range of 190-700 nm, 1200 L/mm spectrophotometer, Analytical technologies, India. To know the possible bio-molecules responsible for reduction and stabilization of AgNPs by Fourier transform infra-red (FTIR) spectra in the scan range of 4,000 to 500 cm−1 transmittance with an ALPHA interferometer, Bruker, Ettlingen, Karlsruhe, Germany by KBr pellet method. X-ray diffraction of synthesized nanoparticles is examined by an X-ray diffractometer (XRD) (Shimadzu, XRD-6000) equipped with Cu Ka radiation source using Ni as a filter at a setting of 30 kV/30 mA to know the crystalline nature of AgNPs. Purity of AgNPs was analyzed by FEI Quanta 200 FEG high resolution (HR)-scanning electron microscopy (SEM) machine equipped with EDAX instrument. To know the size, shape, agglomeration pattern, and dispersed nature of the nanoparticles are done by atomic force microscopy (AFM) by NOVA NT-MDT SOLVER NEXT, Russia. SEM by FEI Quanta 200 FEG HR-SEM machine. Transmission electron microscopy (TEM) using HF-3300 advanced 300 kV TEM/STEM from Hitachi.
Antimicrobial Studies of AgNPs
The antimicrobial activity of biologically synthesized nanoparticles are analyzed on seven pathogenic bacteria such as Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538 (Gram-positive), Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 43816, Proteus vulgaris ATCC 13315, Pseudomonas aeruginosa ATCC 15442, Salmonella typhimurium ATCC 14028 (Gram-negative), and five fungal pathogens such as Alternaria solani ATCC 32904, Aspergillus flavus ATCC 9643, Aspergillus niger ATCC 16404, Penicillium chrysogenum ATCC 11709, and Trichoderma harzianum ATCC 20476 procured from Department of Microbiology, Sri Venkateswara University, Tirupati. Disc diffusion method [31] was followed for testing antimicrobial activity against biologically synthesized AgNPs, and comparative studies were made with plant pulp extract, 1 mM AgNO3 as negative controls and streptomycin or fluconazole as a standard for bacteria and fungi, respectively. 7 mm sterile discs are prepared from Whatman no. 1 filter paper and 20 µl of plant extract, 1 mM AgNO3 solutions, and 10 µg/disc streptomycin/fluconazole are loaded on separate discs and allowed to air dry for 1 h at sterile conditions. Apart from these, a 5, 10, 20, 40, 60, 80 µg/ml concentrations of synthesized AgNPs are tested separately to know the minimum inhibitory concentration. 20 and 40 µg/ml concentration of AgNPs show minimum inhibitory effect and 80 µg/ml concentrations of AgNPs show maximum inhibitory concentration. Due to this, we prefer 80 µg/ml concentrations of AgNPs to check the antimicrobial activity on different microbial organisms. Freshly prepared nutrient agar media for bacteria and potato dextrose agar media for fungi are poured into sterile petri plates, allowed to 30 min for solidification. The plates are swabbed with 100 µl of microbial cultures and placed the previously prepared discs; the experiment is carried out in triplicates. The plates are incubated at 37°C for 24-48 h, and then the zone of inhibition is measured with the help of a scale and tabulated the results.
RESULTS
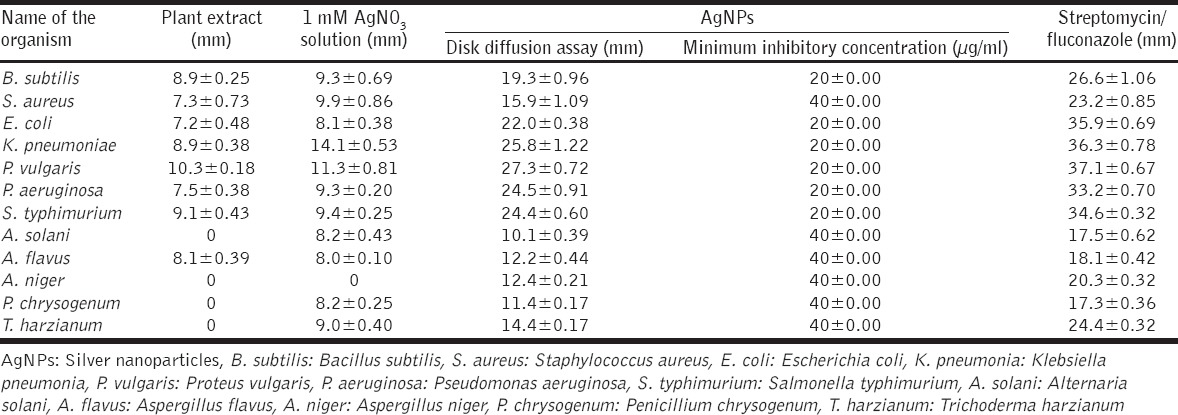
Reduction of Ag+ into Ag0 nanoparticles was observed visually by means of a color change pattern of the reaction medium. A. digitata fruit pulp having thick cream color, upon synthesis the color change from creamy to light yellow indicates the formation of nanoparticles. This color changed nanoparticles solution was analyzed by UV-vis spectrophotometry shows a broad absorption peak at 434 nm further confirms the formation of nanoparticles are AgNPs [Figure 1]. FTIR spectroscopic studies of these synthesized nanoparticles show broad transmittance peaks at 3322 cm-1 assigned for an O-H bond of phenols and 1636 cm−1 assigned for an N-H bond of primary amines [Figure 2]. XRD spectrum of synthesized AgNPs shows the crystalline nature of AgNPs and gives four intensive peaks at 38.1°, 46.2°, 64.5°, and 77.3° of 2θ degrees of X-axis corresponds to 111, 200, 220, and 311 Bragg reflections of Y-axis [Figure 3]. These peaks are indexing the face-centered cubic nature of AgNPs. The mean particle diameter of synthesized AgNPs is 44 nm, calculated according to Debye-Scherrer equation (D = kλ/β cos θ) and it coincides with powder diffraction file of Joint Committee on Powder Diffraction Standards file No. 04-0783 [32]. The full width at half maximum values, i.e. k = 0.44 was derived from 38, 46, 64, and 77 Bragg reflections of the X-axis. 2 µm resolution studies of biologically synthesized AgNPs with AFM reveal the particles are polydispersed, spherical in shape, having the size range from 25 to 57 nm, and there is no agglomeration observed between the particles [Figure 4a]. Raw data obtained from this AFM microscope is treated with a specially designed image processing software (NOVA-TX) to further exploit the three-dimensional (3D) image of nanoparticles [Figure 4b]. 500 nm resolution studies with 20 kV electron energy passing through the nanoparticles coated thin films on a clean glass slide reveals the nanoparticles are spherical in shape, polydispersed and having the size range from 18 to 32 nm [Figure 5a]. The same sample was analyzed with the help of EDAX instrument shows 33.28% presence of Ag0 in the sample [Figure 5b] along with 05.29% of carbon, 03.28% of nitrogen, 31.80% of oxygen, 08.37% of sodium, 02.57% of magnesium, 01.01% of aluminum, 00.78% of silicon, 07.87% of aurum, and 05.74% of calcium [Table 1]. 33.28% of Ag metal in the sample indicates the high purity of synthesized nanoparticles. Crystalline nature of synthesized AgNPs was analyzed using selected area electron diffraction (SAED) by directing the electron beam perpendicular to nanoparticles. The SAED pattern of synthesized AgNPs shows the spots had been corresponding to 111, 200, 220, and 311 of the crystallographic nature of face-centered cubic structures [Figure 6a]. 20 nm resolution studies with 300 kV electron energy passing through nanoparticles coated thin films on copper grid show the nanoparticles are polydispersed, spherical in shape and size range from 3 to 7 nm [Figure 6b]. To know the antimicrobial potency of biologically synthesized AgNPs from fruit pulp of A. digitata was analyzed against two Gram-positive, five Gram-negative, and five fungal pathogens by disc diffusion method. Among the bacterial pathogens, the highest inhibition zones were observed in P. vulgaris followed by K. pneumoniae, P. aeruginosa, S. typhimurium, E. coli, B. subtilis, and S. aureus. Whereas in the case of fungi, the highest inhibition zones were observed in T. harzianum, followed by A. niger, A. flavus, P. chrysogenum, and A. solani. [Figure 7 and Table 2].
Figure 1.
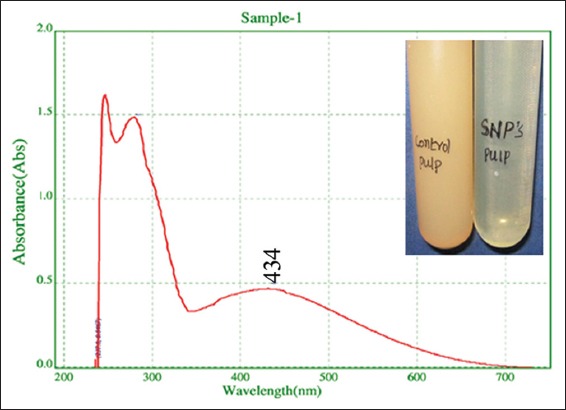
Ultraviolet-vis spectrum of synthesized silver nanoparticles shows broad peak at 434, 280, and 247 nm of narrow peaks is due interference of phytoconstituents in the medium. Inset figure shows color change pattern from cream to light yellow
Figure 2.
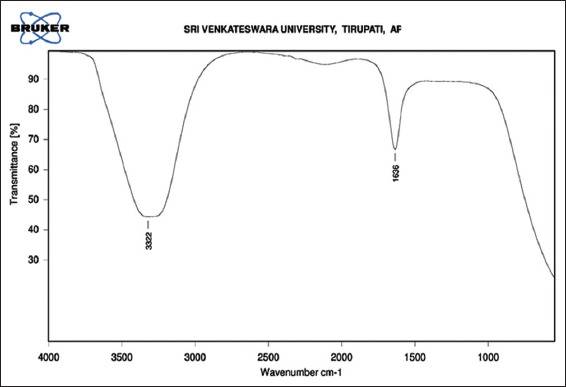
Fourier transform infra-red spectrum of synthesized silver nanoparticles shows broad peaks at 3322 cm−1 and 1636 cm−1
Figure 3.
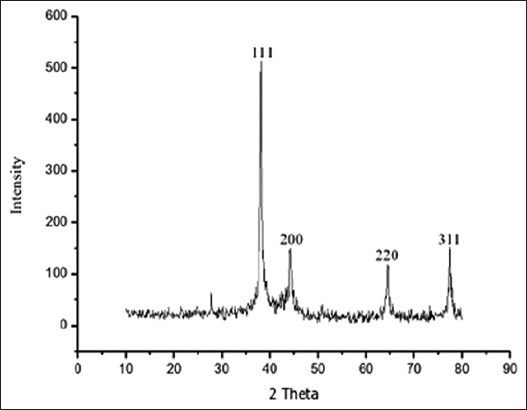
X-ray diffractometer pattern of synthesized silver nanoparticles shows four intensive peaks
Figure 4.
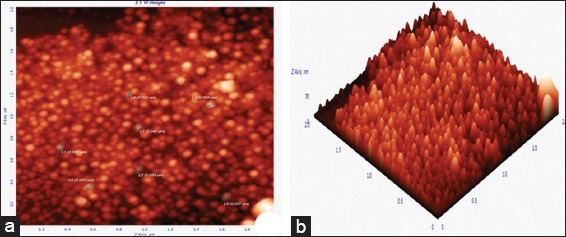
Atomic force microscopy micrograph of synthesized silver nanoparticles (AgNPs), (a) 2 µm resolution studies 25-57 nm size, spherical shaped, polydispersed particles, (b) three-dimensional image of AgNPs analyzed by NOVA-TX software
Figure 5.
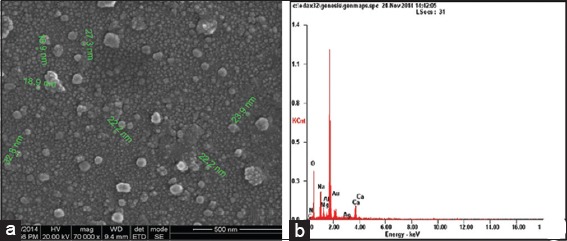
Scanning electron microscopy micrograph of synthesized silver nanoparticles (AgNPs) (a) 500 nm resolution studies shows 18-32 nm size, spherical shaped particles, (b) EDAX spectrum of synthesized AgNPs shows 33.28 weight percent of Ag metal in the sample
Table 1.
Metal analysis by EDAX spectrum shows different weight percentages of sample

Figure 6.
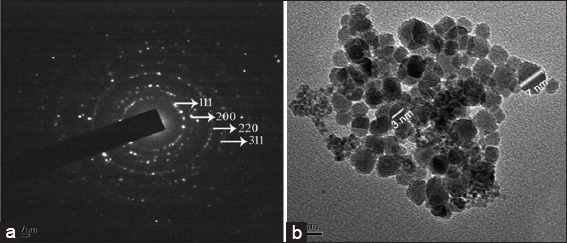
Transmission electron microscopy micrograph of synthesized silver nanoparticles (AgNPs), (a) selected area electron diffraction pattern shows characteristic crystal spots of elemental silver, (b) 20 nm resolution studies of AgNPs shows 3-7 nm size, spherical shaped nanoparticles
Figure 7.
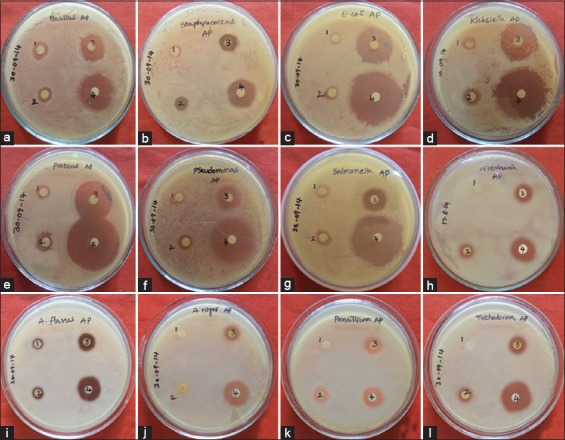
Antimicrobial studies of biologically synthesized silver nanoparticles (AgNPs) from Adansonia digitata pulp extract, (a) Bacillus subtilis, (b) Staphylococcus aureus, (c) Escherichia coli, (d) Klebsiella pneumoniae, (e) Proteus vulgaris, (f) Pseudomonas aeruginosa, (g) Salmonella typhimurium, (h) Alternaria solani, (i) Aspergillus flavus, (j) Aspergillus niger, (k) Penicillium chrysogenum, (l) Trichoderma harzianum, (1) Plant extract, (2) AgNO3, (3) AgNPs, (4) streptomycin/fluconazole
Table 2.
Zone of inhibition (mm) of AgNPs on microbial pathogens and comparative studies with different controls
DISCUSSION
When the addition of AgNO3 solution to the plant extract the color of the plant extract is changed gradually according to the quantitative addition of AgNO3 solution. This color change is due to the reduction of nanoparticles with the help of electrons present in the fruit pulp extract coming from NAD and ascorbic acid as electron donors present in the plant extracts [33]. Significantly higher amount of ascorbic acid, i.e., >300 mg/100 g was reported in fruit pulp of A. digitata [34]. This solution was subjected to UV-vis spectrophotometry between the scan ranges of 190-750 nm, a broad peak is obtained at 434 nm. The same type of results was observed in leaf extract mediated synthesis of AgNPs from Couroupita guianensis [35]. The color change pattern and broad peak obtained in UV-vis spectrophotometry are due to Surface Plasmon Resonance nature of AgNPs present in the medium. These nanoparticles absorb light at different wavelengths and excited due to charge density at the interface between conductor and insulator to give a respective peak on UV-vis spectrophotometry. FT-IR is a sensitive tool to analyze functional groups present in the biological samples. It relies on the light absorbance between 4000 cm−1 to 500 cm−1 of the electromagnetic, infrared region. In our case, the synthesized nanoparticles show broad peaks at 3322 cm−1 and 1636 cm−1. The same type of results was observed in Myristica fragrans seed extract mediated synthesis of AgNPs [36]. From this FTIR study, clearly reveals hydroxyl groups of phenols and amide groups of proteins from plant extract forming a layer to the nanoparticles and acting as a capping agent to prevent agglomeration and providing stability in the medium. Based on these FTIR studies, we suggest that the bio-molecules present in plant extracts play dual role in formation and stabilization to AgNPs.
AFM is a primary tool for analyzing size, shape, agglomeration pattern and also offers visualizations of 3D views of the nanoparticles, unlike the electron microscopes. It has an advantage over combination of HR, samples do not have to be conductive and does not require the high-pressure vacuum conditions. Better resolution and percentage presence of nanoparticles were demonstrated by SEM with EDAX instrument provides reliable characterization and morphology of the particles when compare to AFM. The four intensive Bragg reflections are appear in XRD pattern [Figure 3] is correlated with SAED pattern [Figure 6a] of AgNPs, clearly indicates the nanoparticles are crystalline in nature. Higher resolution studies with TEM analysis shows size, shape, and agglomeration pattern of nanoparticles. It achieves better resolution than SEM due to electron energies higher than 20 kV used in SEM. Finally, all the microscopic studies reveal the biologically synthesized AgNPs are spherical in shape, having the size range from 3 to 57 nm polydispersed and no agglomerations were found between the particles.
To test the inhibitory effect of biologically synthesized nanoparticles on different microorganisms shows potential antimicrobial activity. Bacterial species show the highest inhibitory activity when compare to fungi because the cell walls of fungi are made up of chitin is more rigid when compare to bacterial cell walls containing peptidoglycans. Among the bacteria, Gram-negative species show the highest inhibitory zones when to compare to Gram-positive species, because the Gram-negative species containing a less amount of peptidoglycans. Silver is a precious metal used as an effective antimicrobial agent before the advent of AgNPs. Due to overuse of silver products, decreased the efficiency of silver agents as antibiotic. In recent times with the advancement of nanotechnology, the interest in the use of AgNPs as antibacterial agents had been rekindled [37]. Antimicrobial activities of AgNPs are dependent on the size and shape of the particles. Small sized nanoparticles have higher antimicrobial activity than larger particles because they have large surface area to interact bacteria efficiently [38]. In recent times, the scientists produce 30 to 40 nm size, spherical shaped AgNPs from fruit extract of Vitis vinifera shows excellent antimicrobial activity against B. Subtilis and K. planticola [39]. 10 to 70 nm size, spherical shaped AgNPs synthesized from Emblica officinalis fruit extract shows potential antimicrobial activity against S. aureus, B. subtilis, E. coli, and K. Pneumoniae [40]. Euphorbia hirta leaf mediated synthesis of AgNPs having a spherical shape with 40-45 nm size shows good antifungal efficacy [41]. In our studies also 3-57 nm size, spherical shaped AgNPs produced from A. digitata pulp extract confirmed by HR microscopic studies with AFM, SEM, and TEM proved these AgNPs are eco-friendly antimicrobial agents against different microorganisms.
CONCLUSION
In the present study, we report an eco-friendly, non-toxic, cost-effective method for synthesis of AgNPs from A. digitata pulp extract as reducing agent. In this method, naturally occurring materials are acts as reducing agents such as bio-molecules such as phenols and proteins present in plant extract as a simple and alternative to complex physical or chemical synthetic procedures. 3 to 57 nm size, spherical shaped, polydispersed nanoparticles are produced from A. digitata pulp extract confirmed by AFM, SEM, and TEM prove this plant extract as an effective reducing agent for the production of narrow range of nanoparticles by industrial scale. Further antimicrobial studies reveal that small size, spherical shaped particles have immense activity against different microbial pathogens and acts as eco-friendly antimicrobial agents.
ACKNOWLEDGMENTS
The authors are highly thankful DST-PURSE, Sri Venkateswara University - Tirupati, JNTU-Hyderabad and IIT Madras- Chennai for providing characterization facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yugandhar P, Savithramma N. Biosynthesis, characterization and antimicrobial studies of green synthesized silver nanoparticles from fruit extract of Syzygium alternifolium (Wt.). Walp. An endemic, endangered medicinal tree taxon. Appl Nanosci. 2016;6:223–33. DOI: 10.1007/s13204-015-0428-4. [Google Scholar]
- 2.Herrera-Becerra R, Zorrilla C, Ascencio JA. Production of iron oxide nanoparticles by a biosynthesis method: An environmentally friendly route. J Phys Chem C. 2007;111:16147–53. [Google Scholar]
- 3.Lee HJ, Song JY, Kim BS. Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J Chem Technol Biotechnol. 2013;88:1971–7. [Google Scholar]
- 4.Yugandhar P, Savithramma N. Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.). Hepper. Int J Adv Res. 2013;1:89–103. [Google Scholar]
- 5.Veronica A, Isaac H, Jose RP, Miguel JY, Horacio T, Patricia S, et al. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J Nanopart Res. 2014;6:377–82. [Google Scholar]
- 6.Bhumi G, Savithramma N. Biological synthesis of zinc oxide nanoparticles from Catharanthus roseus (L.). G. Don. Leaf extract and validation for antibacterial activity. Int J Drug Dev Res. 2014;6:208–14. [Google Scholar]
- 7.Yugandhar P, Haribabu R, Savithramma N. Synthesis, characterization and antimicrobial properties of green-synthesised silver nanoparticles from stem bark extract of Syzygium alternifolium (Wt.). Walp. 3 Biotech. 2015;5:1031–9. doi: 10.1007/s13205-015-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobley CM, Skrabalak SE, Campbell DJ, Xia Y. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics. 2009;4:171–9. [Google Scholar]
- 9.Yugandhar P, Savithramma N. Leaf assisted green synthesis of silver nanoparticles from Syzygium alternifolium (Wt.). Walp. Characterization and antimicrobial studies. Nano Biomed Eng. 2015;7:29–37. doi: 10.1007/s13205-015-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seema G, Amrish C, Avijit M, Rupa M. Green synthesis of silver nanoparticles using Arnebia nobilis root extract and wound healing potential of its hydrogel. Asian J Pharm. 2014;8:95–101. [Google Scholar]
- 11.Sundaravadivelan C, Nalini Padmanabhan M, Sivaprasath P, Kishmu L. Biosynthesized silver nanoparticles from Pedilanthus tithymaloides leaf extract with anti-developmental activity against larval instars of Aedes aegypti L. (Diptera; Culicidae) Parasitol Res. 2013;112:303–11. doi: 10.1007/s00436-012-3138-9. [DOI] [PubMed] [Google Scholar]
- 12.Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci. 2014;5:73–81. [Google Scholar]
- 13.Vasanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces. 2014;117:354–9. doi: 10.1016/j.colsurfb.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Rafie HM, Hamed MA. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv Nat Sci Nanosci Nanotechnol. 2014;5:1–11. [Google Scholar]
- 15.Bhuvaneswari R, Chidambaranathan N, Jegatheesan K. Hepatoprotective effect of Embilica officinalis and its silver nanoparticles against ccl4 induced hepatotoxicity in wistar albino rats. Dig J Nanomater Biostruct. 2014;9:223–35. [Google Scholar]
- 16.Veerasamy R, Xin TZ, Gunasagaran S, Xiang TF, Yang EF, Jeyakumar N, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15:113–20. [Google Scholar]
- 17.Anamika M, Sanjukta C, Prashant MR, Geeta W. Evidence based green synthesis of nanoparticles. Adv Mater Lett. 2012;3:519–25. [Google Scholar]
- 18.Ahmad N, Sharma S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain Chem. 2012;2:141–7. [Google Scholar]
- 19.Sidibe M, Williams JT. Baobab - Adansonia digitata. Fruits for the Future. Southampton, UK: International Centre Underutilised Crops; 2002. p. 96. [Google Scholar]
- 20.Chadare FJ, Linnemann AR, Hounhouigan JD, Nout MJ, Van Boekel MA. Baobab food products: A review on their composition and nutritional value. Crit Rev Food Sci Nutr. 2009;49:254–74. doi: 10.1080/10408390701856330. [DOI] [PubMed] [Google Scholar]
- 21.Qarawi AA, Al-Damegh MA, El-Mougy SA. Hepatoprotective influence of Adansonia digitata Pulp. J Herbs Spices Med Plants. 2003;10:1–6. [Google Scholar]
- 22.Afolabi OR, Popoola TO. The effects of baobab pulp powder on the micro flora involved in temple fermentation. Eur Food Res Technol. 2005;220:187–90. [Google Scholar]
- 23.Vimalanathan S, Hudson JB. Multiple inflammatory and antiviral activities in Adansonia digitata (Baobab) leaves, fruits and seeds. J Med Plants Res. 2009;3:576–82. [Google Scholar]
- 24.Elsaid FG. The effect of seeds and fruit pulp of Adansonia digitata L. (Baobab) on ehrlich ascites carcinoma. Food Nutr Sci. 2013;4:38–46. [Google Scholar]
- 25.Abdelrahim MY, Elamin BM, Khalil DJ, El Badwi SM. Antidiarrhoeal activity of ethanolic extract of Adansonia digitata fruit pulp in rats. J Physiol Pharmacol Adv. 2013;3:172–8. [Google Scholar]
- 26.Gwarzo MY, Bako HY. Hypoglycemic activity of methanolic fruit pulp extract of Adansonia digitata on blood glucose levels of alloxan induced diabetic rats. Int J Anim Vet Adv. 2013;5:108–13. [Google Scholar]
- 27.Ibrahim AY, Mahmoud MG, Aske MM. Anti-inflammatory and antioxidant activities of polysaccharide from Adansonia digitata : An in vitro study. Int J Pharm Sci Rev Res. 2014;25:174–82. [Google Scholar]
- 28.Singh S, Parasharami V, Rai S. Medicinal uses of Adansonia digitata L.: An endangered tree species. J Pharm Sci Innov. 2013;2:14–6. [Google Scholar]
- 29.Kumar CH, Yugandhar P, Suhrulatha D, Savithramma N. Synthesis, characterization and antimicrobial studies of stem bark mediated synthesis of silver nanoparticles from Adansonia digitata (L.) J Pharm Sci Res. 2015;7:76–82. [Google Scholar]
- 30.Chennareddy MK, Yugandhar P, Nataru S. Adansonia digitata leaf extract mediated synthesis of silver nanoparticles;characterization and antimicrobial studies. J Appl Pharm Sci. 2015;5:82–9. [Google Scholar]
- 31.Cruickshank R. Medical Microbiology: A Guide to Diagnosis and Control of Infection. Edinburgh: Livingston Publishers; 1986. [Google Scholar]
- 32.Jana J, Gauri SS, Ganguly M, Dey S, Pal T. Silver nanoparticle anchored carbon dots for improved sensing, catalytic and intriguing antimicrobial activity. Dalton Trans. 2015;44:20692–707. doi: 10.1039/c5dt03858h. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad N, Sharma S, Singh VN, Shamsi SF, Fatma A, Mehta BR. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol Res Int. 2011;2011:454090. doi: 10.4061/2011/454090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalla AA, Mohammed MA, Mudawi HA. Production and quality assessment of instant baobab (Adansonia digitata L.) Adv J Food Sci Technol. 2010;2:125–33. [Google Scholar]
- 35.Devaraj P, Kumari P, Arti C, Renganathan A. Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against MCF-7 cell line. J Nanotechnol. 2013;2013:1–5. [Google Scholar]
- 36.Sharma G, Sharma AR, Kurian M, Bhavesh R, Nam JS, Lee SS. Green synthesis of silver nanoparticle using Myristica fragrans (nutmeg) seed extract and its biological activity. Digest J Nanomater Biostruct. 2014;9:325–32. [Google Scholar]
- 37.Wong KK, Liu X. Silver nanoparticles - The real silver bullet in clinical medicine. Med Chem Commun. 2010;1:125–31. [Google Scholar]
- 38.Vanaja M, Annadurai G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci. 2013;3:217–23. [Google Scholar]
- 39.Gnanajobitha G, Paulkumar K, Vanaja M, Rajeshkumar S, Malarkodi C, Annadurai G, Kannan C. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostruct Chem. 2013;3:1–6. [Google Scholar]
- 40.Ramesh PS, Kokila T, Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim Acta A Mol Biomol Spectrosc. 2015;142:339–43. doi: 10.1016/j.saa.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 41.Elumalai EK, Prasad TN, Kambala V, Nagajyothi PC, David E. Green synthesis of silver nanoparticle using Euphorbia hirta L and their antifungal activities. Arch Appl Sci Res. 2010;2:76–81. [Google Scholar]


