ABSTRACT
Nervous systems exhibit many forms of neuronal plasticity during growth, learning and memory consolidation, as well as in response to injury. Such plasticity can occur across entire nervous systems as with the case of insect metamorphosis, in individual classes of neurons, or even at the level of a single neuron. A striking example of neuronal plasticity in C. elegans is the synaptic rewiring of the GABAergic Dorsal D-type motor neurons during larval development, termed DD remodeling. DD remodeling entails multi-step coordination to concurrently eliminate pre-existing synapses and form new synapses on different neurites, without changing the overall morphology of the neuron. This mini-review focuses on recent advances in understanding the cellular and molecular mechanisms driving DD remodeling.
KEYWORDS: CDK-5, CED-3, C. elegans, dynamics, DLK-1 MAPKKK, HBL-1, IRX-1, kinesin, lin-14, microtubule, OIG-1, transcriptional regulation, UNC-30, UNC-55
Introduction
Descriptions of the C. elegans nervous system generally include phrases like “invariant connectivity” or “stereotypic position of neurons.” While such descriptions are accurate, little is said concerning how various forms of neuronal plasticity contribute toward establishing a functional adult C. elegans nervous system. The ventral nerve cord in adult worms contains 76 motor neurons that are grouped into 8 classes by morphology, connectivity, and function,1 while the ventral nerve cord in juvenile L1 worms contains only 22 motor neurons belonging to 3 of these classes.2,3 The birth of motor neurons at the end of L1 and their integration into the embryonic nervous system is thus one example of global circuit plasticity in C. elegans.
Described nearly 4 decades ago by John White and colleagues, another example of circuit plasticity in C. elegans is the switch in connectivity of the GABAergic Dorsal D (DD) motor neurons during locomotory circuit development.2 Six DD neurons are positioned evenly along the ventral nerve cord and extend neurites in ventral and dorsal nerve cords, connected by circumferential commissures1 (Fig. 1A). In adult worms, DD neurons receive synaptic inputs from cholinergic motor neurons in the ventral nerve cord and form neuromuscular synapses to dorsal body wall muscles.1 However, in L1 worms, the ventrally innervating cholinergic neurons, as well as the closely related GABAergic Ventral D (VD) neurons are not yet born. This would leave the DD neurons without synaptic inputs and the ventral muscles without innervation. Intrigued by this paradox, White et al. set out to reconstruct 2 L1 larvae using serial electron micrographs, and discovered that L1 DD neurons formed neuromuscular synapses along their ventral neurites, and received synaptic inputs from dorsally innervating cholinergic neurons.2 Their work defined the phenomenon of DD synapse remodeling, a complete switch in connectivity where DD neurons eliminate pre-existing synapses along their ventral neurites and reform synapses along their dorsal neurites by adulthood, surprisingly without major change in overall neuronal morphology (Fig. 1A). The post-embryonically born VD neurons share similar axon morphology as DD neurons, innervate the ventral muscle and do not undergo remodeling.1 Studies over the past 2 decades have revealed multiple genetic factors that regulate the timing of DD remodeling and changes in cellular components that facilitate this structural plasticity, shedding light on our understanding of the molecular mechanisms of synapse reorganization.
Figure 1.
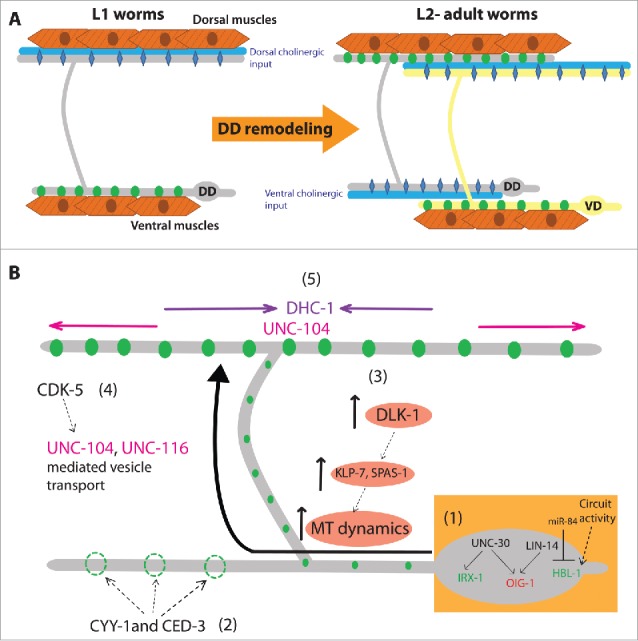
(A) Schematic of DD synapse remodeling. In L1 worms (Left) DD neurons form synapses (green circles) along the ventral neurites and receive cholinergic synaptic inputs (blue) in their dorsal neurites through the ACh receptor (blue diamonds). After DD remodeling in L2 and older animals (right), synapses along the ventral neurite are eliminated, and new synapses are formed with dorsal muscles. The VD neurons now synapse onto the ventral muscles and receive cholinergic synaptic input in ther dorsal neurites. DD neurons now receive cholinergic synaptic input in their ventral neurites (blue). (B) The mechanics of DD remodeling (1) A transcriptional program involving UNC-30, LIN-14, IRX-1 and HBL-1 modulates synapse remodeling in DD neurons, to regulate the expression of factors that promote (green) and inhibit (red) DD remodeling. (2) Ventral synapse elimination requires Cyclin-1/CYY-1 and components of the cell death pathway, including CED-3. (3) Activation of DLK-1 results in an increase in dynamic MTs through the activation of MT catastrophe factors like KLP-7 and SPAS-1. (4) Increased dynamic MTs facilitate UNC-116 mediated synaptic vesicle transport, and CDK-5 stimulates UNC-104 mediated synaptic vesicle transport to the dorsal neurite. (5) Patterning of newly formed synapses is achieved through coordinated activity of UNC-104 and DHC-1.
Factors that regulate the timing of DD remodeling
Using transgenic fluorescent protein reporters to visualize DD neuron axons and synapses, it was determined that DD remodeling begins with new synapses forming at the anterior dorsal neurite of DD1 (the DD neuron closest to the head of the worm) in mid-L1, approximately 10 hours post hatching in wild type animals cultured at 20°C4. Elimination of pre-existing synapses and formation of new synapses proceed through the L1-L2 molt, and DD remodeling is complete by late L2. The heterochronic gene, lin-14,5 was the first factor identified to regulate timing of DD remodeling.4 lin-14 expression decreases at the onset of remodeling, and a reduction in lin-14 activity results in precocious remodeling,4 consistent with the idea that lin-14 acts to prevent remodeling.
The timing of DD remodeling coincides with the birth of the VD neurons, which form synapses to ventral body wall muscles, thereby replacing the larval DD synapses (Fig. 1A). However, the absence of VD or other ventral cholinergic motor neurons does not affect DD remodeling, as evidenced in lin-6 mutants in which postembryonic cell division of late born motor neurons is blocked.2,3 In these mutants, DD remodeling proceeds normally, although their dorsal neurites maintain some larval synaptic inputs from cholinergic neurons.2 The COUP-TF nuclear hormone receptor UNC-55 is expressed in the VD neurons and restricts them from remodeling their synapses.6,7 Ectopic expression of UNC-55 in L1 DD neurons can prevent their remodeling, consistent with a model that lack of UNC-55 in DD neurons enables their ability to remodel.8Transcriptional targets of UNC-55 include the Iroquois-like homeodomain protein IRX-1 and the Hunchback-like transcription factor HBL-1, both of which are normally expressed in the DD neurons and promote remodeling.9,10 Thus UNC-55 inhibits VD synapse remodeling through orchestrating a transcriptional repression program.
Another transcriptional regulator of DD synapse remodeling is the Pitx transcription factor, UNC-30, which is expressed in both DD and VD neurons and functions to specify their GABAergic fate.11-13 UNC-30 promotes the expression of IRX-1 in DD neurons to facilitate remodeling.9 Ultrastructural reconstruction of unc-30 mutants, which was completed by John White 3 decades ago but only recently published, revealed disruption of the synaptic patterns of both adult DD and VD neurons, as well as aberrant innervation of L1 DD neurons.14 Moreover, UNC-30 was shown to co-regulate transcriptional targets with LIN-14 in L1 DD neurons and with UNC-55 in VD neurons.14 This transcriptional strategy involving UNC-30, LIN-14 and UNC-55 promotes the expression of the immunoglobin domain protein OIG-1, which acts to prevent aberrant remodeling in L1 DD neurons and VD neurons in both the larval and adult stages.14
The timing of DD remodeling is also dependent on global circuit activity, as genetic mutants that block or exaggerate synaptic transmission delay or advance the completion of rewiring, respectively.10 However, DD remodeling is not dependent on GABA synaptic transmission.15 Changes in global circuit activity result in corresponding changes in the expression levels of the pro-remodeling gene HBL-1.10 The microRNA miR-84 can repress HBL-1 expression.10 providing an additional layer of regulation on DD remodeling.
While recent studies on DD remodeling have focused largely on remodeling of pre-synaptiC-terminals, John White also observed remodeling of dendritic inputs from the dorsal to ventral DD neurite.2 DD neurons receive cholinergic synaptic inputs, and express acetylcholine receptor subunits, including ACR-12,16 in their dendrites. In L1 DD neurons, OIG-1 prevents remodeling of ACR-12.14,17 Expression of IRX-1 in DD neurons during remodeling represses OIG-1 to promote remodeling of ACR-12.17 In L1 animals, the post-synaptic GABA-A receptor UNC-49 is expressed exclusively in the ventral muscles.18 Following the birth of the VD neurons in L2 animals, UNC-49 receptor clusters appear in both ventral and dorsal muscles.18 Concurrent with DD remodeling, UNC-49 in the ventral body muscles switches from being depolarizing to hyperpolarizing in response to the GABA receptor agonist, muscimol.19 DD remodeling is thus a genetically programmed change in both circuit function and connectivity that occurs at a precise developmental time point, drawing parallels to critical period plasticity in mammalian nervous systems.20 The various factors regulating the timing of DD remodeling are summarized in Figure 1B. While these studies have elegantly elucidated the transcriptional regulation required to modulate circuit rewiring, the cellular changes during the execution of DD remodeling remain unclear.
The cellular changes that facilitate DD remodeling
In developing neurons, synapses are formed either concurrently with axon guidance and elongation, or at the end of axon guidance and target recognition, resulting in en passant or terminal synaptic boutons, respectively. In the worm locomotory circuit, synapses are en passant along neurites, which raises an interesting question as to how DD synapses are formed without axon growth to the new target. As the cytoskeleton is essential for both growth and transport along the neuron processes, we directed our attention to the role of the cellular transport machinery in facilitating DD remodeling.
Microtubules (MTs), polymers of α- and β- tubulin, are the primary cytoskeletal component involved in transport of synaptic material in neurons, and have distinct polarities in axons and dendrites across species. There is much speculation as to whether this difference in MT polarity is a cause or effect of neuronal polarity.21 Much to our surprise, we found that while DD neurites switch polarity after synapse remodeling, with the ventral neurite assuming a dendritic and dorsal neurite an axonal identity, their MT polarity remained unchanged.22 This observation shows that neurite identity could be uncoupled from MT polarity, and suggests that the specificity of axonal and dendritic cargo might be determined by factors besides the orientation of MTs.
Mature neurons contain highly stable MTs, and they also contain an additional population of dynamic MTs, which constantly grow and shrink from the MT plus end. One possible factor regulating cargo trafficking is the number of dynamic MTs, as we observed a dramatic increase in dynamic MTs during DD remodeling.22 Indeed, DD remodeling was dependent on this increase in growing MTs, such that mutant animals with fewer dynamic MTs during remodeling failed to rewire their synapses to the dorsal neurite.22 The increase in number of dynamic MTs facilitates synaptic vesicle transport mediated by plus-end directed motors UNC-104/Kinesin-3 and UNC-116/Kinesin-122 along the DD neuron commissure during remodeling. There is also evidence pointing to the involvement of the cyclin-dependent kinase CDK-5 in modulating UNC-104 activity during remodeling.23 MT polarity remains plus- end out in all DD neuron processes during remodeling, including those in the dorsal neurites.22 Thus a combination of UNC-104 and the minus end directed DHC-1/dynein is required to pattern the newly formed synapses along the dorsal DD neurite.23 Concurrent to new synapse formation to dorsal muscles, existing synapses are eliminated from the ventral neurite of DD neurons, and some synaptic vesicle components appear to be recycled during new synapse formation.23 Emerging evidence implicates both a cyclin Y homolog CYY-123, and the cell death pathway24in DD synapse elimination, but the link between the 2 pathways remains to be addressed.
We also found that activity of the conserved MAP3Kinase DLK-1 is required for new synapse formation during DD remodeling. In conditions where MT structure is compromised due to a genetic alteration in α-tubulin,25 loss of dlk-1 completely blocks DD remodeling.22 The DLK-1 promoter (˜2 kb upstream from the TSS) contains binding sites for LIN-14,26 UNC-308 and UNC-55,8 suggesting possible regulation by these transcription factors. DLK-1 transcript levels are globally upregulated from mid L1-L2 stage,27 and a pulse of DLK-1 at the onset of DD remodeling facilitates new synapse formation.22 DLK-1 localizes to the peri-synaptic region in adult DD neurons,28 and promotes MT growth and growth cone formation in regenerating axons.29,30 During DD remodeling, DLK-1 mediates an increase in the number of dynamic MTs to facilitate synaptic vesicle transport, partly through downstream MT catastrophe factors like KLP-7/Kinesin-13 and SPAS-1/Spastin.22,31However, loss of dlk-1 alone only results in a slight delay in the completion of DD remodeling, whereas loss of dynamic MTs results in a complete block.22 Collectively, these results indicate the existence of one or more redundant pathways that regulate MT growth during DD remodeling, and await further characterization.
Outlook
Compared to most other examples of large scale synapse rewiring involving neurite growth, retraction, or in some cases, even the death of inappropriately connected neurons,32 DD remodeling appears to be unique. However, the discovery of this unique form of neural plasticity is primarily because of our complete understanding of the neural connectivity of C. elegans, made possible by work from John White and colleagues.1,2 Increased technological capability to achieve single cell resolution in other organisms might lead to the description of similar rewiring paradigms. Indeed, an example of synapse refinement in the absence of axon growth was described in the mammalian central nervous system, where imaging of individual axonal arbors of retinal ganglion cells showed that retinogeniculate synapse remodeling can occur without axon retraction.33
Four decades after John White's seminal work, we have learned a great deal about DD remodeling, which has provided a rich platform to study the temporal cues4,6,7,9,10,14,17 as well as the cellular mechanisms that underlie this complete inversion of synaptic connectivity.22-24 Our findings have also revealed a novel role of dynamic MTs in regulating synaptic vesicle transport in the absence of neurite growth or pruning. A recurring theme from these studies is that DD remodeling is guarded by genetic redundancy involving multiple pathways. How each pathway interacts with the rest of the network to coordinate remodeling with such spatio-temporal precision remains a mystery. Since synapse loss is a primary pathophysiology of several neurodegenerative disorders,34,35 our current knowledge and future efforts in elucidating synapse formation and elimination during DD remodeling is important to ultimately understanding the molecular basis of such debilitating conditions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of our labs for their comments on the manuscript. Y.J. is an Investigator of the Howard Hughes Medical Institute.
Funding
This work was supported by HHMI and an NIH grant (NINDS R01 035546 to Y. J.).
References
- [1].White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philosophical Trans Royal Soc B: Biol Sci 1986; 314(1165):1-340; http://dx.doi.org/ 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- [2].White JG, Albertson DG, Anness MAR. Connectivity changes in a class of motorneurons during the development of a nematode. Nature 1978; 271:764-766; PMID:625347; http://dx.doi.org/ 10.1038/271764a0 [DOI] [PubMed] [Google Scholar]
- [3].Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 1983; 100:64-119; PMID:6684600; http://dx.doi.org/ 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- [4].Hallam S, Jin Y. lin-14 regulates the timing of synaptic remodeling in Caenorhabditis elegans. Nature 1998; 395:644-647; http://dx.doi.org/ 10.1038/27091 [DOI] [PubMed] [Google Scholar]
- [5].Ambros V, Horvitz R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 1984; 226(4673):409-16; PMID:6494891; http://dx.doi.org/ 10.1126/science.6494891 [DOI] [PubMed] [Google Scholar]
- [6].Walthall WW, Plunkett JA. Genetic transformation of the synaptic pattern of a motoneuron class in Caenorhabditis elegans. J Neurosci 1995; 15:1035-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou HM, Walthall WW. UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans. J Neurosci 1998; 18(24):10438-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shan G, Kim K, Li C, Walthall WW. Convergent genetic programs regulate similarities and differences between related motor neuron classes in Caenorhabditis elegans. Dev Biol 2005; 280(2):494-503; PMID:15882588; http://dx.doi.org/ 10.1016/j.ydbio.2005.01.032 [DOI] [PubMed] [Google Scholar]
- [9].Petersen SC, Watson JD, Richmond JE, Sarov M, Walthall WW, Miller DM. A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. J Neurosci 2011; 31(43):15362-75; PMID:22031882; http://dx.doi.org/ 10.1523/JNEUROSCI.3181-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thompson-Peer KL, Bai J, Hu Z, Kaplan JM. HBL-1 Patterns Synaptic Remodeling in C. elegans. Neuron 2012; 73(3):453-465; PMID:22325199; http://dx.doi.org/ 10.1016/j.neuron.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jin Y, Hoskins R, Horvitz HR. Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 1994; 372:780-783; PMID:7997265; http://dx.doi.org/ 10.1038/372780a0 [DOI] [PubMed] [Google Scholar]
- [12].Eastman C, Horvitz HR, Jin Y. Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J Neurosci 1999; 19(15):6225-6234; PMID:10414952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cinar H, Keles S, Jin Y. Expression profiling of GABAergic motor neurons in Caenorhabditis elegans. Curr Biol 2005; 15:340-346; PMID:15723795; http://dx.doi.org/ 10.1016/j.cub.2005.02.025 [DOI] [PubMed] [Google Scholar]
- [14].Howell K, White JG, Hobert O. Spatiotemporal control of a novel synaptic organizer molecule. Nature 2015; 523(7558):83-87; PMID:26083757; http://dx.doi.org/ 10.1038/nature14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci 1999; 19(2):539-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petrash HA, Philbrook A, Haburcak M, Barbagallo B, Francis MM. ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans. J Neurosci 2013; 33(13):5524-5532; http://dx.doi.org/ 10.1523/JNEUROSCI.4384-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He S, Philbrook A, Mcwhirter R, Gabel CV, Taub DG, Carter MH, Hanna IM, Francis MM, Miller DM 3rd. Transcriptional control of synaptic remodeling hrough regulated expression of an Immunoglobulin superfamily protein. Curr Biol 2015; 25(19):2541-2548; PMID:26387713; http://dx.doi.org/ 10.1016/j.cub.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gally C, Bessereau JL. GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. J Neurosci 2003; 23(7);2591-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Han B, Bellemer A, Koelle MR. An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans. Genetics 2015; 199:1159-1172; PMID:25644702; http://dx.doi.org/ 10.1534/genetics.114.173963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hensch TK. Critical period regulation. Ann Rev Neurosci 2004; 27:549-79; PMID:15217343; http://dx.doi.org/ 10.1146/annurev.neuro.27.070203.144327 [DOI] [PubMed] [Google Scholar]
- [21].Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol 2011; 71(6):403-18; PMID:21557497; http://dx.doi.org/ 10.1002/dneu.20818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kurup N, Yan D, Goncharov A, Jin Y. Dynamic microtubules drive circuit rewiring in the absence of neurite remodeling. Curr Biol 2015; 25(12):1594-1605; PMID:26051896; http://dx.doi.org/ 10.1016/j.cub.2015.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park M, Watanabe S, Poon VYN, Ou C-Y, Jorgensen EM, Shen K. CYY-1/cyclin Y and CDK-5 differentially regulate synapse elimination and formation for rewiring neural circuits. Neuron 2011; 70(4):742-57; PMID:21609829; http://dx.doi.org/ 10.1016/j.neuron.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meng L, Mulcahy B, Cook SJ, Neubauer M, Wan A, Jin Y, Yan D. The cell death pathway regulates synapse elimination through cleavage of gelsolin in Caenorhabditis elegans neurons. Cell Rep 2015; 11(11):1737-1748; PMID:26074078; http://dx.doi.org/ 10.1016/j.celrep.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baran R, Castelblanco L, Tang G, Shapiro I, Goncharov A, Jin Y. Motor neuron synapse and axon defects in a C. elegans alpha-tubulin mutant. PloS One 2010; 5:e9655; PMID:20300184; http://dx.doi.org/ 10.1371/journal.pone.0009655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hristova M, Birse D, Hong Y, Ambros V. The caenorhabditis elegans heterochronic regulator LIN-14 Is a novel transcription factor that controls the developmental timing of transcription from the insulin / insulin-like growth factor gene ins-33 by direct DNA binding. Mol Cell Biol 2005; 25(24):11059-11072; PMID:16314527; http://dx.doi.org/ 10.1128/MCB.25.24.11059-11072.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grun D, Kirchner M, Thierfelder N, Stoeckius M, Selbach M. Conservation of mRNA and protein expression during development of C. elegans. Cell Rep 2014; 6(3):565-577; PMID:24462290; http://dx.doi.org/ 10.1016/j.celrep.2014.01.001 [DOI] [PubMed] [Google Scholar]
- [28].Nakata K, Abrams B, Gril B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 2005; 120(3):407-20; PMID:15707898; http://dx.doi.org/ 10.1016/j.cell.2004.12.017 [DOI] [PubMed] [Google Scholar]
- [29].Hammarlund M, Nix P, Hauth L, Jorgensen EM, BM . Axon Regeneration requires a conserved MAP Kinase Pathway. Science 2009; 323:802-806; PMID:19164707; http://dx.doi.org/ 10.1126/science.1165527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ghosh-Roy A, Goncharov A, Jin Y, Chisholm AD. Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell 2012; 23(4):716-28; PMID:23000142; http://dx.doi.org/ 10.1016/j.devcel.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roll-mecak A, Mcnally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol 2010; 22:96-103; PMID:19963362; http://dx.doi.org/ 10.1016/j.ceb.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol 2009; 25:161-95; PMID:19575668; http://dx.doi.org/ 10.1146/annurev.cellbio.24.110707.175402 [DOI] [PubMed] [Google Scholar]
- [33].Hong YK, Park S, Litvina EY, Morales J, Sanes JR, Chen C. Refinement of the retinogeniculate synapse by bouton clustering. Neuron 2014; 84(2):332-339; PMID:25284005; http://dx.doi.org/ 10.1016/j.neuron.2014.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: synapses gone cold. Mol Neurodegeneration 2011; 6(1):63; PMID:21871088; http://dx.doi.org/ 10.1186/1750-1326-6-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration. Trends Neurosci 2010; 33(11):513-523; PMID:20850189; http://dx.doi.org/ 10.1016/j.tins.2010.08.002 [DOI] [PubMed] [Google Scholar]


