Abstract
Hepatitis A virus (HAV) is transmitted via the fecal-oral route from contaminated food or water. As part of the most recent survey of viral hepatitis burden in Thailand, we analyzed the current seroprevalence of HAV in the country and compared with data dating back to 1971. From March to October, 2014, a total of 4,260 individuals between one month and 71 years of age from different geographical regions (North = 961; Central = 1,125; Northeast = 1,109; South = 1,065) were screened for anti-HAV IgG antibody using an automated chemiluminescent microparticle immunoassay. Overall, 34.53% (1,471/4,260) possessed anti-HAV IgG antibody, and the age-standardized seroprevalence was 48.6%. Seroprevalence rates were 27.3% (North), 30.8% (Central), 33.8% (Northeast) and 45.8% (South) and were markedly lower than in the past studies especially among younger age groups. The overall trend showed an increase in the age by which 50% of the population were anti-HAV IgG antibody: 4.48 years (1971–1972), 6 (1976), 12.49 (1990), 36.02 (2004) and 42.03 (2014).This suggests that Thailand is transitioning from low to very low HAV endemicity. Lower prevalence of HAV correlated with improved healthcare system as measured by decreased infant mortality rate and improved national economy based on increased GDP per capita. The aging HAV immuno-naïve population may be rendered susceptible to potential HAV outbreaks similar to those in industrialized countries and may benefit from targeted vaccination of high-risk groups.
Introduction
Hepatitis A virus (HAV) infection is estimated at 1.5 million individuals annually worldwide [1]. HAV belongs to the family Picornaviridae and the genus Hepatovirus [2]. It is a non-enveloped virus with a 7.5 kb single-stranded positive-sense RNA genome. It is classified into six genotypes, designated I-VI. Genotypes I, II and III have been identified in humans, while genotypes IV, V and VI are simian [3]. Despite genome diversity, all genotypes represent a single serotype [4]. The major transmission route for HAV is through consumption of contaminated food or water, although occasional transmission from person to person (through sexual intercourse or blood transfusion) has been reported [5–9]. High prevalence of HAV is often associated with poor hygiene, lack of sanitation and low socio-economic status [10].
In addition to improved hygiene and sanitation, the incidence of HAV infection can be substantially reduced using an inactivated HAV vaccine, which can effectively protect from all genotypes. Administration of two doses of HAV vaccine provides a long-term immunity from infection for up to 15 years [11,12]. Among the endemic regions of Africa and Asia, most individuals acquire immunity early in life [13]. Therefore, the prevalence of HAV infection in developing countries with low socioeconomic status remains high because infection occurs in early childhood and vaccination is often not feasible [14–15].
As a developing nation in Southeast Asia, Thailand has attained an upper- to middle-income economy in the past decade concommittant with continued improvement in sanitation and hygiene [16]. Consequently, the proportion of the population with immunity to HAV as measured by anti-HAV antibodies demonstrated a steady decline [17–18]. To assess the most current HAV seroprevalence in the general population in association with socio-economic factors, we evaluated serum samples obtained from individuals living in different geographical regions of Thailand for HAV antibodies and determined the correlates of immunity to national healthcare and economic parameters.
Materials and Methods
This study was part of the overall research consortium to assess the status of viral hepatitis in the country by examining the seroprevalence of hepatitis A, B and C in Thailand and was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No. 419/56). Individuals were informed of the study objective and written consents were obtained from all participants or their parents. Basic demographic information (age, gender, and address) was also obtained. All samples were de-identified and anonymous.
Study population
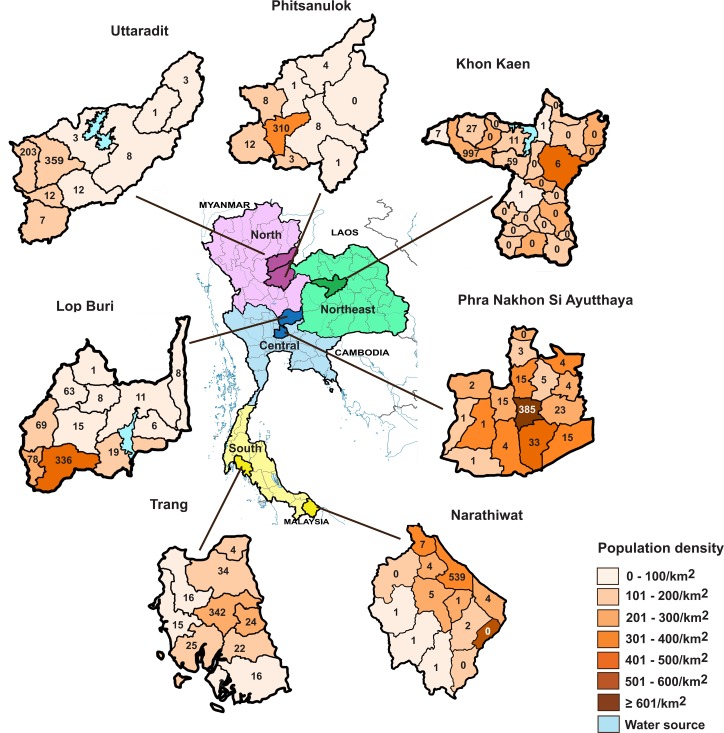
Blood samples were collected between March 1 and October 31, 2014 from patients during scheduled pediatric health check-up or outpatient clinic at hospitals in seven provinces representing four regions of Thailand (Fig 1). The provinces of Uttaradit and Phitsanulok represented the north, Lop Buri and Phra Nakhon Si Ayutthaya represented the central region, Khon Kaen represented the northeast, and Narathiwat and Trang represented the south. These provincial and district hospitals were chosen for their relatively good facilities and locations in generally comparable population densities. Samples were obtained from 4,260 individuals (1,792 males and 2,468 females; North = 961, Central = 1,125, Northeast = 1,109, and South = 1,065) (Table 1). The inclusion criteria were Thais residing in their respective provinces age between 1 month and 71 years of age. Exclusion criteria were immunological or hematologic disorder (e.g. HIV-positive status and hemophilia), malignancy, history of chronic diseases (e.g. heart and liver diseases), and congenital defects. Individuals who were hospitalized for longer than one month during the past year, undergoing steroid treatment, immunosuppressive drugs or chemotherapy were also excluded. Blood samples were separated into serum within 24 hours, transported to the Center of Excellence in Clinical Virology at King Chulalongkorn Memorial Hospital, and stored at -20°C until tested.
Fig 1. Map of Thailand and the domicile of study participants.
Seven provinces from the four regions (North, violet; Northeast, green; Central, blue; and South, yellow) are shown. Districts within a given province are denoted in different colors depending on the population density (range 0 to ≥ 601 individuals/km2). Number of study participants from each district is indicated.
Table 1. Regions and demographics of individuals assessed for immunity to HAV.
| Region | Province | Hospital name | District | Participants | Male | Female | Age range | Mean age ± SD (yr) |
|---|---|---|---|---|---|---|---|---|
| North | Uttaradit | Uttaradit Hospital | Mueang Uttaradit | 614 | 268 | 346 | 6 mo–62 yr | 22.9 ± 17.9 |
| (n = 961) | Laplae Hospital | Laplae | ||||||
| Phitsanulok | Naresuan University Hospital | Mueang Phitsanulok | 347 | 126 | 221 | 5 mo–68 yr | 27.8 ± 18.7 | |
| Center | Lop Buri | King Narai Hospital | Khao Sam Yot | 614 | 299 | 315 | 7 mo–71 yr | 26.5 ± 18.4 |
| (n = 1,125) | Phra Nakhon Si Ayutthaya | Phra Nakhon Si Ayutthaya Hospital | Phra Nakhon Si Ayutthaya | 511 | 209 | 302 | 6 mo–60 yr | 26.3 ± 17.7 |
| Northeast | Khon Kaen | Chumphae Hospital | Chumphae | 1,109 | 526 | 583 | 6 mo–61 yr | 25.4 ± 17.7 |
| (n = 1,109) | ||||||||
| South | Narathiwat | Narathiwat Ratchanakarin Hospital | Mueang Narathiwat | 565 | 157 | 408 | 7 mo–60 yr | 31.0 ± 16.9 |
| (n = 1,065) | Trang | Trang Hospital | Mueang Trang | 500 | 207 | 293 | 1 mo–62 yr | 25.1 ± 25.1 |
| TOTAL | 4,260 | 1,792 | 2,468 | 1 mo–71 yr | 26.5 ± 18.2 |
Serological assay
Anti-HAV IgG was determined using the automated chemiluminescent microparticle immunoassay on the ARCHITECT i2000SR instrument (ARCHITECT HAVab-IgG, Abbott, Wiesbaden, Germany) according to the manufacturer’s instructions. The sensitivity and specificity of the test were > 98% and > 99.17%, respectively. Samples with the signal-to-cut-off (S/CO) ratio of > 1.00 were considered HAV antibody-positive by the automated assay, while samples with S/CO ratio < 1.00 were considered negative.
Comparison of anti-HAV seroprevalence
Anti-HAV IgG prevalence obtained from this study was compared to the HAV seroprevalence data from past studies (Table 2). Briefly, the studies conducted in central region of Thailand in 1971–1972 and 1976 surveyed the urban and rural regions, respectively [16]. The 1991 study was conducted in the predominantly rural central region [17], while the 2007 study surveyed all four regions of the country similar to this study [18].
Table 2. Previous studies of anti-HAV IgG prevalence in Thailand.
| Previous studies conducted | Year | Sample size | Location | % overall anti-HAV IgG positive |
|---|---|---|---|---|
| Burke et al. (1981) [16] | 1971 to 1972 | 308 | Huay Khwang, Bangkok | 86.4 |
| Burke et al. (1981) [16] | 1976 | 206 | Ban Tablan, Prachin Buri | 59.7 |
| Poovorawan et al. (1991) [17] | 1990 | 364 | Pong Nam Ron, Chanthaburi | 64.5 |
| 236 | Bo Thong, Chon Buri | |||
| Chatproedprai et al. (2007) [18] | 2004 | 1,336 | Chiang Rai | 27.35 |
| 985 | Udon Thani | |||
| 846 | Nakhon Si Thammarat | |||
| 830 | Chon Buri |
To calculate the approximate age group with 50% detectable anti-HAV IgG, a line chart indicating the percentage of anti-HAV IgG seropositivity and age was constructed. The 50th percentile of the anti-HAV-IgG seropositive rates allowed the determination of the approximate age group at which half of the population was HAV seropositive. Subsequently, a linear equation (y = mx + c) was derived from the chart trend line of each study, which gave a modifying factor used to allow the extrapolation of a more precise mean age with standard deviation (SD) at which 50% of the population possessed anti-HAV IgG antibodies.
Economic and national health data
To determine the relationship between socio-economic status, health and HAV seroprevalence, the population of Thailand and other factors were considered. Gross domestic product (GDP) per capita in U.S. dollars [19] represented an economic indicator reflective of the standard of living. Infant mortality rate (defined as the number of infant deaths before one year of age per 1,000 live births in a given year) was used as an indicator for the quality of national healthcare [20]. The HAV annual morbidity rate per 100,000 individuals and location-specific HAV outbreak data were derived from the Bureau of Epidemiology, Department of Disease Control, Thai Ministry of Public Health [21].
Statistical analysis
Statistical analysis (mean, SD and 95% CI) was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Seroprevalence of anti-HAV IgG
Of the 4,260 persons sampled in this study, there were more women than men (57.9% versus 42.1%, respectively). The distribution of males and females were approximately equal for persons ≤ 14 years, but there were substantially more females than males ≥ 15 years (S1 Table).
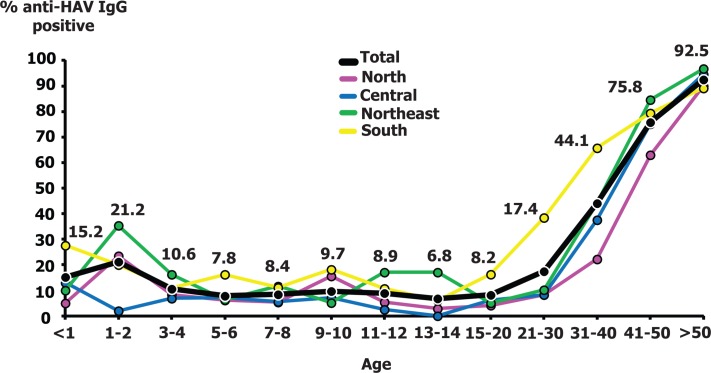
Seroprevalence progressively increased with age and was highest in individuals > 50 years (92.5%) in all regions (96.8% Northeast, 94.6% Central, 90.0% North, and 89.2% South). Regionally, we found the lowest anti-HAV IgG prevalence in the North (27.3%), while the highest was in the South (45.8%) especially among individuals 15–40 year-olds (Fig 2). The age-standardized anti-HAV IgG positive rate was 48.6% (Table 3).
Fig 2. The frequency of anti-HAV IgG positivity in each age group.
Numbers above the line charts indicate the total seroprevalence for each age group.
Table 3. Expected number of age-specific anti-HAV antibody-positive Thais based on the anti-HAV IgG-seropositive rates in 2014.
| Age group | Population | % anti-HAV positive rate | Anticipated anti-HAV IgG seropositive individuals |
|---|---|---|---|
| < 1 | 685,829 | 15.2 | 104,246 |
| 1–2 | 1,522,482 | 21.2 | 322,766 |
| 3–4 | 1,527,526 | 10.6 | 161,917 |
| 5–6 | 1,552,929 | 7.84 | 121,749 |
| 7–8 | 1,586,457 | 8.4 | 133,262 |
| 9–10 | 1,610,751 | 9.69 | 156,081 |
| 11–12 | 1,579,273 | 8.9 | 140,555 |
| 13–14 | 1,634,052 | 6.8 | 111,115 |
| 15–20 | 5,615,735 | 8.19 | 459,928 |
| 21–30 | 8,384,422 | 17.4 | 1,462,243 |
| 31–40 | 10,346,437 | 44.1 | 4,561,744 |
| 41–50 | 10,465,811 | 75.8 | 7,934,131 |
| > 50 | 16,484,653 | 92.5 | 15,253,249 |
| Total | 62,996,357 | 48.6* | 30,600,220 |
* Calculated from anticipated seropositive individuals divided by the population.
Trends of anti-HAV IgG positivity from 1971 to 2014
Seroprevalence of anti-HAV IgG in each age group was compared to age-specific prevalence of antibody to HAV from 4 previous studies (1971–1972, 1976, 1990 and 2004), which utilized similar immunoassay (Fig 3). Although studies conducted prior to the year 2000 only surveyed individuals residing in the central region of the country, serosurvey conducted in 2004 involved provinces spanning all four geographical regions and represented the most comprehensive seroprevalence similar to this study. Prior to 1990, nearly all young adults possessed anti-HAV antibody. This general pattern changed beginning in 2004 when most young adults were likely to be seronegative for HAV. To better characterize the delayed HAV seroconversion over the past 45 years, we determined the average age at which roughly half of the individuals in the population possessed anti-HAV antibody. Analysis showed that the HAV seropositive mean age progressively increased from ~4.5 years in the early 1970’s to ~42 years in 2014.
Fig 3. Comparison of anti-HAV IgG positivity from 1971 to 2014.
Seroprevalence data from this and other studies were plotted as line charts. The dotted line denotes 50% anti-HAV IgG positivity. Intersection with the seroprevalence curve indicates the mean age at which 50% of the individuals in the population possessed anti-HAV IgG (denoted by the numbers on the line graphs) [16–18].
Healthcare system and economic status correlated with changing HAV burden
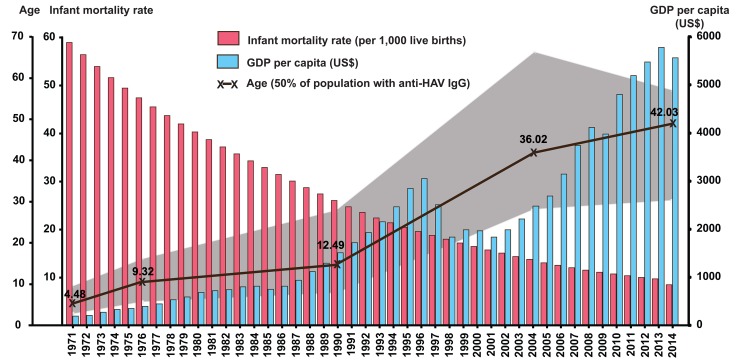
Since provincial and regional socio-economic data were not available for correlation with the seroprevalence finding, we used the available national data to analyze for potential association. While seroprevalence of HAV declined over the past 45 years, the infant mortality rate also decreased from ~60 per 1,000 to ~10 per 1,000 live births per year (Fig 4). Meanwhile, the GDP per capita steadily increased over the same period, suggesting an overall improved national economic status. The morbidity rates (per 100,000 individuals) associated with HAV infection available beginning in 2003 from the Bureau of Epidemiology, Thai Ministry of Public Health, indicated that between 2003 and 2014, HAV morbidity rate was less than 1 per 100,000 persons annually (Fig 5). Death associated with HAV infection is extremely rare, with the average mortality rate less than 0 per 100,000 persons annually during this period [21].
Fig 4. Correlation between infant mortality rate (per 1,000 live births), GDP, and age at which 50% of the population possessed anti-HAV antibody.
Infant mortality rates (pink graphs), GDP per capita (blue graphs), and the mean age at 50% anti-HAV IgG positivity in the population (black line) are indicated. Half of the population around the mean age is denoted in gray areas.
Fig 5. Total hepatitis A infection cases and morbidity rates in Thailand.
Data available from 2003 to 2014 (excluding 2004) from the Bureau of Epidemiology, Thai Ministry of Public Health, are presented as line charts. Rates are indicated per 100,000 persons.
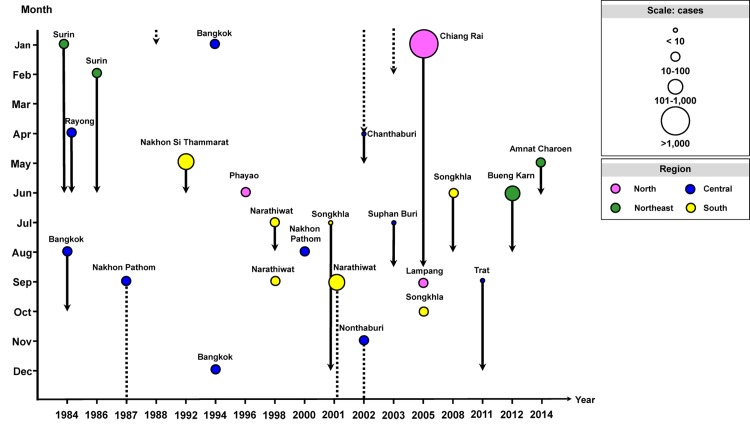
There were two recorded HAV outbreaks in the northern province of Chiang Rai and the northeastern province of Bueng Karn, which resulted in the spike in morbidity in 2005 (3.98) and 2012 (2.32). Epidemiological data also showed documented sporadic incidence of HAV outbreaks of varying scale over the past 30 years, which occurred in various provinces in Thailand (Fig 6).
Fig 6. Documented hepatitis A outbreaks in Thailand from 1984 to 2014.
Circles denote outbreak events in different regions (North, purple; Central, blue; Northeast, green; South, yellow). Circle sizes correspond to the number of individuals affected. Outbreak in a calendar year is denoted as solid lines; those that continued to the following year are denoted in dashed lines. Line length corresponds to the recorded outbreak period.
Discussion
A study published 40 years ago found that most Thais were infected with HAV before reaching adulthood [17]. Our study revealed that as the healthcare and economic status of Thailand improved, the prevalence of HAV antibodies in the population steadily declined. Although some middle-income countries in Southeast Asia represent low endemicity region as defined by the World Health Organization (≥ 50% of the population immune by age 30) [22–24], these most current seroprevalence data suggest that Thailand is transitioning to a very low HAV endemicity (defined by <50% having immunity by age 30 years).
Presently, most children and young adults are unlikely to possess immunity to HAV due to the observed low anti-HAV seroprevalence in the 3–20 year age groups (6.8–10.6%). Consequently, they are expected to be more susceptible to HAV infection should it occur later in life. Due to placental antibody transfer from mother to child, we continued to find a slight increase in the anti-HAV seroprevalence in children age < 1 year old. This passive antibody transfer will likely convey protection should infants be exposed to HAV early in life, but this is transient as the majority of acquired maternal antibodies declined with age [25, 26]. Consistent with previous sero-surveillance data, most individuals above 40 years possessed HAV antibodies, which may be attributed to the region’s lower standard of living and hygiene conditions in the first half of the century as well as higher exposure to HAV during childhood. Although under-reporting may have contributed to the very low morbidity and mortality rates for hepatitis A infection in Thailand, susceptibility to local HAV outbreaks in 2005 and 2012 (Figs 5 and 6) is consistent with the available scientific evidence that HAV is not highly endemic in this region. Nevertheless, morbidity rates can increase significantly in such instance as the majority of patients were adults.
The trend of lower HAV infection rates in recent years is underscored by the steady increase in the mean age with 50% anti-HAV IgG seropositivity in the population. The most significant change occurred between 1990 and 2004, from 12.49 years to 36.02 years. As uninfected children become adults, the HAV seroprevalence is predicted to further decline, which would shift the seroprevalence curved steadily to the right. The shift in the mean age in which 50% of the population possessed anti-HAV IgG was associated with several indicators, including higher socio-economic status as measured by the declining infant mortality rate, improved sanitation facilities and hygiene practices, and increased GDP per capita to support better living standards. It is envisioned that future seroprevalence will continue to decline due to lower HAV endemicity, although this will render the population susceptible to occasional outbreaks as seen in very low endemic and developed countries [27–29].
HAV infection is vaccine-preventable. However, vaccination is not universally implemented in many underdeveloped and developing countries because infection often through contaminated water and food is commonly asymptomatic and occurs at an early age, thus preventing symptomatic infection as adults. As sanitation and living conditions improved, limited exposure to HAV in children over time leads to susceptibility in adulthood. In developed countries with low rates of HAV infection, increased mobidity and mortality particularly in the unvaccinated elderly have been reported [30]. Since the hepatitis A vaccine is currently not included into the national Expanded Program of Immunization (EPI), the shift in seroprevalence trends we observed in this study are not due to vaccine implementation. Although the efficacy of HAV vaccination can lead to the prevention of infection in up to 94–100%, cost-benefit analysis does not appear to justify its widespread use in the country [31]. In low to very low endemic countries, targeted vaccination of high-risk groups has been recommended [32], which may prevent complications associated with hospitalization, morbidity and mortality [30].
There were several limitations to this study. More females than males in this hospital-based study may reflect the fact that women were more more likely to seek medical care when needed compare to men. Although questionnaires were administered to participants to gather socio-economic data, incomplete reporting of information prevented analyzable classification of socio-economic status that would have been useful. The observed fluctuation in the seroprevalence may be subjected to the differences in the cohort sampled over the years. In addition, declined HAV antibody titers in older individuals may also account for varying seroprevalence rates and that the specificity of immunological assay may underdetect individuals who have low anti-HAV antibodies. We also cannot determine if detectable HAV antibody in the serum resulted from past or active infection, although it is unlikely from immunization. Nevertheless, this most current HAV seroprevalence survey should assist in formulating policy and possible consideration for HAV vaccination in susceptible age groups to further facilitate the control and prevention of HAV.
Supporting Information
(DOCX)
Acknowledgments
We thank the staff of Chumphae Hospital, Uttaradit Hospital, Laplae Hosptial, Laplae Hospital, Naresuan University Hospital, Phra Nakhon Si Ayutthaya Hospital, King Narai Hospital, Trang Hospital, and the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University, for their excellent technical and administrative assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Research Council of Thailand (NRCT), the National Research University Project, Office of Higher Education Commission (WCU-58-006-HR), the Research Chair Grant from the National Science and Technology Development Agency, Chulalongkorn University Centenary Academic Development Project (CU56-HR01), Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530093), the Outstanding Professor of Thailand Research Fund (DPG5480002), the Center of Excellence in Clinical Virology, Chulalongkorn University (GCE58-014-30-004) and King Chulalongkorn Memorial Hospital. This research is also supported by the Postdoctoral Fellowship under Rachadapisaek Sompote Fund, the Graduate School, Chulalongkorn University to Pattaratida Sa-nguanmoo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hepatitis A vaccines. Wkly Epidemiol Rec. 2000;75: 38–44. [PubMed] [Google Scholar]
- 2.Hollinger FB, Martin A. Hepatitis A virus In: Knipe DM, Howley PM, editors. Fields Virology, 6th ed. New York: Lippincott Williams & Wilkins; 2013. pp. 550–581. [Google Scholar]
- 3.Robertson BH, Jansen RW, Khanna B, Totsuka A, Nainan OV, Siegl G, et al. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J Gen Virol. 1992;73: 1365–1377. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Ticehurst JR, Purcell RH, Buckler-White A, Baroudy BM. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987;61: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ. 2009;58: 1–27. [PubMed] [Google Scholar]
- 6.Lima LR, De Almeida AJ, Tourinho Rdos S, Hasselmann B, Ximenez LL, De Paula VS. Evidence of hepatitis A virus person-to-person transmission in household outbreaks. PLoS One. 2014;9:e102925 10.1371/journal.pone.0102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Andrea L, Pérez-Rodríguez FJ, de Castellarnau M, Manzanares S, Lite J, Guix S, et al. Hepatitis A virus genotype distribution during a decade of universal vaccination of preadolescents. Int J Mol Sci. 2015;16: 6842–6854. 10.3390/ijms16046842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stene-Johansen K, Tjon G, Schreier E, Bremer V, Bruisten S, Ngui SL, et al. Molecular epidemiological studies show that hepatitis A virus is endemic among active homosexual men in Europe. J Med Virol. 2007;79: 356–365. [DOI] [PubMed] [Google Scholar]
- 9.Gossner CM, Severi E. Three simultaneous, food-borne, multi-country outbreaks of hepatitis A virus infection reported in EPIS-FWD in 2013: what does it mean for the European Union? Euro Surveill. 2014;19. pii: 20941. [DOI] [PubMed] [Google Scholar]
- 10.Melnick JL. History and epidemiology of hepatitis A virus. J Infect Dis. 1995. March;171 Suppl 1: S2–8. [DOI] [PubMed] [Google Scholar]
- 11.Pintó RM, D'Andrea L, Pérez-Rodriguez FJ, Costafreda MI, Ribes E, Guix S, et al. Hepatitis A virus evolution and the potential emergence of new variants escaping the presently available vaccines. Future Microbiol. 2012;7: 331–346. 10.2217/fmb.12.5 [DOI] [PubMed] [Google Scholar]
- 12.López EL, Contrini MM, Mistchenko A, Kieffer A, Baggaley RF, Di Tanna GL, et al. Modeling the long-term persistence of hepatitis A antibody after a two-dose vaccination schedule in Argentinean children. Pediatr Infect Dis J. 2015;34: 417–425. 10.1097/INF.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 13.Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, et al. Hepatitis A virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol. 2014;21: 227–243. 10.1016/j.meegid.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132: 1005–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunasol P, Cooksley G, Chan VF, Isahak I, John J, Loleka S, et al. Hepatitis A virus: declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 1998. June;29: 255–262. [PubMed] [Google Scholar]
- 16.Burke DS, Snitbhan R, Johnson DE, Scott RM. Age-specific prevalence of hepatitis A virus antibody in Thailand. Am J Epidemiol. 1981;113: 245–249. [DOI] [PubMed] [Google Scholar]
- 17.Poovorawan Y, Paiboonkasemsuthi S, Theamboonlers A, Kamolratanakul P, Chumdermpadetsuk S. Seroepidemiology of antibody to hepatitis A in the rural eastern part of Thailand. Southeast Asian J Trop Med Public Health. 1991;22: 35–38. [PubMed] [Google Scholar]
- 18.Chatproedprai S, Chongsrisawat V, Chatchatee P, Theamboonlers A, Yoocharoen P, Warinsathien P, et al. Declining trend in the seroprevalence of infection with hepatitis A virus in Thailand. Ann Trop Med Parasitol. 2007;101: 61–68. [DOI] [PubMed] [Google Scholar]
- 19.The World Bank. GDP per captita (current US$); 2015. Database: The World Bank [Internet]. Accessed: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- 20.The World Bank. Mortality rate, infant (per 1,000 live births); 2015. Database: The World Bank [Internet]. Accessed: http://data.worldbank.org/indicator/SP.DYN.IMRT.IN.
- 21.Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand. Hepatitis A; 2015. Database: Bureau of Epidemiology, Department of Disease Control, [Internet] Accessed: http://www.boe.moph.go.th/boedb/surdata/disease.php?dcontent=old&ds=11. [Article in Thai].
- 22.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28: 6653–6657. 10.1016/j.vaccine.2010.08.037 [DOI] [PubMed] [Google Scholar]
- 23.Suwantika AA, Yagenoglu S, Riewpaiboon A, Tu HA, Postma MJ. Economic evaluations of hepatitis A vaccination in middle-income countries. Expert Rev Vaccines. 2013;12: 1479–1494. 10.1586/14760584.2013.851008 [DOI] [PubMed] [Google Scholar]
- 24.WHO position paper on hepatitis A vaccines–June 2012. Wkly Epidemiol Rec. 2012;87: 261–276. [PubMed] [Google Scholar]
- 25.Burke DS, Nimmannitya S. Passively acquired antibody to hepatitis A virus in Thai infants. Southeast Asian J Trop Med Public Health. 1980;11: 415–416. [PubMed] [Google Scholar]
- 26.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012: 985646 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, et al. An outbreak of hepatitis A associated with green onions. N Engl J Med. 2005; 353:890–897. [DOI] [PubMed] [Google Scholar]
- 28.Dentinger CM, Bower WA, Nainan OV, Cotter SM, Myers G, Dubusky LM, et al. An outbreak of hepatitis A associated with green onions. J Infect Dis. 2001; 183:1273–1276. [DOI] [PubMed] [Google Scholar]
- 29.Montano-Remacha C, Ricotta L, Alfonsi V, Bella A, Tosti M, Ciccaglione A, et al. Hepatitis A outbreak in Italy, 2013: a matched case-control study. Euro Surveill. 2014; 19: pii: 20906. [PubMed] [Google Scholar]
- 30.Ly KN, Klevens RM. Trends in disease and complications of hepatitis A virus infection in the United States, 1999–2011: a new concern for adults. J Infect Dis. 2015;212: 176–182. 10.1093/infdis/jiu834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teppakdee A, Tangwitoon A, Khemasuwan D, Tangdhanakanond K, Suramaethakul N, Sriratanaban J, et al. Cost-benefit analysis of hepatitis a vaccination in Thailand. Southeast Asian J Trop Med Public Health. 2002; 33: 118–127. [PubMed] [Google Scholar]
- 32.WHO position paper on hepatitis A vaccines: June 2012- recommendations. Vaccine. 2013;31: 285–286. 10.1016/j.vaccine.2012.10.102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.