Abstract

Mechanical point-chirality in a [2]rotaxane is utilized for asymmetric catalysis. Stable enantiomers of the rotaxane result from a bulky group in the middle of the thread preventing a benzylic amide macrocycle shuttling between different sides of a prochiral center, creating point chirality in the vicinity of a secondary amine group. The resulting mechanochirogenesis delivers enantioselective organocatalysis via both enamine (up to 71:29 er) and iminium (up to 68:32 er) activation modes.
Distinctive features imparted by the mechanical bonding in rotaxanes1 (such as their dynamic properties and the ability of the ring to mask regions of the thread) are beginning to be investigated in various aspects of catalysis.2−7 The position of the macrocycle on rotaxane threads has been used to conceal or expose amine groups that can promote different aminocatalysis activation modes,3 and threaded molecular structures have been exploited in processive4 and sequence-specific5 catalysis. A rotaxane has been demonstrated to be an effective chiral ligand in nickel-catalyzed Michael addition reactions,6 and a rotaxane complex was recently employed7 in a gold-mediated cyclopropanation. In both of the latter two cases, embedding the coordinated metal ion within the well-defined binding pocket of the rotaxane resulted in higher stereoselectivity of the products than when using the noninterlocked components as ligands (enantioselectivity in the case of the chiral rotaxane-nickel catalyst;6 diastereoselectivity with the achiral rotaxane-gold catalyst7). However, to date asymmetric catalysis with rotaxanes has only utilized conventional chiral elements.2b,2f,3b,6,7 Here we report on the use of a previously unexplored feature of rotaxane architectures in catalysis, that of chirality induced by the mechanical bond.8,9
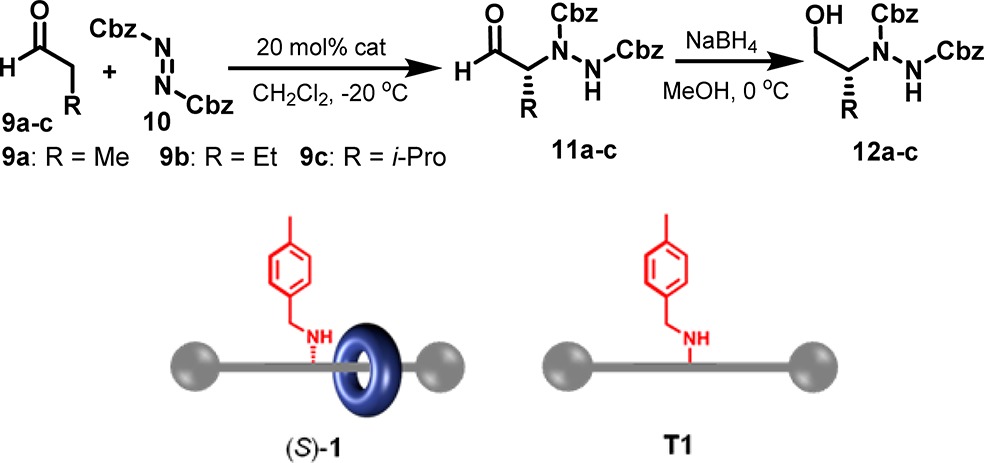
The chemical structures of the rotaxane, (S)-1, and its parent thread, T1, are shown in Figure 1. Thread T1 is achiral, possessing a mirror place through the prochiral center labeled C11. In the rotaxane, however, the bulky 4-tolylamine group (shown in red, Figure 1) prevents macrocycle shuttling between the two succinamide stations (shown in green) resulting in a loss of symmetry. Accordingly, rotaxane (S)-1 has a center of point chirality (C11) with the two succinamide groups of the thread in different chemical environments, one accessible to the macrocycle and one not. Establishing point chiral centers through mechanical bonding is rare,10 and chiral systems of this type have not previously been investigated for potential applications.
Figure 1.

Chemical structures of rotaxane (S)-1 and thread T1, showing the mechanically induced chirality of the rotaxane. The carbon labeled C11 is a prochiral center in the noninterlocked thread (T1) but a chiral center in the rotaxane ((S)-1) as the 4-tolylamine group blocks shuttling of the macrocycle to the other side of the thread.
Two rotaxanes, (S)-1 with the 4-tolylamine group and (S)-2 bearing an analogous 3,5-bis(trifluoromethyl)benzylamine barrier, were prepared in enantioenriched form (84% ee in each case, as indicated by chiral HPLC, see Supporting Information) by unambiguous synthetic routes (see Supporting Information). In each rotaxane synthesis, the key steps involve the five-component macrocyclization about a chiral succinamide thread (e.g., 3) to form a [2]rotaxane intermediate (e.g., 4), followed by extension of the stopper (with 5) to form the second, inaccessible, succinamide group of the axle, shown in Scheme 1 for the synthesis of (S)-1.
Scheme 1. Synthesis of Mechanically Point-Chiral Rotaxane (S)-1.

Reagents and conditions: (i) isophthaloyl chloride, p-xylylenediamine, NEt3, CHCl3, RT, 20 h, 33%; (ii) Pd/C, H2, CH2Cl2, MeOH, RT, 16 h, 91%; (iii) 5, N,N′-dicyclohexylcarbodiimide, dimethylaminopyridine, THF:CH2Cl2, RT, 16 h, 81%; (iv) CF3CO2H, CH2Cl2, RT, 12 h, 73%.
Comparison of the 1H NMR spectrum of rotaxane (S)-1 with that of the component thread, T1, in CD2Cl2 shows an upfield shift of one set of succinamide CH2CH2 protons (ΔδH = −1.25 ppm) in the rotaxane due to shielding by the aromatic rings of the encapsulating macrocycle (Figure 2, H7,8 in the thread splits into two sets of protons, H7,8 and H7′,8′, in the rotaxane). This is a result of the amine group being too bulky for the macrocycle to pass.
Figure 2.
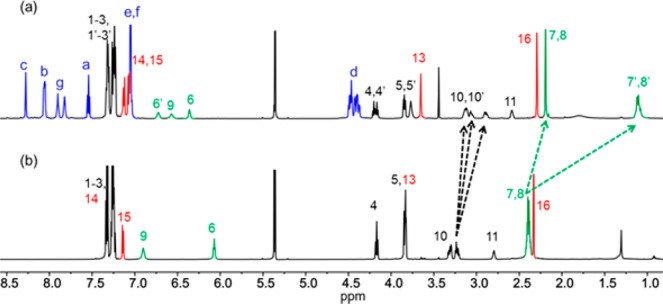
1H NMR spectra (600 MHz, CD2Cl2, 298 K) of (a) rotaxane (S)-1 and (b) thread T1. The letter, number, and color assignments correspond to those shown in Figure 1.
Desymmetrization of the rotaxane axle through the mechanically locked location of the macrocycle creates point chirality at carbon C11. The circular dichroism (CD) spectrum of (S)-1 in CHCl3 confirms this mechanical chirogenesis (Figure S3). In contrast, the achiral thread, T1, does not display a CD response.
Space-filling models of rotaxane (S)-1 suggest that the presence of the macrocycle on one side of the axle should create a well-expressed chiral environment around the amine group (Figure S4). We therefore investigated the efficacy of the chiral rotaxane in asymmetric organocatalysis, first through iminium activation with the Michael addition of 1,3-diphenyl-1,3-propanedione to α,β-unsaturated aldehydes (Table 1). At −20 °C in CH2Cl2 using 20 mol % of the achiral threads as catalyst (T1 and T2), we obtained a racemic mixture of products (entries 1 and 6, Table 1). In contrast, using (S)-1 as catalyst, we obtained an excess of the (S)-Michael adducts (entries 2–5, Table 1). Aldehydes with smaller alkyl substituents (e.g., 6) gave the best enantiomeric ratios, the best being 68:32 (R = Me) in favor of the (S)-product (entry 2, Table 1). Rotaxane (S)-2, which possesses a different aryl substituent, gave similar enantioselectivities (entry 7, Table 1). Decreasing the polarity of the reaction solvent (CH2Cl2) by adding less polar solvents (CCl4, Et2O, PhCH3) had no significant effect on the enantiomeric ratio of the products (Table S1). Decreasing the reaction temperature below −20 °C led to modest increases in enantioselectivity (69:31 er for 8a), whereas at room temperature, racemic mixtures of products were obtained (Table S2).
Table 1. Enantioselective Michael Addition of 1,3-Diphenyl-1,3-propanedione to α,β-Unsaturated Aldehydes via Iminium Catalysis Using Rotaxanes (S)-1, (S)-2, and the Parent Threads T1 or T2 as Catalysts.

| entrya | cat. | R (6) | conv. (%) (24/48 h)b | er of 8 (S:R)c |
|---|---|---|---|---|
| 1 | T1 | Me | 63/95 | 50:50 |
| 2 | (S)-1d | Me | 50/77 | 68:32 |
| 3 | (S)-1d | Et | -/58 | 58:42 |
| 4 | (S)-1d | Pr | -/36 | 55:45 |
| 5 | (S)-1d | CH2CH2Ph | 90/- | 57:43 |
| 6 | T2 | Me | 29/44e | 50:50 |
| 7 | (S)-2d | Me | 70/95 | 67:33 |
Reactions were run with 12.5 μmol 7, 25.0 μmol unsaturated aldehyde (6a–d) in 50 μL of CH2Cl2 at −20 °C with 20% catalyst loading.
Conversions estimated by 1H NMR.
er values determined by HPLC after 24 h.
84% ee.
Low conversions with the T2 catalyst may be due to the poor solubility of this compound in the reaction medium.
To investigate the effectiveness of these mechanically point-chiral catalysts using a different activation mode, we examined the efficacy of (S)-1 in an enamine-mediated α-amination reaction (Table 2). Dibenzyl azodicarboxylate (10) was employed as a nitrogen electrophile and reacted with aldehydes possessing various substituents (9a–c, Table 2). Due to the configurational instability of the α-aminated products (11a–c), the enantioselectivity of the rotaxane-catalyzed reaction was assessed after reduction to the corresponding alcohol (12a–c).11 The result was enantiomeric ratios of up to 71:29 er (entry 3, Table 2), similar to those obtained in the iminium activation mode catalyzed reaction (Table 1).
Table 2. Enantioselective Enamine Catalysis by (S)-1 or T1: Asymmetric α-Amination of Aldehydes with Dibenzyl Azodicarboxylate.
| entrya | cat. | R (9) | conv. (%)b | er of 12 (S:R)c |
|---|---|---|---|---|
| 1 | T1 | Me | >95 | 50:50 |
| 2 | (S)-1d | Me | >95 | 70:30 |
| 3 | (S)-1d | Et | >95 | 71:29 |
| 4 | (S)-1d | i-Pr | 95 | 67:33 |
Reactions were run with 12.5 μmol 10, 25.0 μmol unsaturated aldehyde (9a–c) in 50 μL of CH2Cl2 at −20 °C with 20% catalyst loading. After 24 h, reactions were quenched with MeOH and excess NaBH4 at 0 °C.
Conversions are for compound 11, estimated by 1H NMR.
er values determined by HPLC after 24 h.
84% ee.
The point chirality previously exploited in asymmetric catalysis has invariably arisen from four different covalent groups attached to the tetrahedral center. Our results show that point chirality induced by mechanical bonding between an achiral macrocycle and an achiral thread can also be used to generate a chiral space suitable for asymmetric catalysis. We anticipate that the expression of mechanically generated chirality for asymmetric catalysis, either by ligated metals or through organocatalysis, may be further enhanced through the structural optimization of the rotaxane components.
Acknowledgments
Y.C. and S.E.-C. are grateful to the EU for Marie Curie Action Intra-European Postdoctoral Fellowships. We thank the ERC and the EPSRC for funding and the EPSRC National Mass Spectrometry Centre (Swansea, U.K.) for high resolution mass spectrometry.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b00303.
Additional figures and tables, experimental details, synthetic procedures and characterization data (PDF)
Author Contributions
† Y.C. and S.E.-C. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Neal E. A.; Goldup S. M. Chem. Commun. 2014, 50, 5128. 10.1039/c3cc47842d. [DOI] [PubMed] [Google Scholar]; b van Dongen S. F. M.; Cantekin S.; Elemans J. A. A. W.; Rowan A. E.; Nolte R. J. M. Chem. Soc. Rev. 2014, 43, 99. 10.1039/C3CS60178A. [DOI] [PubMed] [Google Scholar]; c Xue M.; Yang Y.; Chi X.; Yan X.; Huang F. Chem. Rev. 2015, 115, 7398. 10.1021/cr5005869. [DOI] [PubMed] [Google Scholar]; d Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Chem. Rev. 2015, 115, 10081. 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Leigh D. A.; Marcos V.; Wilson M. R. ACS Catal. 2014, 4, 4490. 10.1021/cs5013415. [DOI] [Google Scholar]; For examples of rotaxane catalysts, see refs (3)–7 and ; b Tachibana Y.; Kihara N.; Takata T. J. Am. Chem. Soc. 2004, 126, 3438. 10.1021/ja039461l. [DOI] [PubMed] [Google Scholar]; c Osaki M.; Takashima Y.; Yamaguchi H.; Harada A. J. Am. Chem. Soc. 2007, 129, 14452. 10.1021/ja075140o. [DOI] [PubMed] [Google Scholar]; d Berná J.; Alajarín M.; Orenes R.-A. J. Am. Chem. Soc. 2010, 132, 10741. 10.1021/ja101151t. [DOI] [PubMed] [Google Scholar]; e Miyagawa N.; Watanabe M.; Matsuyama T.; Koyama Y.; Moriuchi T.; Hirao T.; Furusho Y.; Takata T. Chem. Commun. 2010, 46, 1920. 10.1039/b917053g. [DOI] [PubMed] [Google Scholar]; f Tachibana Y.; Kihara N.; Nakazono K.; Takata T. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 1182. 10.1080/10426501003773589. [DOI] [Google Scholar]; g Suzaki Y.; Shimada K.; Chihara E.; Saito T.; Tsuchido Y.; Osakada K. Org. Lett. 2011, 13, 3774. 10.1021/ol201357b. [DOI] [PubMed] [Google Scholar]; h Takashima Y.; Osaki M.; Ishimaru Y.; Yamaguchi H.; Harada A. Angew. Chem., Int. Ed. 2011, 50, 7524. 10.1002/anie.201102834. [DOI] [PubMed] [Google Scholar]; i Caputo C. B.; Zhu K.; Vukotic V. N.; Loeb S. J.; Stephan D. W. Angew. Chem., Int. Ed. 2013, 52, 960. 10.1002/anie.201207783. [DOI] [PubMed] [Google Scholar]
- a Blanco V.; Carlone A.; Hänni K. D.; Leigh D. A.; Lewandowski B. Angew. Chem., Int. Ed. 2012, 51, 5166. 10.1002/anie.201201364. [DOI] [PubMed] [Google Scholar]; b Blanco V.; Leigh D. A.; Marcos V.; Morales-Serna J. A.; Nussbaumer A. L. J. Am. Chem. Soc. 2014, 136, 4905. 10.1021/ja501561c. [DOI] [PubMed] [Google Scholar]; c Blanco V.; Leigh D. A.; Lewandowska U.; Lewandowski B.; Marcos V. J. Am. Chem. Soc. 2014, 136, 15775. 10.1021/ja509236u. [DOI] [PubMed] [Google Scholar]; d Beswick J.; Blanco V.; De Bo G.; Leigh D. A.; Lewandowska U.; Lewandowski B.; Mishiro K. Chem. Sci. 2015, 6, 140. 10.1039/C4SC03279A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Thordarson P.; Bijsterveld E. J. A.; Rowan A. E.; Nolte R. J. M. Nature 2003, 424, 915. 10.1038/nature01925. [DOI] [PubMed] [Google Scholar]; b Coumans R. G. E.; Elemans J. A. A. W.; Nolte R. J. M.; Rowan A. E. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 19647. 10.1073/pnas.0603036103. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Deutman A. B. C.; Monnereau C.; Elemans J. A. A. W.; Ercolani G.; Nolte R. J. M.; Rowan A. E. Science 2008, 322, 1668. 10.1126/science.1164647. [DOI] [PubMed] [Google Scholar]; d Miyagawa N.; Watanabe M.; Matsuyama T.; Koyama Y.; Moriuchi T.; Hirao T.; Furusho Y.; Takata T. Chem. Commun. 2010, 46, 1920. 10.1039/b917053g. [DOI] [PubMed] [Google Scholar]; e van Dongen S. F. M.; Clerx J.; Nørgaard K.; Bloemberg T. G.; Cornelissen J. J. L. M.; Trakselis M. A.; Nelson S. W.; Benkovic S. J.; Rowan A. E.; Nolte R. J. M. Nat. Chem. 2013, 5, 945. 10.1038/nchem.1752. [DOI] [PubMed] [Google Scholar]
- a Lewandowski B.; De Bo G.; Ward J. W.; Papmeyer M.; Kuschel S.; Aldegunde M. J.; Gramlich P. M. E.; Heckmann D.; Goldup S. M.; D’Souza D. M.; Fernandes A. E.; Leigh D. A. Science 2013, 339, 189. 10.1126/science.1229753. [DOI] [PubMed] [Google Scholar]; b De Bo G.; Kuschel S.; Leigh D. A.; Lewandowski B.; Papmeyer M.; Ward J. W. J. Am. Chem. Soc. 2014, 136, 5811. 10.1021/ja5022415. [DOI] [PubMed] [Google Scholar]
- Hoekman S.; Kitching M. O.; Leigh D. A.; Papmeyer M.; Roke D. J. Am. Chem. Soc. 2015, 137, 7656. 10.1021/jacs.5b04726. [DOI] [PubMed] [Google Scholar]
- a Galli M.; Lewis J. E. M.; Goldup S. M. Angew. Chem., Int. Ed. 2015, 54, 13545. 10.1002/anie.201505464. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee A.-L. Nat. Chem. 2016, 8, 8. 10.1038/nchem.2403. [DOI] [PubMed] [Google Scholar]
- For examples of rotaxanes that are mechanically chiral by virtue of the two ends of the axle being inequivalent and the macrocycle rotationally unsymmetrical (‘mechanical planar chirality’ or ‘cyclochirality’), see:; a Yamamoto C.; Okamoto Y.; Schmidt T.; Jäger R.; Vögtle F. J. Am. Chem. Soc. 1997, 119, 10547. 10.1021/ja971764q. [DOI] [Google Scholar]; b Schmieder R.; Hübner G.; Seel C.; Vögtle F. Angew. Chem., Int. Ed. 1999, 38, 3528.. [DOI] [PubMed] [Google Scholar]; c Reuter C.; Seel C.; Nieger M.; Vögtle F. Helv. Chim. Acta 2000, 83, 630.. [DOI] [Google Scholar]; d Kishan M. R.; Parham A.; Schelhase F.; Yoneva A.; Silva G.; Chen X.; Okamoto Y.; Vögtle F. Angew. Chem., Int. Ed. 2006, 45, 7296. 10.1002/anie.200602002. [DOI] [PubMed] [Google Scholar]; e Kameta N.; Nagawa Y.; Karikomi M.; Hiratani K. Chem. Commun. 2006, 3714. 10.1039/b607251h. [DOI] [PubMed] [Google Scholar]; f Makita Y.; Kihara N.; Nakakoji N.; Takata T.; Inagaki S.; Yamamoto C.; Okamoto Y. Chem. Lett. 2007, 36, 162. 10.1246/cl.2007.162. [DOI] [Google Scholar]; g Glen P. E.; O’Neill J. A. T.; Lee A.-L. Tetrahedron 2013, 69, 57. 10.1016/j.tet.2012.10.069. [DOI] [Google Scholar]; h Bordoli R.; Goldup S. M. J. Am. Chem. Soc. 2014, 136, 4817. 10.1021/ja412715m. [DOI] [PMC free article] [PubMed] [Google Scholar]; For rotaxanes that exhibit mechanical sequence isomerism, see:; i Fuller A.-M. L.; Leigh D. A.; Lusby P. J. J. Am. Chem. Soc. 2010, 132, 4954. 10.1021/ja1006838. [DOI] [PubMed] [Google Scholar]
- Stereoisomerism that arises through the restriction of component dynamics in mechanically interlocked structures, as opposed to that arising through sequence information in the constitution of the components (see refs (8a−8h)), is conceptually similar to atropisomerism, which formally only refers to restricted rotation about single bonds.
- a Chatterjee M. N.; Kay E. R.; Leigh D. A. J. Am. Chem. Soc. 2006, 128, 4058. 10.1021/ja057664z. [DOI] [PubMed] [Google Scholar]; b Alvarez-Pérez M.; Goldup S. M.; Leigh D. A.; Slawin A. M. Z. J. Am. Chem. Soc. 2008, 130, 1836. 10.1021/ja7102394. [DOI] [PubMed] [Google Scholar]; c Carlone A.; Goldup S. M.; Lebrasseur N.; Leigh D. A.; Wilson A. J. Am. Chem. Soc. 2012, 134, 8321. 10.1021/ja302711z. [DOI] [PubMed] [Google Scholar]
- a List B. J. Am. Chem. Soc. 2002, 124, 5656. 10.1021/ja0261325. [DOI] [PubMed] [Google Scholar]; b Bøgevig A.; Juhl K.; Kumaragurubaran N.; Zhuang W.; Jørgensen K. A. Angew. Chem., Int. Ed. 2002, 41, 1790.. [DOI] [PubMed] [Google Scholar]; c Mukherjee S.; Jang J. W.; Hoffmann S.; List B. Chem. Rev. 2007, 107, 5471. 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


