Abstract
Deep vein thrombosis and common complications, including pulmonary embolism and post thrombotic syndrome, represent a major source of morbidity and mortality worldwide. Experimental models of venous thrombosis have provided considerable insight into the cellular and molecular mechanisms that regulate thrombus formation and subsequent resolution. Here we critically appraise the ex vivo and in vivo techniques used to assess venous thrombosis in these models. Particular attention is paid to imaging modalities, including magnetic resonance imaging, micro computed tomography and high frequency ultrasound that facilitate longitudinal assessment of thrombus size and composition.
Keywords: venous thrombosis, animal model, imaging
INTRODUCTION
Deep vein thrombosis (DVT) is a common condition with an annual incidence of approximately 1 in 1000 in the general population and can lead to fatal pulmonary embolism (PE). Together these conditions account for a greater number of deaths in the United Kingdom than those caused by breast cancer, road traffic accidents, and AIDS combined 1, 2. Approximately one third of patients with DVT develop post-thrombotic syndrome, a chronic condition characterised by persistent limb pain, swelling, and ulceration, which carries a significant health and economic burden, and is associated with a reduced quality of life 3.
Treatment of DVT currently involves systemic anticoagulation, aimed at preventing secondary thrombotic events, and catheter directed thrombolysis, which in the acute setting has proven effective in removal of the thrombus. However, both of these therapeutic strategies give rise to pathological bleeding in a significant number of cases and may be contraindicated in specific patient subpopulations. Novel treatments that either prevent thrombus formation or hasten resolution without these side effects are desirable, and are likely to arise from a better understanding of the molecular and cellular mechanisms that control venous thrombosis.
Experimental models of DVT have been developed in a variety of animals, including the mouse 4, rat 5, rabbit 6, dog 7, pig 8, and non-human primates 9. Spontaneous, symptomatic DVT is not observed in these animals with researchers instead relying on a number of physical or chemical interventions on a given vessel (such as ligation or ferric chloride) to induce thrombosis. Although the coagulation and fibrinolytic systems in non-human primates most closely resemble those in man, the use of these species present researchers with both financial and ethical dilemmas. Other large species such as pigs have a similar fibrinolytic responses to man, lending themselves to assessment of thrombolytics whilst the close resemblance of the coagulation system in sheep to that of man may be of particular utility when assessing novel antithrombotic agents 10. However, murine models particularly those involving the infrarenal vena cava (IVC) currently predominate; owing to their technical simplicity, compatibility with available imaging platforms and the availability of transgenic strains in this species. These murine models have proven especially useful in elucidating the molecular and cellular determinants of venous thrombosis 11.
Comparison of data from different studies is complicated by the variety of models used (both animal species and mechanisms of induction) in conjunction with the wide array of analytical techniques employed. Whilst the relative merits of respective models have been the source of critical review 12 there remains no consensus on the best method(s) to accurately quantify venous thrombosis in the pre-clinical setting. In this review we critically evaluate ex vivo and in vivo methods currently used in the assessment of venous thrombosis and describe emerging imaging techniques that may prove useful in studying this dynamic condition.
EX VIVO ASSESSMENT OF VENOUS THROMBOSIS
Weight
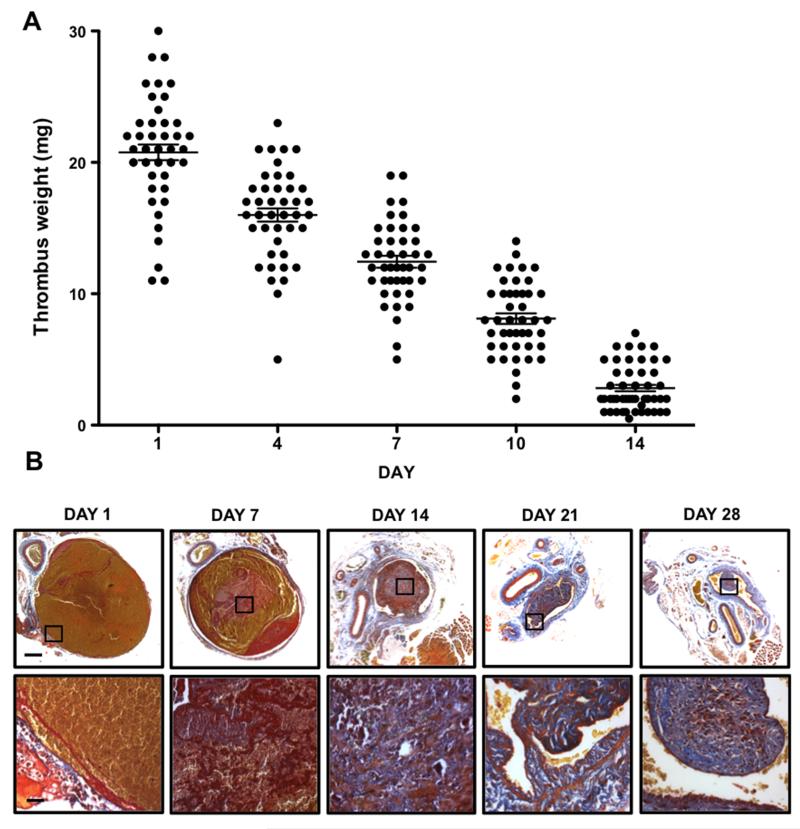
Thrombus weight is a simple, quantitative and inexpensive measure of thrombus formation and subsequent resolution; with weight decreasing as the thrombus resolves (Figure 1A). Thrombus weight can be obtained with or without excision from the surrounding vessel 13, 14. Measurements of thrombus weight without excision from the vessel may introduce variability associated with the inclusion of the vein wall and extraneous adherent tissue. Excision of the thrombus provides a more direct measure of weight, but during later stages of thrombus resolution, thrombus and vein wall become difficult to separate. Importantly, measurement of thrombus weight permits further biochemical and cellular analysis of the thrombus. It has been proposed that adjusting for thrombus length reduces apparent intra-group variability when measuring thrombus weight 15, 16. In our experience such adjustments are not always sensitive to changes in thrombus size; for example, when thrombus weight decreases proportionally to thrombus length, similar values of length-to-weight ratio will be obtained.
Figure 1. Weight and morphology of resolving murine venous thrombi.
(A) Thrombus weight in the St Thomas’ model of IVC stenosis measured at days 1, 4, 7, 10 and 14 post- induction, bars represent mean ± SEM. (B) Representative micrographs of transverse thrombus sections stained with Martius scarlet blue (MSB) at days 1, 7, 14, 21 and 28 post-induction. MSB detects collagen (blue), fibrin (red) and erythrocytes (yellow), scale bars 200μm (low power) and 25μm (high power). Adapted from Saha et. al. 2013 23.
Histological analysis
Histological techniques are commonly used to provide estimates of both thrombus size and composition. Thrombus is excised in situ with surrounding vein wall and prepared for histological sectioning. Estimation of thrombus size is carried out by analysis of thrombus cross-sectional area in sections taken at set intervals (300-500μm) along the entire length of the thrombus. The summed area multiplied by the distance between sections provides an estimate of thrombus volume 17, 18. We believe that reconstitution of thrombus volume provides the best estimate of overall thrombus burden. Measurements of representative cross-sectional area or scoring for the presence of thrombus at set intervals may not adequately account for changes in thrombus length or area respectively 4, 19, 20. Histological analysis does, however, present a number of technical challenges. Sectioning of thrombus, particularly at early time-points, can prove difficult because of the friable nature of this tissue. Sectioning individual thrombi at defined intervals along their length, with subsequent staining and analysis of sections at each level is also time consuming. A further limitation of this technique is that processing of thrombus for wax embedding results in significant shrinkage that may distort differences between groups 21.
Histological preparation of venous thrombi also enables parallel analysis of thrombus composition by either tinctorial or immunohistochemical staining. Thrombus resolution is characterised by extensive remodelling of the extracellular matrix by cells that infiltrate the thrombus. Extracellular matrix deposition can be readily detected with a number of tinctoral stains such as Picrosirius Red (collagen), Van Giessen (elastin), Alcian Blue (proteoglycans) and Martius Scarlet Blue (fibrin and collagen, Figure 1B). Although these techniques are well established, conditions must be tightly controlled to ensure tissue structures are appropriately and consistently stained. Immunohistochemical localisation of the cellular infiltrate (e.g. leukocytes, endothelial cells, myofibroblasts) can also be used to assess thrombus organisation (Table 1). Careful consideration of cell specific markers and extensive optimisation of antigen binding are required.
Table 1. Cell types present in the venous thrombus.
In murine models of vena cava thrombosis, ‘early’, and ‘mid-late’ stages of thrombus resolution refer to days 1-7 and days 7-28 post-induction respectively.
IN VIVO ASSESSMENT OF VENOUS THROMBOSIS
The terminal nature of ex vivo assessment is a significant limitation in the study of thrombus resolution as it precludes longitudinal measurements of changes in thrombus size in the same animal. Imaging techniques that facilitate longitudinal and reproducible quantification of thrombus burden overcome this limitation and provide powerful analysis through the generation of paired data. This approach can also limit the number of animals required for experimentation consistent with the principles laid out by the national centre for the replacement, reduction and refinement of animals in research.
Magnetic resonance imaging (MRI)
Non-contrast MRI
MRI can be used to diagnose DVT in man without the need for contrast agents 22. MRI time-of-flight venography is a rapid contrast-free technique for assessing thrombus volume in pre-clinical studies23. Phased sequences are used to allow specific visualisation of the venous system, where thrombus appears as a flow deficit that can be segmented to allow quantification of thrombus volume (Figure 2A). MR longitudinal relaxation time (T1) mapping can also be used to image thrombus, which has a shorter T1 compared with surrounding blood and tissue 23, 24. Shortened T1 times are likely caused by the accumulation of paramagnetic iron (Fe3+) in the thrombus23. Temporal changes in thrombus T1 have been observed during murine venous thrombus resolution and are associated with increasing thrombus organisation (Figure 2B) 23, 25. Magnetization transfer and diffusion weighted MR sequences have also been developed to characterize the age and protein composition of the thrombus 26. Combining the T1, magnetisation transfer and diffusion weighted sequences, whilst time consuming to acquire, could provide valuable information of both thrombus size and organisation. A major benefit of non-contrast MRI is that newly developed sequences are rapidly translatable from the laboratory to the clinic without need for regulatory approval. The time restrictions imposed by MRI, and multisequence imaging in particular, limit the utility of this technique to assessment of thrombus resolution that occurs over a period of weeks, rather than formation in which the thrombus forms in the order of minutes.
Figure 2. Imaging of the resolving venous thrombus by MRI.
(A) Venous phase time-of-flight scans used for imaging of the IVC, presence of thrombus results in a filling defect in the vessel which can be used to estimate thrombus volume. (B) Generation of T1 maps demonstrates a temporal shortening in T1 relaxivity (red shift) as the thrombus resolves. Adapted from Saha et. al. 2013 23.
Contrast MRI
Agents, such as gadolinium, have been used to image thrombi in experimental models. In a baboon model of IVC thrombosis, gadolinium enhanced MR venography was effective in identifying the thrombosed segment 9. A number of peptide-conjugated contrast agents have been developed that target matrix components present in the acute and resolving venous thrombus, such as fibrin and collagen 27, 28. A fibrin targeted contrast agent, EP-2104R, has been used to visualise intracranial thrombosis in pre-clinical models of stroke 29 and is currently under evaluation for the detection of thrombi in man 30. Furthermore, EP-2104R allows visualisation of the component of the venous thrombus that is susceptible to lysis in a low flow mouse model and could find clinical utility in stratifying patients for thrombolysis 31. A significant limitation of contrast-based approaches is the need for extensive validation of target specificity and the need for regulatory approval before use in human subjects, requiring costly clinical trials. Use of MRI in the pre-clinical setting has a number of limitations that include; the availability of scan time on much in demand clinical 3-Tesla scanners, the cost of scan time and the extended length of time required for an individual scan.
Micro computed tomography (microCT)
Technological advances in the field of computed tomography (CT) have facilitated the development of high-resolution microCT imaging platforms suitable for pre-clinical use. Contrast-enhanced microCT has been used extensively in the study of murine models of cardiovascular pathologies including critical limb ischaemia, abdominal aortic aneurysms and myocardial infarction 32-34.
Visualisation of the vasculature by microCT requires intravenous administration of high molecular-weight blood-pool contrast agents such as Iopremol, ExiTron nano 12000 or Aurovist 35. Contrast enhanced microCT enables longitudinal measurements of thrombus resolution as demonstrated in a murine model of IVC stenosis 36. This technique requires segmentation of thrombus from surrounding tissue, allowing 3D reconstruction and extraction of volumetric data. A strength of contrast-enhanced microCT is the ability to obtain high-resolution images with reconstructed voxel dimensions in the range of 20-60μm 36. Limitations of this technique for thrombus imaging include the high doses of ionising radiation required, the use of expensive and potentially nephrotoxic contrast agents and the cost of the imaging time. In addition, contrast enhanced microCT provides only anatomical data and not information on thrombus composition as can be obtained by MRI.
The use of fusion imaging modalities, such as CT-fluorescence resonance tomography (CT-FMT) enables the concurrent collection of anatomical and biological data in a non-invasive manner. CT-FMT provides imaging of cells, proteins and enzymatic activity through the use of targeted and activatable near infrared (NIR) probes. This technique has been used to evaluate thrombus composition with respect to macrophage and fibrin content, and matrix metalloproteinase activity localised to the thrombus 38, 39. Angiosense a NIR blood pool contrast agent used for quantification of tumour vasculature may also be relevant to studies of venous thrombus neovascularisation 40. FMT allows for fast acquisition of data with scan times in the range of 5-8minutes but affords limited spatial resolution in the sub-millimetre to millimetre range dependent on object depth 38, 41.
Radionucleotide Imaging
A number of targeted radionucleotide probes have been developed to localise components of the thrombus in vivo. Initial efforts to visualise fibrin utilised labelled components of the fibrinolytic system including 99mTc-tissue type plasminogen activator, 67Ga-urokinase type plasminogen activator and 99mTc-fibrin fragment E1, imaged by single photon emission computed tomography (SPECT) in a rabbit model of venous thrombosis 6, 45, 46. Integrin αIIbβ3 has also proven to be an attractive target for the generation of SPECT probes with labelled disintegrin-like peptides localising to thrombus in a canine femoral vein model 7, 47. Alternatively, positron emission tomography CT (PET-CT) can also be used to image venous thrombi. The PET tracer 18F-fluorodeoxyglucose localises to the acute thrombus in a novel model of recurrent DVT 42. Other targeted PET probes developed for imaging of arterial thrombi and atherosclerosis, which bind fibrin and the platelet surface receptor glycoprotein VI, may be directly applicable to the study of venous thrombosis 43, 44. Advantages of SPECT over PET include; simpler probe generation, longer prober half-lives and higher-resolution images (approximate voxel dimensions of 350μm and 850μm respectively). However, PET provides higher sensitivity compared to SPECT that may be of particular importance when quantifying fibrin in mature venous thrombi.
Ultrasonography
This technique is commonly used in the assessment of venous thrombosis in the clinical setting and can be used to assess thrombus formation and its resolution in the experimental setting.
High frequency ultrasound (HFUS)
Duplex ultrasonography is a commonly used non-contrast imaging modality for the diagnosis of venous thrombosis in man. Pre-clinical HFUS systems have been developed that allow two-dimensional high-resolution imaging of murine venous thrombi with pixel dimensions in range of 40-70μm 48, 49. IVC thrombus can be identified in transverse and longitudinal planes due to the hyper-echoic nature of the thrombus compared with surrounding blood (Figure 3). The echogenicity of the thrombus periphery is, however, similar to that of blood making accurate segmentation of the thrombus difficult. The absence of flow in the thrombus can be used to improve thrombus segmentation through the use of the colour doppler modality 48, 49. To further improve contrast between the thrombus and blood transpulmonary circulating micro-bubble contrast agents, such as Sonovue can be administered intravenously with the thrombus presenting as a hypo-echoic structure within the vessel lumen50.
Figure 3. Visualisation of venous thrombi by high frequency ultrasound.
Transverse view of (A) sham operated mouse with a patent IVC and (B) after IVC ligation with hyperechoic thrombus present in the lumen. Adapted from Aghourain et. al. 2012 48.
Ultrasonic imaging techniques can also be used to provide surrogate measures of thrombus composition. Ultrasound elastography of venous thrombi shows a consistent increase in strain measurements over time indicative of thrombus ‘hardening’, and is taken as thrombus organisation. This technique has been used to accurately estimate clot age in a rat IVC stasis model 51. Measurements of thrombus size using HFUS have so far been two dimensional limiting data acquisition to either length or cross-sectional area. Available 3D acquisition systems commonly utilised to measure subcutaneous tumour development could be used to reconstruct the thrombus and provide a better measure of thrombus burden 52.
Antibody-targeted microbubble contrast agents have been developed to identify fibrin and platelet integrin αIIbβ3 at sites of arterial thrombus formation and these agents may also be applicable to the study of venous thrombosis 53, 54. Alternative agents that provide measures of endothelial cell activation at the sites of atherogenesis through binding cell surface markers such as vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 have also been developed 55, 56. These agents may be of use when studying venous thrombus formation given the role of the endothelium during initiation 11.
Ultrasonic flow probes
Measurements of blood flow in the vessel lumen have been used in the assessment of venous thrombus formation. After induction, blood flow in the thrombosed segment can be monitored using an ultrasonic flow probe over a period of 30-40mins 57, 58. Time to occlusion of the vessel can be used as a measure of thrombus formation. Calculation of time to occlusion may, however, be complicated by residual flow through the thrombosed vessel. Alternatively, flow can be monitored at fixed time-points to observe the degree of vessel stenosis, a technique that has proven robust to the effects of residual blood flow in the arterial system 59. Changes in blood flow whilst not providing a direct measure of thrombus size, do act as a measure of vessel stenosis, which may be a more clinically relevant endpoint than measurement of thrombus size. The invasive and terminal nature of this procedure precludes the use of ultrasonic flow measures in longitudinal studies of the same animal.
Intra-vital microscopy
A variety of intra-vital microscopy (IVM) techniques have been used to image experimental thrombus formation in vivo including wide-field video and confocal microscopy. The relatively small depth of field achieved by these techniques has limited the majority of studies to in vivo imaging of thrombosis in the mesenteric and cremaster muscle micro-vasculature. Thrombi, formed in venules can be visualised by fluorescently-labelled antibodies to thrombus constituents such as fibrin and platelets, the accumulation of which allows temporal quantification of thrombus formation 60, 61. Accurate estimation of the spatial resolution of current wide-field IVM systems is complicated by movement and light scatter of tissue during capture, however, the ability of this technique to resolve single platelets suggests that observed resolutions are in the sub-micrometer range62. This intra-vital approach has been adapted to study the dynamics of thrombus formation in the femoral vein after localised electrolytic injury of the vessel wall 63, 64. One of the major benefits of IVM based studies of the microvasculature is that multiple thrombotic events can be initiated in a single animal (~10 per mouse), greatly reducing the numbers needed for experimentation. While flow rate and leukocyte rolling differ between the micro and macro-vasculature findings have been largely complementary with respect to thrombosis 60, 61.
IVM has also been used to study the cooperation between neutrophils monocytes and platelets during thrombus initiation in large vessels such as the femoral vein and IVC 37. IVM of the macro-vasculature allows imaging of the luminal vein surface and the thrombus periphery, but because of limitations in tissue penetrance it is not possible to image the thrombus in its entirety. NIR probes have distinct advantages for imaging of macro-vascular thrombosis by IVM owing to the greater penetrance of light through biological tissues in the range of 700-900nm. Confocal IVM with a novel NIR probe has been used to localise fibrin deposition in femoral vein thrombi, demonstrating the potential utility of this technique in informing thrombus structure 39. The terminal nature of the procedure, however, prevents longitudinal measurements in a single animal.
CONCLUSIONS
The most commonly used methods of analysing venous thrombosis in experimental models remain physical measurement of thrombus weight and histological estimates of thrombus size and composition. These techniques are limited to studies that are cross-sectional in design because of their terminal nature. Developments in imaging modalities (such as MRI, microCT and HFUS) have enabled longitudinal in vivo analysis of not only of thrombus burden, but also of thrombus composition that is beginning to rival the information provided by histology. Further developments in these imaging techniques will facilitate concomitant physical, cellular, and molecular analysis of experimental venous thrombi. These will significantly enhance our capacity to investigate mechanisms that regulate the formation and resolution of venous thrombi.
Table 2. Methods of assessing venous thrombus formation and resolution.
| W | H | MRI | CT | RI | HFUS | IVM | |
|---|---|---|---|---|---|---|---|
| Real-time | • | • | |||||
| Cell tracking | • | ||||||
| Co-localisation | • | • | • | • | • | ||
| Large vesselsa | • | • | • | • | • | • | • |
| Small vesselsb | • | ||||||
| Non-invasive | • | • | • | • | |||
| Quantitative | • | • | • | • | • | • | • |
| Rapid | • | • | • | • | |||
| Longitudinal | • | • | • | • | |||
| Clinically relevant | • | • | • | • | |||
| Reported in mice | • | • | • | • | • | • | • |
| Resolution (μm) | N/A | N/A | 100-500 | 20-60 | 350-850 | 40-70 | <1 |
Abbreviations: W, weight; H, histology; MRI, magnetic resonance imaging; CT, computed tomography; RI, radionucleotide imaging; HFUS, high frequency ultrasound; IVM, intravital microscopy.
large vessels > 1mm in diameter,
small vessels <100μm in diameter.
SIGNIFICANCE.
Animal models have contributed significantly to our understanding of the mechanisms that govern formation and subsequent resolution of venous thrombi. Here we critically appraise the techniques commonly used to assess thrombosis in these models. Particular attention is paid to imaging modalities that enable longitudinal measurements of thrombus burden and that provide data on composition in a non-invasive manner.
Acknowledgments
SOURCES OF FUNDING
SG and CE were funded by non-clinical PhD studentships from the British Heart Foundation. PS and AP are funded by clinical lectureships from the National Institute for Health Research. BM is funded by the British Heart Foundation and the Circulation Foundation.65, 66
Nonstandard Abbreviations and Acronyms
- CT
Computed tomography
- DVT
Deep vein thrombosis
- FMT
Fluorescence molecular tomography
- HFUS
High frequency ultrasound
- IVC
Infrarenal vena cava
- IVM
Intravital microscopy
- MRI
Molecular resonance imaging
- NIR
Near infrared
- PET
Positron emission tomography
- SPECT
Single photon emission computed tomography
Footnotes
DISCLOSURES
Authors declare that they have no conflict of interest.
REFERENCES
- 1.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M. Venous thromboembolism (vte) in europe. The number of vte events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Hunt BJ. Awareness and politics of venous thromboembolism in the united kingdom. Arterioscler Thromb Vasc Biol. 2008;28:398–399. doi: 10.1161/ATVBAHA.108.162586. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: Rescue by normal bone marrow-derived cells. Circulation. 2003;107:869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- 5.McGuinness CL, Humphries J, Waltham M, Burnand KG, Collins M, Smith A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85:1018–1024. [PubMed] [Google Scholar]
- 6.Itoh K, Ieko M, Hiraguchi E, Kitayama H, Tsukamoto E. In vivo kinetics of 99mtc labeled recombinant tissue plasminogen activator in rabbits. Annals of nuclear medicine. 1994;8:193–199. doi: 10.1007/BF03164997. [DOI] [PubMed] [Google Scholar]
- 7.Knight LC, Baidoo KE, Romano JE, Gabriel JL, Maurer AH. Imaging pulmonary emboli and deep venous thrombi with 99mtc-bitistatin, a platelet-binding polypeptide from viper venom. J Nucl Med. 2000;41:1056–1064. [PubMed] [Google Scholar]
- 8.Kang C, Bonneau M, Brouland JP, Bal dit Sollier C, Drouet L. In vivo pig models of venous thrombosis mimicking human disease. Thromb Haemost. 2003;89:256–263. [PubMed] [Google Scholar]
- 9.Wakefield TW, Strieter RM, Schaub R, Myers DD, Prince MR, Wrobleski SK, Londy FJ, Kadell AM, Brown SL, Henke PK, Greenfield LJ. Venous thrombosis prophylaxis by inflammatory inhibition without anticoagulation therapy. J Vasc Surg. 2000;31:309–324. doi: 10.1016/s0741-5214(00)90162-9. [DOI] [PubMed] [Google Scholar]
- 10.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. [PubMed] [Google Scholar]
- 11.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz JA, Obi AT, Myers DD, Jr., Wrobleski SK, Henke PK, Mackman N, Wakefield TW. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:556–562. doi: 10.1161/ATVBAHA.111.244608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabre J, Chabasse C, Cao C, Mukhopadhyay S, Siefert S, Bi Y, Netzel-Arnett S, Sarkar R, Zhang L. Activated protein c accelerates venous thrombus resolution through heme oxygenase-1 induction. J Thromb Haemost. 2014;12:93–102. doi: 10.1111/jth.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. 2011;31:43–49. doi: 10.1161/ATVBAHA.110.216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosaka M, Ishida Y, Kimura A, Kuninaka Y, Inui M, Mukaida N, Kondo T. Absence of ifn-gamma accelerates thrombus resolution through enhanced mmp-9 and vegf expression in mice. J Clin Invest. 2011;121:2911–2920. doi: 10.1172/JCI40782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans CE, Grover SP, Humphries J, Saha P, Patel AP, Patel AS, Lyons OT, Waltham M, Modarai B, Smith A. Antiangiogenic therapy inhibits venous thrombus resolution. Arterioscler Thromb Vasc Biol. 2014;34:565–570. doi: 10.1161/ATVBAHA.113.302998. [DOI] [PubMed] [Google Scholar]
- 18.Evans CE, Humphries J, Mattock K, Waltham M, Wadoodi A, Saha P, Modarai B, Maxwell PH, Smith A. Hypoxia and upregulation of hypoxia-inducible factor 1{alpha} stimulate venous thrombus recanalization. Arterioscler Thromb Vasc Biol. 2010;30:2443–2451. doi: 10.1161/ATVBAHA.110.215038. [DOI] [PubMed] [Google Scholar]
- 19.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte cxcr2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 20.Modarai B, Humphries J, Burnand KG, Gossage JA, Waltham M, Wadoodi A, Kanaganayagam GS, Afuwape A, Paleolog E, Smith A. Adenovirus-mediated vegf gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol. 2008;28:1753–1759. doi: 10.1161/ATVBAHA.108.170571. [DOI] [PubMed] [Google Scholar]
- 21.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 22.Kanne JP, Lalani TA. Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism. Circulation. 2004;109:I15–21. doi: 10.1161/01.CIR.0000122871.86662.72. [DOI] [PubMed] [Google Scholar]
- 23.Saha P, Andia ME, Modarai B, Blume U, Humphries J, Patel AS, Phinikaridou A, Evans CE, Mattock K, Grover S, Ahmad A, Lyons OT, Attia RQ, Renne T, Premaratne S, Wiethoff AJ, Botnar RM, Schaeffter T, Waltham M, Smith A. Magnetic resonance t1-relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation. 2013;128:729–736. doi: 10.1161/CIRCULATIONAHA.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blume U, Orbell J, Waltham M, Smith A, Razavi R, Schaeffter T. 3d t(1)-mapping for the characterization of deep vein thrombosis. Magma. 2009;22:375–383. doi: 10.1007/s10334-009-0189-8. [DOI] [PubMed] [Google Scholar]
- 25.Ichiki M, Sakai Y, Nango M, Nakamura K, Matsui H, Cho H, Kitayama T, Sahara T, Otani N, Inoue Y, Miki Y. Experimental venous thrombi: Mri characteristics with histopathological correlation. Br J Radiol. 2012;85:331–338. doi: 10.1259/bjr/37592039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinikaridou A, Andia ME, Saha P, Modarai B, Smith A, Botnar RM. In vivo magnetization transfer and diffusion weighted mri detects thrombus composition in a mouse model of deep vein thrombosis. Circ Cardiovasc Imaging. 2013;6:433–440. doi: 10.1161/CIRCIMAGING.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overoye-Chan K, Koerner S, Looby RJ, Kolodziej AF, Zech SG, Deng Q, Chasse JM, McMurry TJ, Caravan P. Ep-2104r: A fibrin-specific gadolinium-based mri contrast agent for detection of thrombus. J Am Chem Soc. 2008;130:6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 28.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, Zhang Z. Collagen-targeted mri contrast agent for molecular imaging of fibrosis. Angewandte Chemie. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 29.Uppal R, Ay I, Dai G, Kim YR, Sorensen AG, Caravan P. Molecular mri of intracranial thrombus in a rat ischemic stroke model. Stroke; a journal of cerebral circulation. 2010;41:1271–1277. doi: 10.1161/STROKEAHA.109.575662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC., Jr. Thrombus imaging with fibrin-specific gadolinium-based mr contrast agent ep-2104r: Results of a phase ii clinical study of feasibility. Investigative radiology. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 31.Andia ME, Saha P, Jenkins J, Modarai B, Wiethoff AJ, Phinikaridou A, Grover SP, Patel AS, Schaeffter T, Smith A, Botnar RM. Fibrin-targeted magnetic resonance imaging allows in vivo quantification of thrombus fibrin content and identifies thrombi amenable for thrombolysis. Arterioscler Thromb Vasc Biol. 2014;34:1193–1198. doi: 10.1161/ATVBAHA.113.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Badea C, Hedlund LW, Figueiredo JL, Sosnovik DE, Johnson GA, Weissleder R. High-resolution imaging of murine myocardial infarction with delayed-enhancement cine micro-ct. Am J Physiol Heart Circ Physiol. 2007;292:H3172–3178. doi: 10.1152/ajpheart.01307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nebuloni L, Kuhn GA, Muller R. A comparative analysis of water-soluble and blood-pool contrast agents for in vivo vascular imaging with micro-ct. Academic radiology. 2013;20:1247–1255. doi: 10.1016/j.acra.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Grover SP, Saha P, Jenkins J, Mukkavilli A, Lyons OT, Patel AS, Sunassee K, Modarai B, Smith A. Quantification of experimental venous thrombus resolution by longitudinal nanogold-enhanced micro-computed tomography. Thromb Res. 2015 doi: 10.1016/j.thromres.2015.10.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ripplinger CM, Kessinger CW, Li C, Kim JW, McCarthy JR, Weissleder R, Henke PK, Lin CP, Jaffer FA. Inflammation modulates murine venous thrombosis resolution in vivo: Assessment by multimodal fluorescence molecular imaging. Arterioscler Thromb Vasc Biol. 2012;32:2616–2624. doi: 10.1161/ATVBAHA.112.251983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, Weissleder R, Lin CP, Tearney GJ, Jaffer FA. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC. Cardiovascular imaging. 2012;5:607–615. doi: 10.1016/j.jcmg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montet X, Figueiredo JL, Alencar H, Ntziachristos V, Mahmood U, Weissleder R. Tomographic fluorescence imaging of tumor vascular volume in mice. Radiology. 2007;242:751–758. doi: 10.1148/radiol.2423052065. [DOI] [PubMed] [Google Scholar]
- 41.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, Marinelli B, Aikawa E, Pittet MJ, Swirski FK, Weissleder R. Hybrid in vivo fmt-ct imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara T, Truelove J, Tawakol A, Wojtkiewicz GR, Hucker WJ, MacNabb MH, Brownell AL, Jokivarsi K, Kessinger CW, Jaff MR, Henke PK, Weissleder R, Jaffer FA. Fdg-pet/ct enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation. 2014;130:1044–1052. doi: 10.1161/CIRCULATIONAHA.114.008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ay I, Blasi F, Rietz TA, Rotile NJ, Kura S, Brownell AL, Day H, Oliveira BL, Looby RJ, Caravan P. In vivo molecular imaging of thrombosis and thrombolysis using a fibrin-binding positron emission tomographic probe. Circ Cardiovasc Imaging. 2014;7:697–705. doi: 10.1161/CIRCIMAGING.113.001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bigalke B, Phinikaridou A, Andia ME, Cooper MS, Schuster A, Schonberger T, Griessinger CM, Wurster T, Onthank D, Ungerer M, Gawaz M, Nagel E, Botnar RM. Positron emission tomography/computed tomographic and magnetic resonance imaging in a murine model of progressive atherosclerosis using (64)cu-labeled glycoprotein vi-fc. Circ Cardiovasc Imaging. 2013;6:957–964. doi: 10.1161/CIRCIMAGING.113.000488. [DOI] [PubMed] [Google Scholar]
- 45.Ohmomo Y, Yokoyama A, Yamauchi Y, Horiuchi K, Saji H, Tanaka C, Torizuka K. In vivo kinetics and thrombus accumulation of 67ga-labeled urokinase. International journal of nuclear medicine and biology. 1985;12:47–52. doi: 10.1016/0047-0740(85)90012-9. [DOI] [PubMed] [Google Scholar]
- 46.Knight LC, Abrams MJ, Schwartz DA, Hauser MM, Kollman M, Gaul FE, Rauh DA, Maurer AH. Preparation and preliminary evaluation of technetium-99m-labeled fragment e1 for thrombus imaging. J Nucl Med. 1992;33:710–715. [PubMed] [Google Scholar]
- 47.Lister-James J, Vallabhajosula S, Moyer BR, Pearson DA, McBride BJ, De Rosch MA, Bush LR, Machac J, Dean RT. Pre-clinical evaluation of technetium-99m platelet receptor-binding peptide. J Nucl Med. 1997;38:105–111. [PubMed] [Google Scholar]
- 48.Aghourian MN, Lemarie CA, Blostein MD. In vivo monitoring of venous thrombosis in mice. J Thromb Haemost. 2012;10:447–452. doi: 10.1111/j.1538-7836.2011.04615.x. [DOI] [PubMed] [Google Scholar]
- 49.Geddings J, Aleman MM, Wolberg A, von Bruhl ML, Massberg S, Mackman N. Strengths and weaknesses of a new mouse model of thrombosis induced by inferior vena cava stenosis: Communication from the ssc of the isth. J Thromb Haemost. 2014;12:571–573. doi: 10.1111/jth.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guenther F, Herr N, Mauler M, Witsch T, Roming F, Hein L, Boeynaems JM, Robaye B, Idzko M, Bode C, Von Zur Muhlen C, Duerschmied D. Contrast ultrasound for the quantification of deep vein thrombosis in living mice: Effects of enoxaparin and p2y12 receptor inhibition. J Thromb Haemost. 2013;11:1154–1162. doi: 10.1111/jth.12206. [DOI] [PubMed] [Google Scholar]
- 51.Emelianov SY, Chen X, O’Donnell M, Knipp B, Myers D, Wakefield TW, Rubin JM. Triplex ultrasound: Elasticity imaging to age deep venous thrombosis. Ultrasound Med Biol. 2002;28:757–767. doi: 10.1016/s0301-5629(02)00516-1. [DOI] [PubMed] [Google Scholar]
- 52.Ingram N, Macnab SA, Marston G, Scott N, Carr IM, Markham AF, Whitehouse A, Coletta PL. The use of high-frequency ultrasound imaging and biofluorescence for in vivo evaluation of gene therapy vectors. BMC medical imaging. 2013;13:35. doi: 10.1186/1471-2342-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanza GM, Wallace KD, Scott MJ, Cacheris WP, Abendschein DR, Christy DH, Sharkey AM, Miller JG, Gaffney PJ, Wickline SA. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94:3334–3340. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Hagemeyer CE, Hohmann JD, Leitner E, Armstrong PC, Jia F, Olschewski M, Needles A, Peter K, Ahrens I. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: Validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125:3117–3126. doi: 10.1161/CIRCULATIONAHA.111.030312. [DOI] [PubMed] [Google Scholar]
- 55.Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, Caplan ES, Khangura J, Bullens S, Bunting S, Lindner JR. Molecular imaging of the initial inflammatory response in atherosclerosis: Implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller GE, Lu E, Csikari MM, Klibanov AL, Fischer D, Wagner WR, Villanueva FS. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108:218–224. doi: 10.1161/01.CIR.0000080287.74762.60. [DOI] [PubMed] [Google Scholar]
- 57.Cardenas JC, Owens AP, 3rd, Krishnamurthy J, Sharpless NE, Whinna HC, Church FC. Overexpression of the cell cycle inhibitor p16ink4a promotes a prothrombotic phenotype following vascular injury in mice. Arterioscler Thromb Vasc Biol. 2011;31:827–833. doi: 10.1161/ATVBAHA.110.221721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Smith PL, Hsu MY, Ogletree ML, Schumacher WA. Murine model of ferric chloride-induced vena cava thrombosis: Evidence for effect of potato carboxypeptidase inhibitor. J Thromb Haemost. 2006;4:403–410. doi: 10.1111/j.1538-7836.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- 60.Greene TK, Wang C, Hirsch JD, Zhai L, Gewirtz J, Thornton MA, Miao HZZ, Pipe SW, Kaufman RJ, Camire RM, Arruda VR, Kowalska MA, Poncz M. In vivo efficacy of platelet-delivered, high specific activity factor viii variants. Blood. 2010;116:6114–6122. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stolla M, Stefanini L, Roden RC, Chavez M, Hirsch J, Greene T, Ouellette TD, Maloney SF, Diamond SL, Poncz M, Woulfe DS, Bergmeier W. The kinetics of alphaiibbeta3 activation determines the size and stability of thrombi in mice: Implications for antiplatelet therapy. Blood. 2011;117:1005–1013. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vwf independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aleman MM, Walton BL, Byrnes JR, Wang JG, Heisler MJ, Machlus KR, Cooley BC, Wolberg AS. Elevated prothrombin promotes venous, but not arterial, thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:1829–1836. doi: 10.1161/ATVBAHA.113.301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alias S, Redwan B, Panzenboeck A, Winter MP, Schubert U, Voswinckel R, Frey MK, Jakowitsch J, Alimohammadi A, Hobohm L, Mangold A, Bergmeister H, Sibilia M, Wagner EF, Mayer E, Klepetko W, Hoelzenbein TJ, Preissner KT, Lang IM. Defective angiogenesis delays thrombus resolution: A potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014;34:810–819. doi: 10.1161/ATVBAHA.113.302991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kellermair J, Redwan B, Alias S, Jabkowski J, Panzenboeck A, Kellermair L, Winter MP, Weltermann A, Lang IM. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood. 2013;122:3376–3384. doi: 10.1182/blood-2013-04-499558. [DOI] [PubMed] [Google Scholar]