Abstract
Epilepsy is a major morbidity in Sturge Weber syndrome, a segmental vascular neurocutaneous disorder classically associated with facial angiomas, glaucoma, and leptomeningeal capillary-venous type vascular malformations. The extent of the latter correlates with neurological outcome. Post-zygotic mosaicism for the activating mutation p.R183Q of the GNAQ gene has been identified as the major cause. GNAQ encodes for an alpha subunit of a heterotrimeric G protein critical to blood vessel development. The earlier the timing of the mutation in development, the more severe the involvement, e.g. from isolated port-wine stains to the full syndrome. The strongest predictors of adverse outcomes are MRI and the presence of angiomas involving any part of the forehead, delineated inferiorly from the outer canthus of the eye to the top of the ear, and including the upper eyelid. The neurological course may be progressive and the typical constellation of symptoms is focal onset seizures, hemiparesis, headache, stroke-like episodes, behavior problems, intellectual disability, and visual field deficits. Antiseizure medications are effective in about half of patients. The presence of localized seizures, focal neurological deficits, and drug resistant epilepsy indicate epilepsy surgical evaluation. Earlier seizure onset, i.e. before six months of age, is associated with a more severe course with significant residual deficits. Factors contributing to epileptogenesis include decreased brain tissue perfusion due to abnormal venous drainage, anoxic injury contributing to cerebral calcification, breakdown of the blood-brain barrier, and the presence of developmental cortical malformations. Pre-symptomatic prophylactic treatment may be a future option to modify the course of the disease including the associated epileptogenesis.
Keywords: epilepsy, Sturge Weber syndrome, seizures, epileptogenesis, neurocutaneous syndromes
Introduction
The phakomatoses or neurocutaneous syndromes comprise a heterogeneous group of diseases that are mostly hereditary and characterized by the association of skin lesions with a variety of central and/or peripheral nervous system manifestations. Of the common phakomatoses, tuberous sclerosis and Sturge-Weber syndrome (SWS) feature epilepsy as a major morbidity to a complex phenotype of variable severity. SWS is a segmental vascular neurocutaneous disorder classically associated with facial angiomas known as port-wine stains, glaucoma associated with vascular ocular abnormalities, and leptomeningeal capillary-venous type vascular malformations. The extent of the latter correlates with the neurological outcome.
Recent groundbreaking research identified a somatic activating mutation of the gene GNAQ as the likely cause of the majority of cases of SWS as well as non-syndromic port-wine stains. Interestingly, the timing of the somatic mutation in GNAQ during development likely impacts the clinical phenotype 1.
The neurological manifestations in SWS are often progressive. Brain involvement is common with the capillary malformation causing progressive epilepsy and cerebral atrophy. The extent of the capillary malformation is correlated with the severity of seizures, extent of atrophy, and cognitive outcome. The pathophysiological processes leading to epileptogenesis and atrophy are not entirely known. This review outlines possible mechanisms of epileptogenesis in SWS 2.
Pathophysiology
A somatic activating mutation in the GNAQ (p.R183Q) gene was identified in the affected skin of individuals with non-syndromic port-wine stains and in SWS patients 1. Thus, post-zygotic mosaicism for this GNAQ mutation has been described as the major cause of SWS.
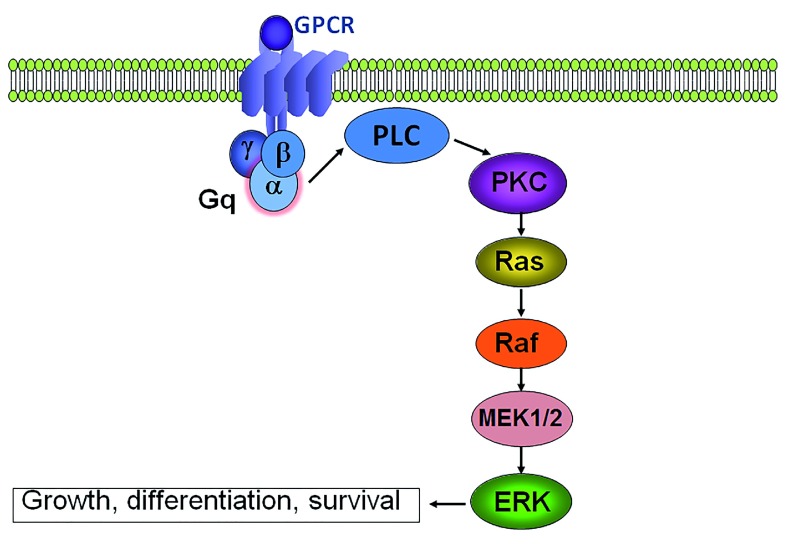
GNAQ encodes Gαq, an alpha subunit of the heterotrimeric G-protein that links G-protein-coupled receptors to activation of phospholipase C (PLC), transient increases in cytosolic calcium, and activation of Rac and Rho. The arginine (R) residue at position 183 in Gαq is a conserved amino acid in the GTP-binding pocket. R183Q mutation leads to a decrease in function of the GTPase and to constitutive activation of downstream effector pathways. Several of the G-protein-coupled receptors linked to Gαq, such as Gβ and Gγ subunits, are critical to blood vessel development and function; therefore, the abnormal signaling may result in vascular malformations 3.
Gαq effectors increase downstream signaling through the RAS signaling pathway ( Figure 1), and this is an implicated mechanism to explain the increased cell proliferation and inhibition of apoptosis in the affected skin and leptomeningeal capillary malformation samples in patients with SWS. The cell of origin affected by the mutation is not yet known 4, 5. Recent research showed that endothelial cells in capillary malformations are enriched for GNAQ mutations and are likely responsible for the pathophysiology underlying capillary malformations 6. It is likely that the mutation occurs earlier in development in SWS than in isolated port-wine stains, thus affecting an earlier progenitor with wider potential downstream effects. Somatic mutations in GNAQ at other amino acids are also seen in uveal melanoma and more recently in the extended spectrum of clinical presentation from phakomatosis pigmentovascularis (PPV) to extensive dermal melanocytosis 7. Based on the diversity of conditions and spectrum of severity, it seems that the mutation occurring at different times in development will influence the phenotype and severity of the condition. Microscopic examination of SWS brain tissue shows deposition of calcium in the cortex, hypoplastic blood vessels, gliosis, and sometimes loss of neurons or focal cortical dysgenesis 8, 9. The current evidence suggests that observed malformation of brain development in patients with SWS is likely secondary to abnormal vascular development concomitant to the cortical developmental stages.
Figure 1. Schematic representation of GNAQ (encodes for Gαq) signaling via RAS/ERK pathway.
Clinical considerations on neurological aspects of SWS
The clinical course of SWS is variable and can be devastating. In a cohort of 192 patients with facial port-wine stains, a strong predictor of adverse outcomes was an angioma involving any part of the forehead, delineated at its inferior border by a line joining the outer canthus of the eye to the top of the ear, and including the upper eyelid 10. Abnormal MRI was a better predictor of all clinical adverse outcome measures than port-wine stain distribution, although for practical reasons guidelines based on clinical phenotype are proposed. Typically, the progressive neurological problems evolve over time causing medication-resistant epilepsy and brain atrophy. The constellation of symptoms is characterized by focal onset of seizures, hemiparesis, headache, stroke-like episodes, behavioral problems, intellectual disability, and visual field defects.
Seizures occur in 75–80% of all SWS patients and in over 90% of patients with bilateral involvement. Onset of seizures occurs in 75% of children before 1 year of age, 86% by 2 years of age, and 95% by the age of 5 years. Medications are effective in preventing seizures in approximately 50% of patients. Patients with localized seizures, focal neurological deficits, and drug-resistant epilepsy should be considered for epilepsy surgery 11, 12.
Based on the revised International League Against Epilepsy (ILAE) classification, the most common seizure type is focal seizures with an observable motor component and without impairment of awareness. Seizures with variable degrees of impairment of consciousness are also frequently observed along with autonomic features and evolution to bilateral convulsive events. Seizures can be subtle, and prompt recognition of an epileptic episode is important because prolonged seizures and status epilepticus are commonly seen 13.
About 30% of cases may have onset of seizures during febrile episodes, and there is an increased susceptibility for fever-induced seizures at any age 14. Apneic seizures have been associated with SWS and present in nearly half of drug-resistant patients who require epilepsy surgery (Pinto et al., in press). Unusual seizure types such as ictal laughter have also been described in SWS patients 15. Children with epilepsy due to focal lesions can develop secondary bilateral synchrony. There are also reports of myoclonic astatic seizures, infantile spasms including asymmetric features, and prolonged post-ictal paralysis 15, 16.
Some patients with SWS may present with initial clusters of frequent epileptic events and then remain seizure-free for several months or years. Remissions may last several months before recurrence occurs. The variable course of the epilepsy renders surgical disposition difficult, and the trajectory of associated developmental progress or decline is often a deciding factor 14.
A descriptive study of 77 children and adults with epilepsy secondary to SWS disclosed a dichotomy based on age at seizure onset. Early onset patients (onset of seizures before 6 months of age) had a severe early course with significant residual deficits, while late-onset patients (onset of seizures after 6 months of age) did better. Focal cerebral atrophy worsened in early onset cases. The course of the epilepsy in the late-onset cohort stabilized after 5 years of age in most cases. The authors described that aspirin use correlated with stable course of epilepsy in six patients. Leptomeningeal enhancement appears to increase on imaging studies during acute events before returning to baseline, suggesting that extent of disease is probably best judged during the interictal state. The use of aspirin routinely is still controversial 17, 18.
Electroencephalography in SWS
The typical electroencephalogram (EEG) in patients with SWS consists of asymmetric background frequencies and voltages 19. A large cohort study with emphasis on EEG evolution analyzed 81 EEGs from 44 children and adults with SWS. This study documented the evolutional changes that have been previously described. Recordings evolve to show asymmetric slowing over the course of 1 year; 2 to 3 years later, interictal epileptiform activity with focal sharp waves and increasingly frequent spike discharges appear. One limitation of the study was the potential modification of the EEG pattern due to modern anti-seizure medications or treatment with low-dose aspirin. The detailed EEG analysis did not, however, show a correlation between the EEG severity score and clinical function or seizure control 20.
Atypical findings in ictal EEG have been reported. For instance, patients with unilateral brain insults have shown ictal contralateral slow waves. A patient reported with this pattern became seizure free after hemispherectomy, and the abnormalities seen in the healthy contralateral hemisphere were not a sign of an ictal hemisphere but instead could indicate prominent ischemic changes resulting from a vascular steal phenomenon during the seizure 21.
Most recently, analysis of infraslow EEG activity (ISA) was effective in identifying refractory subclinical focal status epilepticus in a pediatric patient who presented with a 96-hour refractory encephalopathy and non-ischemic hemiparesis, which successfully resolved after midazolam administration. In general, ISA has shown potential in the evaluation of patients with epilepsy and in the differentiation between focal and generalized epilepsies 22.
Neuroimaging in SWS
Contrast-enhanced MR studies are the most accurate single imaging studies to demonstrate the extent of brain involvement; contrast enhanced T2-weighted FLAIR images improve detection of leptomeningeal disease compared to post-contrast T1-weighted images 22– 24. Other typical features include enlargement of the choroid plexus, cortical calcifications, and cerebral atrophy. The demonstration of the extent of the capillary malformation is critical in determining the patient’s prognosis and the necessary approach for cortical resection for epilepsy surgery 24, 25. Dynamic MR perfusion studies suggest hypoperfusion in the underlying brain is connected to the abnormal pial angioma. Cerebral hypoperfusion is essentially secondary to impaired venous drainage. Perfusion imaging reveals increased mean transit time and in more severe cases reduced regional blood flow 25– 27. Proton MR spectroscopy of the affected brain region reveals elevated choline, reduced N-acetylaspartate, and slightly elevated lactate, probably resulting from ongoing ischemia and secondary gray and white matter injury. The increased choline peak is possibly related to accelerated myelination observed in early stages of SWS. Multimodality neuroimaging can be of use to identify areas at risk for future metabolic and functional deterioration 24.
Positron emission tomography/single photon emission computed tomography
Patients with refractory seizures, including those with SWS, often undergo functional studies in preparation for surgery. Given the unique neurovascular coupling mechanism in SWS, functional studies should be interpreted carefully because the vascular malformation in SWS is associated with impairment of the cerebral hemodynamic response to seizure activity.
Increased glucose metabolism detected by positron emission tomography (PET) has been observed in the lesioned hemisphere. A prospective cohort study was designed with the objective of verifying the significance of interictal hypermetabolism on PET images of children with SWS 28. The authors studied the prevalence and clinical correlates of focal increases in cerebral glucose metabolism with seizure onset as well as evolution to drug-resistant epilepsy. Patients with foci of increased cortical glucose metabolism were significantly younger than those with decreased cortical metabolism. Interictal glucose hypermetabolism in young children with SWS is most often seen within a short time before or after the onset of first clinical seizures, and this finding was associated with the presumed period of epileptogenesis. Increased glucose metabolism detected by PET may predict future demise of the affected cortex based on a progressive loss of metabolism and may be an imaging marker of the most malignant cases of intractable epilepsy requiring surgery in SWS 28.
Single photon emission computed tomography (SPECT) detects cerebral blood flow (CBF) asymmetry in infants with SWS, which tends to shift with age. The cortex involved in the vascular malformation is hyperperfused during the first year of life before seizure onset. The classic hypoperfusion appears after 1 year of age, even in patients who do not experience seizures 29. The hypoperfusion seems to result from post-ictal phenomena as well as chronic ischemia. SPECT imaging generally demonstrates hypoperfusion in the diseased tissue. The affected cerebral tissue typically shows increased CBF during the ictal state. Decreased blood flow during seizures, however, can also be observed. These findings point to the variable results of functional studies in SWS that might lead to miscalculation of the lesioned area while planning for surgery 30.
Previous studies using SPECT imaging have shown massive steal phenomena in affected areas during seizures, which could lead to a critical ischemic condition in remote brain regions. Therefore, progressive worsening could be partially explained by repeated seizures with severe ischemia of previously unaffected brain parenchyma 21.
Role of ischemia in epileptogenesis
The hallmark signs of SWS are tortuous and abnormal vascular structures in thickened leptomeninges. These vessels all have similar appearance, caliber, and morphological characteristics. The areas of capillary malformation are described as capillary-venous type vascular malformations. Underlying brain tissue can be atrophic and display neuronal loss, astrogliosis, dysgenic cortex, and calcification in the cortical layers. The cortical vessels underlying the meningeal angiomas are thin-walled, narrowed by hyalinization and subendothelial proliferation, and increased in number. Cerebral angiography demonstrates an aberrant pattern of both the arterial and venous cerebral circulation. Along with areas of arterial thrombosis, there is abnormal venous drainage, with manifest paucity of the superficial draining veins, venous occlusions, and alternative venous flow through deep subependymal channels 11, 12.
Low brain tissue perfusion due to abnormal venous drainage is part of the central mechanism of brain damage in SWS. Decreased quantitative white matter perfusion has been associated with frequent seizures, duration of epilepsy, and brain atrophy 31. The authors used MR perfusion-weighted imaging (PWI) of the affected cerebral white matter to study dynamic perfusion abnormalities in children with SWS. They concluded that increased perfusion in mostly younger patients may represent a transient phenomenon before severe brain atrophy occurs in the affected brain regions. Decreased perfusion is associated with high seizure frequency, long epilepsy duration, and severe brain atrophy, suggesting a detrimental effect of chronic seizures on brain structure and function.
Regional perfusion and cortical metabolic abnormalities can extend beyond lobes affected by leptomeningeal vascular malformations and are related to epilepsy in SWS. PWI along with fluorodeoxyglucose PET (FDG-PET) has been used to study the relationship between regional metabolic and perfusion abnormalities in SWS. While decreased perfusion was associated with hypometabolism in most cases, increased regional CBF was commonly associated with relatively mild or no hypometabolism. Despite a general correlation between perfusion and metabolism, increased white matter perfusion with preserved cortical metabolism in overlying cortex is a common pattern of a perfusion/metabolic mismatch. This may represent a disease stage where cortical function is preserved while increased white matter perfusion provides collateral drainage of cortex via the deep venous system 32.
Calcification as source of seizure
Anoxic injury to endothelial cells secondary to impaired venous drainage may contribute to cerebral calcification. However, enhancement of leptomeningeal vessels and enlarged deep venous vessels suggest breakdown of the blood–brain barrier; thus, calcification could also result from increased blood vessel permeability. The calcification typically occurs exclusively in areas subjacent to the abnormal vasculature. It begins in the subcortical white matter and later develops in the cortex affecting predominantly layers II and III. The common sites are the parietal-occipital regions 33.
In a study of 15 children with unilateral SWS, the degree of cortical calcification was assessed using susceptibility-weighted imaging (SWI) while perfusion status was quantified using dynamic susceptibility contrast PWI (DSC-PWI). Prospective data show that prominent calcification in the affected hemisphere reflects markedly decreased perfusion in underlying white matter and is associated with more severe epilepsy in SWS patients 33.
Epilepsy in SWS associated with cortical malformation
Although some malformations of cortical development are caused by environmental insults that occur during cortical development in utero, genetic factors also play a critical role in the pathogenesis of many cortical malformations 34. Morphological anomalies have been reported on histological examination of surgical samples from SWS patients. One of the common findings associated with developmental cortical malformations in patients with SWS is polymicrogyria. Like other types of cortical malformations, there is a range of severity from very focal, unilateral forms to extensive bilateral involvement. Traditionally, many cases of polymicrogyria were thought to result from environmental insults during later stages of cortical development, typically during cortical organization following neuronal migration. However, some genetic causes have recently been identified 9, 35.
Future research
Given the high incidence of epilepsy in patients with SWS, pre-symptomatic prophylactic treatment has been proposed. A prospective study showed possible improvement in cognitive impairment in a group that were given prophylactic treatment 36. Perhaps the use of anti-seizure drug therapy before clinical seizure onset can modify the course of severe epilepsy in SWS patients. Multicenter studies with appropriate patient selection and medication choices will be helpful to study this possibility.
SWS is a progressive and potentially devastating neurocutaneous disorder that typically evolves over time and may result in drug-resistant epilepsy and brain atrophy. Future research aimed at understanding the mechanisms of this condition and comorbidities will have a high impact on our understanding of early stages of brain involvement and will provide the means to assess responses to novel interventions. Multicenter studies are imperative to determine possible candidates for early biomarkers for brain involvement prior to irreversible structural changes.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jerome Engel Jr, Department of Neurology, David Geffen School of Medicine, UCLA, Los Angeles, California, USA
Jeffrey Noebels, Department of Neurology, Baylor College of Medicine, Houston, Texas, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Shirley MD, Tang H, Gallione CJ, et al. : Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368(21):1971–1979. 10.1056/NEJMoa1213507 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Lo W, Marchuk DA, Ball KL, et al. : Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol. 2012;54(3):214–223. 10.1111/j.1469-8749.2011.04169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimple AJ, Bosch DE, Giguère PM, et al. : Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev. 2011;63(3):728–749. 10.1124/pr.110.003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakashima M, Miyajima M, Sugano H, et al. : The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J Hum Genet. 2014;59(12):691–693. 10.1038/jhg.2014.95 [DOI] [PubMed] [Google Scholar]
- 5. Mizuno N, Itoh H: Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals. 2009;17(1):42–54. 10.1159/000186689 [DOI] [PubMed] [Google Scholar]
- 6. Couto JA, Huang L, Vivero MP, et al. : Endothelial Cells from Capillary Malformations Are Enriched for Somatic GNAQ Mutations. Plast Reconstr Surg. 2016;137(1):77e–82e. 10.1097/PRS.0000000000001868 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Thomas AC, Zeng Z, Rivière JB, et al. : Mosaic activating mutations in GNA11 and GNAQ are associated with Phakomatosis Pigmentovascularis and Extensive Dermal Melanocytosis. J Invest Dermatol. 2016; pii: S0022-202X(16)00332-8. 10.1016/j.jid.2015.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Prayson RA, Bingaman W, Frater JL, et al. : Histopathologic findings in 37 cases of functional hemispherectomy. Ann Diagn Pathol. 1999;3(4):205–212. 10.1016/S1092-9134(99)80052-5 [DOI] [PubMed] [Google Scholar]
- 9. Maton B, Krsek P, Jayakar P, et al. : Medically intractable epilepsy in Sturge-Weber syndrome is associated with cortical malformation: implications for surgical therapy. Epilepsia. 2010;51(2):257–267. 10.1111/j.1528-1167.2009.02304.x [DOI] [PubMed] [Google Scholar]
- 10. Maslin JS, Dorairaj SK, Ritch R: Sturge-Weber Syndrome (Encephalotrigeminal Angiomatosis): Recent Advances and Future Challenges. Asia Pac J Ophthalmol (Phila). 2014;3(6):361–367. 10.1097/APO.0000000000000093 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Comi AM: Presentation, diagnosis, pathophysiology, and treatment of the neurological features of Sturge-Weber syndrome. Neurologist. 2011;17(4):179–184. 10.1097/NRL.0b013e318220c5b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caraballo R, Bartuluchi M, Cersósimo R, et al. : Hemispherectomy in pediatric patients with epilepsy: a study of 45 cases with special emphasis on epileptic syndromes. Childs Nerv Syst. 2011;27(12):2131–2136. 10.1007/s00381-011-1596-5 [DOI] [PubMed] [Google Scholar]
- 13. Berg AT, Millichap JJ: The 2010 revised classification of seizures and epilepsy. Continuum (Minneap Minn). 2013;19(3 Epilepsy):571–597. 10.1212/01.CON.0000431377.44312.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sudarsanam A, Ardern-Holmes SL: Sturge-Weber syndrome: from the past to the present. Eur J Paediatr Neurol. 2014;18(3):257–266. 10.1016/j.ejpn.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 15. Barnett W, Vieregge P, Kömpf D: Gelastic epilepsy: Sturge-Weber syndrome with seizure facilitation. Schweiz Arch Neurol Psychiatr. 1995;146(2):61–63. [PubMed] [Google Scholar]
- 16. Ewen JB, Comi AM, Kossoff EH: Myoclonic-astatic epilepsy in a child with Sturge-Weber syndrome. Pediatr Neurol. 2007;36(2):115–117. 10.1016/j.pediatrneurol.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 17. Kossoff EH, Ferenc L, Comi AM: An infantile-onset, severe, yet sporadic seizure pattern is common in Sturge-Weber syndrome. Epilepsia. 2009;50(9):2154–2157. 10.1111/j.1528-1167.2009.02072.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Lance EI, Sreenivasan AK, Zabel TA, et al. : Aspirin use in Sturge-Weber syndrome: side effects and clinical outcomes. J Child Neurol. 2013;28(2):213–218. 10.1177/0883073812463607 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Ewen JB, Kossoff EH, Crone NE, et al. : Use of quantitative EEG in infants with port-wine birthmark to assess for Sturge-Weber brain involvement. Clin Neurophysiol. 2009;120(8):1433–1440. 10.1016/j.clinph.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kossoff EH, Bachur CD, Quain AM, et al. : EEG evolution in Sturge-Weber syndrome. Epilepsy Res. 2014;108(4):816–819. 10.1016/j.eplepsyres.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Limotai C, Go CY, Baba S, et al. : Steal phenomenon in Sturge-Weber syndrome imitating an ictal electroencephalography change in the contralateral hemisphere: report of 2 cases. J Neurosurg Pediatr. 2015;16(2):212–216. 10.3171/2014.12.PEDS14238 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Bello-Espinosa LE: Infraslow status epilepticus: A new form of subclinical status epilepticus recorded in a child with Sturge-Weber syndrome. Epilepsy Behav. 2015;49:193–197. 10.1016/j.yebeh.2015.04.031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Griffiths PD, Coley SC, Romanowski CA, et al. : Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol. 2003;24(4):719–723. [PMC free article] [PubMed] [Google Scholar]
- 24. Nabbout R, Juhász C: Sturge-Weber syndrome. Handb Clin Neurol. 2013;111:315–321. 10.1016/B978-0-444-52891-9.00037-3 [DOI] [PubMed] [Google Scholar]
- 25. Benedikt RA, Brown DC, Walker R, et al. : Sturge-Weber syndrome: cranial MR imaging with Gd-DTPA. AJNR Am J Neuroradiol. 1993;14(2):409–415. [PMC free article] [PubMed] [Google Scholar]
- 26. Lin DD, Barker PB, Hatfield LA, et al. : Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. J Magn Reson Imaging. 2006;24(2):274–281. 10.1002/jmri.20627 [DOI] [PubMed] [Google Scholar]
- 27. Evans AL, Widjaja E, Connolly DJ, et al. : Cerebral perfusion abnormalities in children with Sturge-Weber syndrome shown by dynamic contrast bolus magnetic resonance perfusion imaging. Pediatrics. 2006;117(6):2119–2125. 10.1542/peds.2005-1815 [DOI] [PubMed] [Google Scholar]
- 28. Alkonyi B, Chugani HT, Juhász C: Transient focal cortical increase of interictal glucose metabolism in Sturge-Weber syndrome: implications for epileptogenesis. Epilepsia. 2011;52(7):1265–1272. 10.1111/j.1528-1167.2011.03066.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Pinton F, Chiron C, Enjolras O, et al. : Early single photon emission computed tomography in Sturge-Weber syndrome. J Neurol Neurosurg Psychiatry. 1997;63(5):616–621. 10.1136/jnnp.63.5.616 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Oguz KK, Senturk S, Ozturk A, et al. : Impact of recent seizures on cerebral blood flow in patients with Sturge-Weber syndrome: study of 2 cases. J Child Neurol. 2007;22(5):617–620. 10.1177/0883073807302597 [DOI] [PubMed] [Google Scholar]
- 31. Miao Y, Juhász C, Wu J, et al. : Clinical correlates of white matter blood flow perfusion changes in Sturge-Weber syndrome: a dynamic MR perfusion-weighted imaging study. AJNR Am J Neuroradiol. 2011;32(7):1280–1285. 10.3174/ajnr.A2540 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Alkonyi B, Miao Y, Wu J, et al. : A perfusion-metabolic mismatch in Sturge-Weber syndrome: a multimodality imaging study. Brain Dev. 2012;34(7):553–562. 10.1016/j.braindev.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Wu J, Tarabishy B, Hu J, et al. : Cortical calcification in Sturge-Weber Syndrome on MRI-SWI: relation to brain perfusion status and seizure severity. J Magn Reson Imaging. 2011;34(4):791–798. 10.1002/jmri.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Guerrini R, Dobyns WB: Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014;13(7):710–726. 10.1016/S1474-4422(14)70040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang DD, Blümcke I, Coras R, et al. : Sturge-Weber Syndrome Is Associated with Cortical Dysplasia ILAE Type IIIc and Excessive Hypertrophic Pyramidal Neurons in Brain Resections for Intractable Epilepsy. Brain Pathol. 2015;25(3):248–255. 10.1111/bpa.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Ville D, Enjolras O, Chiron C, et al. : Prophylactic antiepileptic treatment in Sturge-Weber disease. Seizure. 2002;11(3):145–150. 10.1053/seiz.2001.0629 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation