Abstract
Introduction
Over the past half century, evidence has been accumulating on the emergence of obstructive sleep apnea (OSA), the most prevalent sleep-disordered breathing, as a major risk factor for cardiovascular disease. A significant body of research has been focused on elucidating the complex interplay between OSA and cardiovascular risk factors, including dyslipidemia, obesity, hypertension, and diabetes mellitus that portend increased morbidity and mortality in susceptible individuals.
Conclusion
Although a clear causal relationship of OSA and dyslipidemia is yet to be demonstrated, there is increasing evidence that chronic intermittent hypoxia, a major component of OSA, is independently associated and possibly the root cause of the dyslipidemia via the generation of stearoyl-coenzyme A desaturase-1 and reactive oxygen species, peroxidation of lipids, and sympathetic system dysfunction. The aim of this review is to highlight the relationship between OSA and dyslipidemia in the development of atherosclerosis and present the pathophysiologic mechanisms linking its association to clinical disease. Issues relating to epidemiology, confounding factors, significant gaps in research and future directions are also discussed.
Keywords: Obstructive sleep apnea, Chronic intermittent hypoxia (CIH), Dyslipidemia, Cardiovascular risks
Introduction
Obstructive sleep apnea (OSA) is characterized by frequent arousals from sleep, chronic intermittent hypoxia (CIH), and hemodynamic changes as a result of partial or complete pharyngeal obstruction [1]. Although OSA occurs in all age groups, it is most often found among 40–60 year olds with estimated prevalence of 5–10 % in the US population [2, 3]. The condition has been associated with increased road traffic accidents [4], cardiovascular disease (CVD) [5], stroke [6], mortality [7, 8], days of work lost [9], as well as medical cost burden [10]. The public health impact of OSA is immense, particularly in its association with CVD [11] and death [12].
Recent investigations have suggested links between OSA and CVD, and the implications for therapeutic intervention [13]. Elevations in total cholesterol, triglycerides, and corresponding reduction in HDL have been coupled to oxidative processes commonly found in OSA [14–16]. Basic and clinical data have implicated dyslipidemia in this OSA-related atherosclerosis [17, 18]. Furthermore, research has shown that the metabolic derangements in OSA mirror those in the development of atherosclerosis [19]. In this review, we discuss the relationship between OSA and dyslipidemia in the development of atherosclerosis and examine the pathophysiologic mechanisms linking its association to clinical disease. Issues relating to epidemiology, confounding factors, significant gaps in research, and future directions are also discussed.
Linking obstructive sleep apnea to dyslipidemia
Dyslipidemia, defined as abnormally elevated total cholesterol or triglycerides with or without a corresponding significantly reduced high density lipoprotein (HDL) level, is associated with progressive atherosclerosis in susceptible individuals [20]. Secondary dyslipidemia commonly coexists with positive atherosclerotic derangements, including type 2 diabetes, metabolic syndrome, obesity, coronary artery disease, and hypertension [20]. Recently, there is a rejuvenated interest on the role of OSA in the development of metabolic syndrome including dyslipidemia, a surrogate marker for atherosclerosis [21–28].
Pathophysiological mechanisms
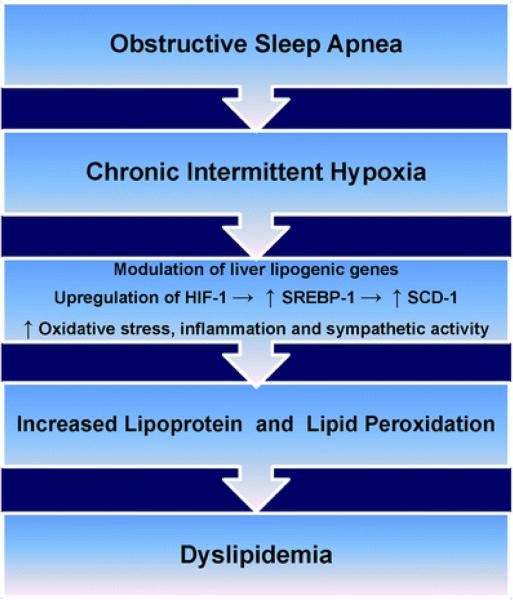
Although the mechanisms responsible for dyslipidemia and adverse cardiovascular outcomes seen in OSA are poorly understood, it appears that CIH is a key factor linking OSA to the progression of dyslipidemia, systemic inflammation, oxidative stress, endothelial dysfunction, and atherosclerosis in both in vitro and in vivo models [29]. In a study involving adult patients with complaints of habitual snoring and without previous diagnosis of sleep-related breathing disorder that were prospectively followed for over 3 years, 85 % was diagnosed with OSA, as defined by apnea hyponea index (AHI) >5/h [30]. The overall prevalence of hypercholesterolemia, hypertriglyceridemia, and hyperuricemia was 61.1 %, 55.3 %, and 25.8%, respectively. Desaturation index, a surrogate marker for hypoxia, was found to be an independent factor contributing to hypercholesterolemia and hypertriglyceridemia [30]. In an animal model, Perry et al. was able to show that only rat exposed to CIH (over 21 days) presented with significant increase in triglyceride compared to control (p<0.05) and those exposed to shorter duration of intermittent hypoxia (over 4 days; p<0.05) [31]. As discussed below and illustrated in Fig. 1, studies have shown that CIH induced by OSA is associated with generation of sterol regulatory element binding protein-1 (SREBP-1) and stearoyl coenzyme A desaturase-1 (SCD-1), peroxidation of lipids, HDL dysfunction, increased total cholesterol level, and sympathetic dysfunction [14, 24, 32–36]. Altogether, these factors create a pro-inflammatory milieu responsible for development of dyslipidemia and propagation of atherosclerosis and CVD in OSA [29].
Figures.
Illustration of pathophysiological pathway by which obstructive sleep apnea via chronic intermittent hypoxia induces dyslipidemia. HIF-1 hypoxia inducible factor 1, SREBP-1 sterol regulatory element binding protein 1,SCD-1 stearoyl coenzyme A desaturase 1
Effect of hypoxia on SREBP and SCD
Experiments in mice have shown that CIH results in hyperlipidemia through up-regulation of genes responsible for hepatic lipid biosynthesis [37]. In another study involving lean mice on regular diet (chow diet), intermittent hypoxiawas associated with increased serum total cholesterol (84 to 94 mg/dl) and triglycerides (34 to 46 mg/dl) within 5 days when matched with control (p<0.001). Similarly, an increase in liver lipid content was noted (18.8±3.3 mg/g vs 9.6± 0.7 mg/g in control; p<0.05) in this study. Of note, the effect of hypoxia raised SREBP-1 level by 26.7±4.0 %; p<0.001 with a striking >2-fold increase in SCD-1 mRNA (and SCD-1 protein levels). These changes were absent in control mice under identical conditions [32]; however, when control mice were subjected to CIH over 12 weeks they produced similar results (p<0.05) without implicating SREBP-2 [37].
Putatively, CIH induces hypoxia inducible factor-1 (HIF-1) in the liver which, in turn, activates both SREBP-1 and SCD-1 [32]. In concert, SREBP-1 induces gene expression of SCD-1, independent of SREBP-2, to enhance triglyceride and phospholipid biosynthesis [32, 33]. More importantly, the degree of hyperlipidemia and changes in hepatic SCD-1 levels were directly dependent on the severity of local hypoxia [14]. Savransky et al. demonstrated both in humans and mice the pivotal role of SCD-1 in the development of dyslipidemia and atherosclerosis in OSA. It was shown that the expression of hepatic SCD-1 significantly correlated with the degree of oxyhemoglobin desaturation in patients with OSA suggesting that CIH induces SCD-1 (r00.68, p<0.001) [24], which may act through direct activation of SREBP-1 or via HIFs [24, 34–36].
Activation of SREBP-1 has also been suggested as a key mediator of dyslipidemia even in the absence of hypoxia [38]. It is known that a classic action of insulin is stimulation of fatty acid synthesis during a time of carbohydrate excess, an action opposed by glucagon via cyclic adenosine monophosphate. Evidently, the fatty liver that is commonly associated with obesity and insulin resistance is due to SREBP-1 which is elevated in response to high insulin levels. Available evidence suggests that this insulin action is mediated via SREBP-1 [38]. Therefore SREBP-1 also increases lipogenic gene expression and enhances fatty acid synthesis and triglyceride accumulation via other non-hypoxic activator [39, 40].
Effect of hypoxia on lipid peroxidation and HDL dysfunction
In addition to affecting the circulating levels of cholesterol, OSA may modulate the functions of lipids leading to generation of oxidized and dysfunctional lipids via oxidative stress [41, 42]. Oxidized form of low-density lipoprotein (LDL) cholesterol is much more atherogenic than the unoxidized form and individuals with OSA have been reported to exhibit lipid peroxidation with higher levels of oxidized LDL cholesterol compared with non-OSA individuals [42]. Carpagnano et al. showed that oxidative stress measured by 8-isopropstane levels, a reliable marker of lipid peroxidation formed by the effect of oxidative stress on arachidonic acid, was increased in the airway and plasma of patients with OSA and that serum level of 8-isoprostane was reduced by bi-level or continuous positive airway pressure (Bi-\CPAP) therapy [43, 44].
In another study, it was observed that increased oxidative stress in OSA is not only associated with increased lipid peroxidation but also with HDL dysfunction: OSA subjects were shown to have a greater degree of HDL dysfunction (p<0.01) and increased oxidized LDL levels (p<0.05) even though they had similar concentrations of plasma lipids and apolipoproteins compared to controls [18]. HDL isolated from subjects with OSA has impaired ability to prevent oxidation of LDL ex vivo [18]. This HDL dysfunction is correlated with the severity of OSA in these subjects (r00.53, p<0.001) [18].
Even though the precise mechanism of HDL in mitigating atherogenesis is uncertain, several hypothesis have been suggested which include: cholesterol efflux from cells in arterial wall [45], binding of oxidant molecules as cholesteryl ester hydroperoxides which are rapidly removed by liver cell [46], destruction of lipid hydroxyperoxides that oxidize LDL phospholipids [47, 48], and more importantly, HDL inhibition of monocyte chemotactic protein (MCP-1) which along with other adhesionmolecules allow stimulated/injured endothelial cells to internalize monocytes that later transform into macrophages in the propagation of atherosclerosis [49]. These critical functions of HDL are mediated by paraoxonases (PON-1 and PON-3: enzymes synthesized by the liver but transported along with HDL in plasma) and apolipoprotein, apoA-1 [47–50], which are dysfunctional in OSA [18].
Research indicates that the protective antioxidant enzyme(PON-1) is not only diminished in OSA, but also inversely correlates with severity of OSA determined by respiratory disturbance index [51]. Evidence also suggests that these antioxidant/anti-inflammatory properties of HDL are superior to HDL concentration in terms of discriminating between those with/without coronary heart disease [52, 53]. Since atherosclerosis is in part initiated by the presence of oxidized LDL in the arterial intima, one may conclude that these antioxidant/anti-inflammatory effects of HDL may account, at least in part, for the antiatherogenic potential of HDL and possibly the role of OSA in dyslipidemia/atherosclerosis.
Effect of hypoxia on sympathetic activity
Neurocardiogenic and neurohormonal dysregulation has been described in OSA as a consequence of hypoxia [54]. 2 Unopposed or hypersympathetic tone may have adverse effects on cholesterol metabolism [55–57]. This observation is supported by the evidence indicating that blockade of beta and alpha receptors have well-described effects on serum HDL and triglyceride levels [55–57]. Agents that block alpha-1 receptor are known to increase HDL and decrease serum triglyceride [46], while beta adrenergic blockers have the opposite effects [56, 57]. However, functional mutations of the beta-2 adrenergic receptor have no proven effect on OSA-induced lipid concentrations, perhaps implying a mechanism attributable to beta-1 receptor downstream the signal pathway [58].
Also, both noradrenaline and cortisol regulate hormone- sensitive lipoprotein and modify HDL synthesis [59, 60]. Thus, the heightened sympathetic milieu in patients with OSA is not only linked to CVD and adverse cardiovascular events [61, 62], but may play a central role in the development of dyslipidemia [53].
Evidence from clinical studies
The clinical evidence linking obstructive sleep apnea with dyslipidemia is limited. Available studies are largely cross-sectional and non-randomized trials [29]. Data compiled by Drager et al. [29] from various studies, however, shows intriguing trends and implies the following:
Virtually all cases without significant association had small sample size. The few studies with significant findings had large sample size and amply powered: Newman et al. (4,491 adults) and Roche et al. (846) [63, 64].
The few randomized studies did not show any clear evidence overall that treatment of OSA with CPAP was effective in improving dyslipidemia, besides non-examined lipids as primary outcome [29].
In 2006, Borgel et al., in a single center study of 470 patients, suggested an association of OSA with cardiovascular risk factors including dyslipidemia [61]. The 6-month study duration found: (1) the number of hypopnea or apnea (AHI) is independently associated with low HDL levels, p<0.001, (2) Bi-/CPAP therapy significantly increased the mean HDL serum levels by 5.8 %(p00.013) within the study period, and (3) the relation between the changes of AHI and HDL or triglyceride indicated some reversibility of dyslipidemia with Bi-/CPAP therapy. In fact, there was improvement in mean lipid/lipoprotein serum levels in all subjects with initial dyslipidemia through Bi-/CPAP therapy [61]. After adjustment for age, gender, BMI, DM, and use of lipid lowering medications, a significant improvement in lipid profiles persisted with OSA therapy [61]. These findings are consistent with observation from sleep heart health study involving 6,440 females >65 years with moderate-to- severe OSA [63].
Recently, the Sleep and Circadian Research Group conducted a randomized, placebo-controlled crossover study evaluating CPAP therapy on postprandial lipidemia over a full 24-h encompassing both sleep and wake periods among 29 patients followed over 2 months. In this study, participants were >21 years old with AHI ≥25/h of sleep ± Oxygen Desaturation Index ≥20/h measured by overnight polysomnography. Hypertriglyceridemia peaks which were found during both wakefulness (2 p.m.) and sleep (3 a.m.) were significantly reduced following CPAP treatment compared with placebo along with reduction in mean 24-h total cholesterol (95 % CI, −0.27 to −0.11; p<0.00001) [65]. Analysis of pooled data from two randomized controlled trials demonstrated that the group treated with therapeutic CPAP for 1 month experienced a significant decrease in serum total cholesterol, but the difference in the fall in total cholesterol between the therapeutic CPAP and the control groups failed to reach statistical significance [66].
Conclusion
Though a direct cause and effect relationship between OSA and dyslipidemia is yet to be established, there is a significant and growing body of evidence that a strong association exists. Population observations, basic science and clinical modalities have begun to unravel the complex interplay involved in the pathophysiology and mechanism of OSA-mediated dyslipidemia. Still there is need for large-scale long-term randomized clinical and translational investigations to explicate the complex relationship between OSA and dyslipidemia and define precise targets for intervention.
Acknowledgment
This research was supported by funding from the NIH (R25HL105444, R01HL095799 and R01MD004113).
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–484. doi: 10.1146/annurev.me.27.020176.002341. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 2.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289(17):2230–2237. doi: 10.1001/jama.289.17.2230. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 4.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos–Santander. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 5.Chami HA, Resnick HE, Quan SF, Gottlieb DJ. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation. 2011;123(12):1280–1286. doi: 10.1161/CIRCULATIONAHA.110.974022. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, Shakar E, O'Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea–hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Won CH, Chun HJ, Chandra SM, Sarinas PS, Chitkara RK, Heidenreich PA. Severe obstructive sleep apnea increases mortality in patients with ischemic heart disease and myocardial injury. Sleep Breath. 2012 doi: 10.1007/s11325-012-0653-y. doi:10.1007/s11325-012-0653-y, PMID: 22294346 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 9.Sjösten N, Vahtera J, Salo P, Oksanen T, Saaresranta T, Virtanen M, Pentti J, Kivimäki M. Increased risk of lost workdays prior to the diagnosis of sleep apnea. Chest. 2009;136(1):130–136. doi: 10.1378/chest.08-2201. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 10.Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, Psaty BM. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749–755. doi: 10.1093/sleep/22.6.749. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Phillipson EA. Sleep apnea -a major public health problem. N Engl J Med. 1993;328(17):1271–1273. doi: 10.1056/NEJM199304293281712. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 12.The National Commission on Sleep Disorders Research . Wake up America: a national sleep alert. US Government Printing Office; Washington: 2002. [Google Scholar]
- 13.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Savransky V, Nanayakkara A, Smith PL, O'donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102(2):557–563. doi: 10.1152/japplphysiol.01081.2006. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 15.Malakasioti G, Alexopoulos E, Befani C, Tanou K, Varlami V, Ziogas D, Liakos P, Gourgoulianis K, Kaditis AG. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath. 2011 doi: 10.1007/s11325-011-0560-7. doi:10.1007/s11325-011-0560-7, PMID: 21811879 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Vatansever E, Surmen-Gur E, Ursavas A, Karadag M. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath. 2011;15(3):275–282. doi: 10.1007/s11325-010-0378-8. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 17.Savransky V, Nanayakkara A, Li J, Bevans S, Smith P, Rodriguez A, Polotosky V. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2005;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan KC, Chow WS, Lam JC, Lam B, Wong WK, Tam S, Ip MS. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184(2):377–382. doi: 10.1016/j.atherosclerosis.2005.04.024. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 19.Lin QC, Zhang XB, Chen GP, Huang DY, Din HB, Tang AZ. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. 2011Sleep Breath:571–578. doi: 10.1007/s11325-011-0544-7. doi:10.1007/s11325-011-0544-7; PMID: 21681412 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 21.Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4(3):261–272. PubMedCentralPubMed. [PMC free article] [PubMed] [Google Scholar]
- 22.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3(5):467–472. PubMedCentralPubMed. [PMC free article] [PubMed] [Google Scholar]
- 23.Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, Kuriyama T. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131(5):1387–1392. doi: 10.1378/chest.06-1807. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 24.Savransky V, Jun J, Nanayakkara A, Fonti S, Moser A, Steele K, Schweitzer M, Patil S, Bhanot S, Schwartz A, Polotsky V. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl Coenzyme A desaturase. Circ Res. 2008;103(10):1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunstein RR, Stenlof K, Hedner J, Sjostrom L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int J Obes Relat Metab Disord. 1995;19(6):410–418. PubMed. [PubMed] [Google Scholar]
- 26.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 27.Davies RJ, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnea and snoring; their comparison with matched controls and response to treatment. J Sleep Res. 1994;3(3):180–185. doi: 10.1111/j.1365-2869.1994.tb00126.x. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 28.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140(6):654–659. doi: 10.1067/mpd.2002.123765. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 29.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):161–165. doi: 10.1097/MED.0b013e3283373624. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou YT, Chuang LP, Li HY, Fu JY, Lin SW, Yang CT, Chen NH. Hyperlipidemia in patients with sleep-related breathing disorders: prevalence & risk factors. Indian J Med Res. 2010;131:121–125. PubMed. [PubMed] [Google Scholar]
- 31.Perry JC, D'Almeida V, Souza FG, Schoorlemmer GH, Colombari E, Tufik S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir Physiol Neurobiol. 2007;156(3):250–258. doi: 10.1016/j.resp.2006.10.004. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97(7):698–706. doi: 10.1161/01.RES.0000183879.60089.a9. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics. 2006;25(3):450–457. doi: 10.1152/physiolgenomics.00293.2005. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AL, Todd BL, Espenshade PJ. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell. 2005;120(6):831–842. doi: 10.1016/j.cell.2005.01.012. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9(9):1391–1396. doi: 10.1089/ars.2007.1691. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91(5):803–806. doi: 10.1113/expphysiol.2006.033498. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99(5):1643–1648. doi: 10.1152/japplphysiol.00522.2005. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 38.Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. PubMedCrossRef. [PubMed] [Google Scholar]
- 40.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty liver in two mouse models of diabetic mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 41.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–570. doi: 10.1164/ajrccm.162.2.9908091. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 42.Barceló A, Miralles C, Barbé F, Vila M, Pons S, Agustí AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16(4):644–647. doi: 10.1034/j.1399-3003.2000.16d13.x. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 43.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122(4):1162–1167. doi: 10.1378/chest.122.4.1162. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 44.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124(4):1386–1392. doi: 10.1378/chest.124.4.1386. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108(6):661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 46.Sattler W, Stocker R. Greater selective uptake by Hep G2 cells of high-density lipoprotein cholesteryl ester hydroperoxides than of unoxidized cholesteryl esters. Biochem J. 1993;294(Pt 3):771–778. doi: 10.1042/bj2940771. PubMedCentralPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275(23):17527–17535. doi: 10.1074/jbc.M910376199. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 48.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106(4):484–490. doi: 10.1161/01.cir.0000023623.87083.4f. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 49.Mackness B, Hine D, Liu Y, Mastorikou M, Mackness M. Paraoxonase-1 inhibits oxidized LDL-induced MCP-1 production by endothelial cells. Biochem Biophys Res Commun. 2004;318(3):680–683. doi: 10.1016/j.bbrc.2004.04.056. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 50.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273(11):6088–6095. doi: 10.1074/jbc.273.11.6088. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 51.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27(1):123–128. PubMed. [PubMed] [Google Scholar]
- 52.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42(8):1308–1317. PubMed. [PubMed] [Google Scholar]
- 53.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108(22):2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 54.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leren P, Eide I, Foss OP, Helgeland A, Hjermann I, Holme I, Kjeldsen SE, Lund-Larsen PG. Antihypertensive drugs and blood lipids: the Oslo study. Br J Clin Pharmacol. 1982;13(Suppl 2):441S–444S. doi: 10.1111/j.1365-2125.1982.tb01954.x. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marrone O, Riccobono L, Salvaggio A. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103(3):722–727. doi: 10.1378/chest.103.3.722. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 57.Lowenstein J. Effects of prazosin on serum lipids in patients with essential hypertension: a review of the findings presented at the Satellite Symposium on coronary heart disease, hypertension and other risk factors, Milan, 1983. Am J Cardiol. 1984;53(3):21A–23A. doi: 10.1016/0002-9149(84)90830-0. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 58.Bartels NK, Börgel J, Wieczorek S, Büchner N, Hanefeld C, Bulut D, Mügge A, Rump LC, Sanner BM, Epplen JT. Risk factors and myocardial infarction in patients with obstructive sleep apnea: impact of β2-adrenergic receptor polymorphisms. BMC Med. 2007;5:1. doi: 10.1186/1741-7015-5-1. PubMedCentralPubMedCrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177(3):385–390. doi: 10.1046/j.1365-201X.2003.01091.x. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 60.Ottosson M, Vikman-Adolfsson K, Enerback S, Olvercrona G, Bjorntorp P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;79(3):820–825. doi: 10.1210/jcem.79.3.8077367. PubMed. [DOI] [PubMed] [Google Scholar]
- 61.Börgel J, Sanner BM, Bittlinsky A, Keskin F, Bartels NK, Buechner N, Huesing A, Rump LC, Mügge A. Obstructive sleep apnea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J. 2006;27(1):121–127. doi: 10.1183/09031936.06.00131304. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 62.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 63.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF. Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–59. doi: 10.1093/aje/154.1.50. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 64.Roche F, Sforza E, Pichot V, Maudoux D, Garcin A, Celle S, Picard-Kossovsky M, Gaspoz JM, Barthélémy JC. PROOF Study Group. Obstructive sleep apnoea/hypopnea influences high-density lipoprotein cholesterol in the elderly. Sleep Med. 2009;10(8):882–886. doi: 10.1016/j.sleep.2008.07.017. PubMedCrossRef. [DOI] [PubMed] [Google Scholar]
- 65.Philips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184(3):355–361. doi: 10.1164/rccm.201102-0316OC. CrossRef. [DOI] [PubMed] [Google Scholar]
- 66.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep data from randomized controlled trials. Thorax. 2004;59(9):777–782. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]