Abstract
Rapidly proliferating solid tumor cells are often dependent on glycolysis for ATP production even in normoxia (the Warburg effect), however it is not yet clear whether acute leukemias have a similarly increased dependence on aerobic glycolysis. We report that all leukemia subtypes (pre-B ALL, T-ALL and AML) demonstrated growth arrest and cell death when treated the novel glycolysis inhibitor 3-BrOP. Potentiated ATP depletion and pro-apoptotic effects were seen for 3-BrOP combinations with the cytochrome-C-reductase inhibitor antimycin A and the mTOR inhibitor rapamycin. These results reveal a potential role for glycolysis inhibition in acute leukemia subtypes and suggest potential combinations.
Keywords: ALL, AML, mTOR, ATP
Introduction
Acute leukemias occur in both adult and pediatric patients. In children, leukemias are the most common form of cancer and are the most frequent cause of disease-related death (1). Acute lymphoblastic leukemia (ALL) is the most common form of leukemia in children, and precursor B cell phenotype accounts for approximately 80% of cases, with T cell phenotype in 20%. Although pediatric ALL protocols have excellent outcomes, those children who relapse and adults with ALL still have <40% long-term survival (1–3). In adults, acute myeloblastic leukemia is more common, and both children and adults have <50% survival, despite intense therapy (4, 5). These acute leukemias represent a significant challenge where intense therapies, including transplant, lead to significant morbidity and mortality with inadequate outcomes. As we explore new therapeutic opportunities, we observe that the common striking feature of all acute leukemias is their potential for rapid proliferation and high metabolic demand.
ATP generation is an essential metabolic process through which cells obtain their energy supply. Mammalian cells have two methods to generate ATP, through glycolysis in the cytoplasm and oxidative phosphorylation (or respiration) in the mitochondria. The glycolysis pathway is relatively inefficient, requiring one glucose molecule for the net production of two ATP molecules. In contrast, oxidative phosphorylation is more efficient, where one glucose molecule will yield thirty-six ATP molecules. Typically mammalian cells rely primarily on oxidative phosphorylation for energy production in aerobic conditions (6, 7). Otto Warburg first described how cancer cells demonstrate increased utilization of glycolysis for ATP production even in aerobic conditions, now known as the Warburg effect (8). In addition to increased glycolytic rates, cancer cells appear to have reduced capacity for oxidative phosphorylation via multiple mechanisms, including down regulation of enzymes involved in oxidative phosphorylation via mitochondrial/nuclear mutations, and dysregulation of metabolic pathways through oncogenic transformation, e.g. BCR-ABL (9, 10). Although there are many examples of solid tumors having altered metabolism with high rates of glucose uptake and glycolysis, it was only recently reported that this occurs in leukemia cells, specifically precursor-B ALL and AML (11, 12). Additional studies have revealed the importance of glycolysis in steroid sensitivity in T-ALL (13), death receptor-induced apoptosis in a T-ALL and an AML line (14), as well as a balance between sensitivity to glycolysis inhibitors and oxidative phosphorylation inhibitors in AML cell lines (15). These studies support the hypothesis that leukemia subtypes (B-ALL, T-ALL and AML) may all demonstrate a dependence on glycolysis in aerobic conditions, providing a potential therapeutic opportunity for the use of glycolysis inhibitors in acute leukemias.
Early glycolysis inhibitors, such as 2-deoxyglucose (2-DG), were effective at inhibiting glycolysis, but only at very high millimolar concentrations and has been shown to induce the expression of P-glycoprotein encoded by the MDR1 gene, possibly increasing a cancer cells ability to develop chemoresistance (16). 2-DG has demonstrated that glycolysis inhibition is a valid therapeutic approach, however the clinical feasibility has been limited with this reagent (9, 16, 17). The next generation of glycolysis inhibitors is represented by 3-bromopyruvate (3-BrPA). 3-BrPA inhibits the enzyme hexokinase II, which is the rate-limiting step of the glycolytic pathway (7). This compound has greater potency but still requires dosing in the high micromolar range and has limited solubility and biodistribution (7, 9). The novel, third-generation glycolysis inhibitor, 3-bromo-2-oxopropionate-1-propyl ester (3-BrOP) has recently been reported. 3-BrOP is a cell permeable ester of 3-bromopyruvate (3-BrPA). Once inside the cell, 3-BrOP is hydrolyzed by cellular esterase to release 3-BrPA. The esterification of 3-BrPA into 3-BrOP has yielded a highly stable and soluble compound that can be readily be used for evaluation of tumor response in vitro and in vivo. Importantly, 3-BrOP may be up to 1000-fold more potent than 2-deoxyglucose, now allowing feasible inhibition of glycolysis at 20mcM rather than 20mM. Previous reports have shown that 3-BrOP as well as its precursor, 3-BrPA can induce cell death in a variety of cancers including human lymphoma, hepatocellular carcinoma, colorectal carcinoma, and the human acute myeloid leukemia cell line HL60 (7, 18, 19). Inhibition of glycolysis with these agents has been highly effective and selective in vivo. For example, in a rabbit model of hepatocellular carcinoma, 3-BrPA induced 90% tumor necrosis with minimal effect on the normal liver tissue (18).
Although cancer cells have increased dependence on glycolysis, many are still able to use mitochondrial oxidative phosphorylation for ATP production which may lead to resistance to a glycolysis inhibitor. Antimycin A is an inhibitor of electron transfer in the mitochondria at complex III, and a potent inhibitor of cytochrome-C-reductase (20, 21). As a single agent, antimycin A has been shown to induce apoptosis at a range of 20 nM to 2 mcM (22) and is associated with growth arrest and increased glycolytic rate (23). We therefore proposed that combining sub-therapeutic doses of antimycin A with 3-BrOP may effectively kill cancer cells through severe ATP depletion while sparing normal cells.
Mammalian target of rapamycin (mTOR) is a sensor of amino acid and ATP homeostasis, and is critical for the cellular response to metabolic stress (24). Inhibition of glycolysis and subsequent ATP depletion will likely lead to compensatory survival signals through the mTOR pathway. Therefore, inhibition of both glycolysis and mTOR together may cooperate to induce severe metabolic dysregulation and cell death. Rapamycin inhibits the function of the mTOR pathway inducing alterations in cellular metabolism and survival signaling (25, 26) and is effective both in vivo and in vitro in precursor-B ALL (25, 27). It has also been shown that 3-BrOP and rapamycin can cooperate in other tumor models (7). Therefore, we propose that inhibition of the mTOR pathway under conditions of ATP depletion by 3-BrOP may prevent compensatory metabolic mechanisms and enhance apoptosis in acute leukemias.
In this report we evaluated the effects of the novel third generation glycolysis inhibitor 3-BrOP on a panel of human acute leukemias, representing precursor-B, T and myeloid subtypes. Furthermore we assessed the effects of combined inhibition of glycolysis and oxidative respiration or the mTOR pathway.
Materials and Methods
Cell Culture
Sixteen human acute leukemia cell lines were maintained in RPMI 1640 containing 10% fetal bovine serum and penicillin/streptomycin. The cell lines included precursor B acute lymphoblastic leukemia (Nalm6, Nalm16, JM1, REH and 697), T cell acute lymphoblastic leukemia (SupT1, Molt4, CCL119, Loucy, and Jurkat), and acute myeloblastic leukemia (HL60, NB4, ML1, U937 (histiocytic sarcoma with myeloid features CD13/15/33), THP1, KG1).
Immunoblot Analysis
Whole cell lysates (25–50 µg) were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes (PVDF, Millipore Corporation), using standard techniques (28). Antibodies were obtained for caspase 3 (Cell Signaling) and beta actin (Cell Signaling). Secondary antibodies conjugated to horseradish peroxidase (SantaCruz, Cell Signaling) were detected using an enhanced chemiluminescence kit (ECLplus, Amersham International) according to the manufacturer's instructions.
Cytotoxicity assays
Cytotoxicity of 3-bromopyruvic acid propyl ester (3-BrOP), rapamycin (Sigma-Aldrich), and antimycin A (Sigma-Aldrich) were assayed following 6 to 72 hours exposure (2.5×104 cells/mL) using AlamarBlue reagent (BioSource) according to manufacturer’s instructions.
Growth Inhibition Assays
3-bromo-2-oxopropionate-1-propyl ester (3-BrOP) was kindly provided by Dr. Peng Huang (M. D. Anderson Cancer Center), dissolved in 1-propanol and stored at 4°C as a 300 mM stock solution. Rapamycin (Sigma-Aldrich) was stored as a 100mM stock in DMSO at −20°C. Antimycin A (Sigma) was stored as a 10mM stock in ethanol at −20°C. Cells were passaged during their exponential growth phase and treated with 3-BrOP, rapamycin, and antimycin A either alone or in combination for 6–96 hours. Cells were counted with a hemocytometer with trypan blue to exclude dead cells.
Cell Cycle and Apoptosis
Cells were treated with 3-BrOP, rapamycin, and antimycin A either alone or in combination for 24–96 hours. Cells were solubilized with triton X-100 and stained with 50 mcg/mL propidium iodide for 1 hour at 4°C. DNA content was measured by flow cytometry. The data presented are from at least three separate experiments.
ATP Assays
Cells were treated with 3-BrOP, antimycin A, or both for 6–72 hours at a range of concentrations. ATP concentrations were measured with the ATPlite Kit according to manufacturer’s instructions (PerkinElmer).
Statistical evaluation
For all assays, unless otherwise noted, mean +/− standard deviation fro 3–5 replicates are used. P-values from comparison of two data points was accomplished using a two-tailed student’s T-test in Microsoft Excel.
Results
3-BrOP induces growth arrest and caspase-mediated cell death in acute leukemia
We tested the effect of 3-BrOP on a panel of fifteen human acute leukemia cell lines including five precursor-B ALL cell lines (Nalm6, Nalm16, 697, REH, and JM1), five T-cell ALL cell lines (SupT1, Molt4, CCL-119, Loucy, and Jurkat), and five AML cell lines (NB4, ML1, U937, THP1, and KG1). The HL60 AML cell line had been previously shown to be susceptible to 3-BrOP with an IC50 of approximately 20 mcM (7).
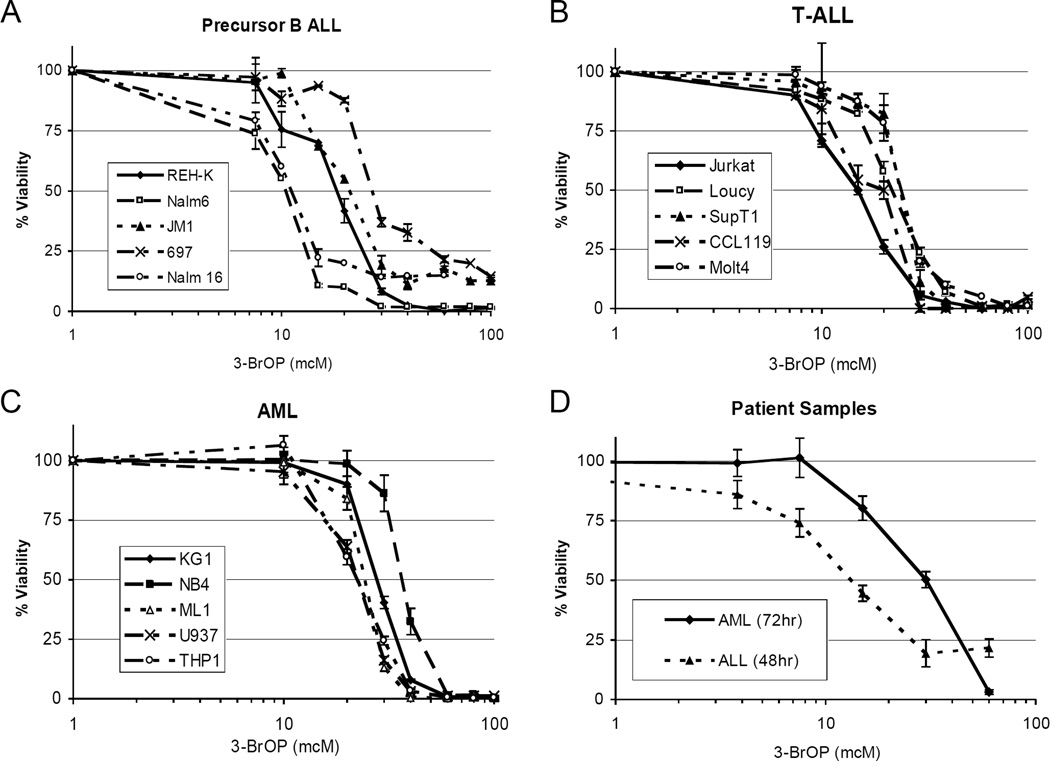
Viability as measured with the AlamarBlue viability assay was markedly diminished in all fifteen lines following 3-BrOP treatment. The inhibitory concentration which induces 50% loss of viability (IC50) at 72 hours of exposure ranged from 10 mcM in the most sensitive pre-B ALL cell lines to 40 mcM in several of the T-ALL and AML cell lines (Figure 1A–C). Importantly, at concentrations above 40 mcM all fifteen human leukemia cell lines from all three subtypes were sensitive to 3-BrOP with >80–100% loss of viability within 72 hours. In addition, two fresh patient samples were also sensitive to 3-BrOP, with an ALL sample IC50 of 15mcM at 48 hours and an AML sample IC50 of 30mcM at 72 hours (Figure 1D). Unfortunately longer ex vivo culture of these primary cells was not possible. These concentrations are consistent with effective doses in other tumor models, where 3-BrOP is effective in vivo (P Huang, personal communication).
Figure 1. Glycolysis inhibition via 3-BrOP inhibits acute leukemia growth.
Cells were exposed to a range of 3-BrOP concentrations for 72 hours and viability was measured using the AlamarBlue reagent. Graphs show average +/− SD for three samples. Normalized values 0% = no cells and 100% = untreated cells. A, precursor-B ALL cell line panel (IC50s 10–30mcM). B, T-ALL cell line panel (IC50s 15–30mcM). C, AML cell line panel (IC50s 20–40mcM). D, patient samples representing pre-B ALL (IC50 15mcM at 48 hours) and AML (IC50 30mcM at 72 hours).
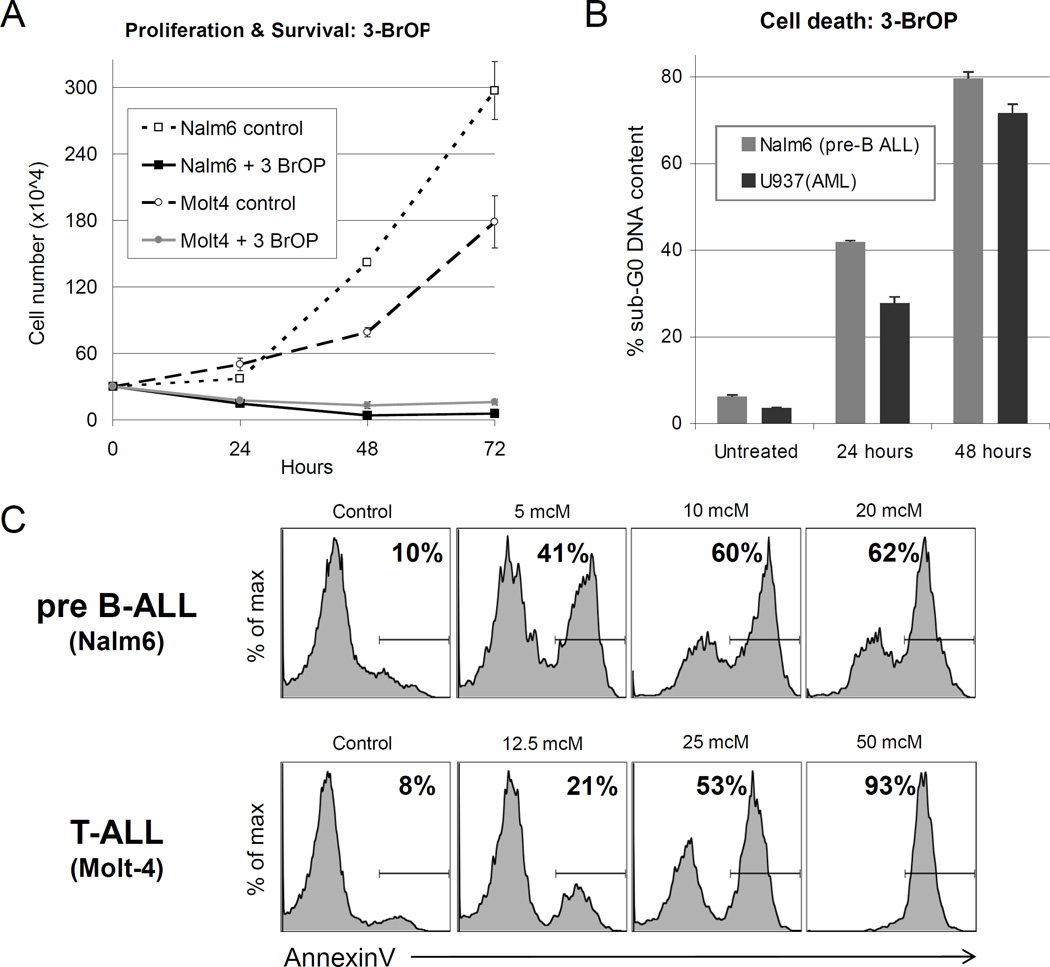
The cells demonstrated significantly decreased survival as early as 24 hours after treatment as measured by cell counts with Trypan blue exclusion (Figure 2A). At 24 hours, there was a rapid reduction in cell numbers in both Nalm6 (pre-B ALL) and Molt4 (T-ALL) lines (60–65%, p<0.001). By 48 hours of treatment, the majority of cells had died (83–97%, p<0.001) (Figure 2A). The remaining cells were dead with >80% of Nalm6 (pre-B ALL) and 72% of U937 (AML) cells having sub-G0 DNA content (p<0.001) (Figure 2B). Cell death was also measured by AnnexinV binding. AnnexinV binding increased in a dose-dependent manner in both B-ALL and T-ALL lines (Figure 2C), which correlates to loss of cell numbers (Figure 2A).
Figure 2. Effects of 3-BrOP on proliferation, survival and cell death.
A, Proliferation and survival was measured via serial cell counts following treatment with 3-BrOP at 10 mcM for Nalm6 (pre-B ALL) and 20 mcM for Molt4 (T-ALL). Average +/− SD of three samples shown. B, Induction of cell death was assessed in Nalm6 (pre-B ALL) and U937 (AML) cells treated with 30mcM 3-BrOP for 24 or 48 hours by measuring the percentage of cells with less than 2N (sub-G0) DNA content by propidium iodide (PI) staining with flow cytometry. Average +/− SD of three samples shown. C, Cell death was also measured after 48 hours exposure to increasing concentrations of 3-BrOP. AnnexinV-APC conjugate was used and the percent of cells which are AnnexinV-positive is indicated.
ATP is depleted by the combination of 3-BrOP and antimycin-A
As 3-BrOP inhibits glycolysis, we expect that cellular ATP levels will be decreased in treated cells. However in aerobic conditions leukemia cells may have adequate oxidative respiration to maintain intracellular ATP, tempering 3-BrOP effects. Therefore we assessed the effects of combining 3-BrOP with an inhibitor of oxidative respiration, antimycin A, in ALL cells. Effects on intracellular ATP concentrations and survival were evaluated at multiple time points following treatment with either 3-BrOP, antimycin A, or the combination (Figure 3A–C).
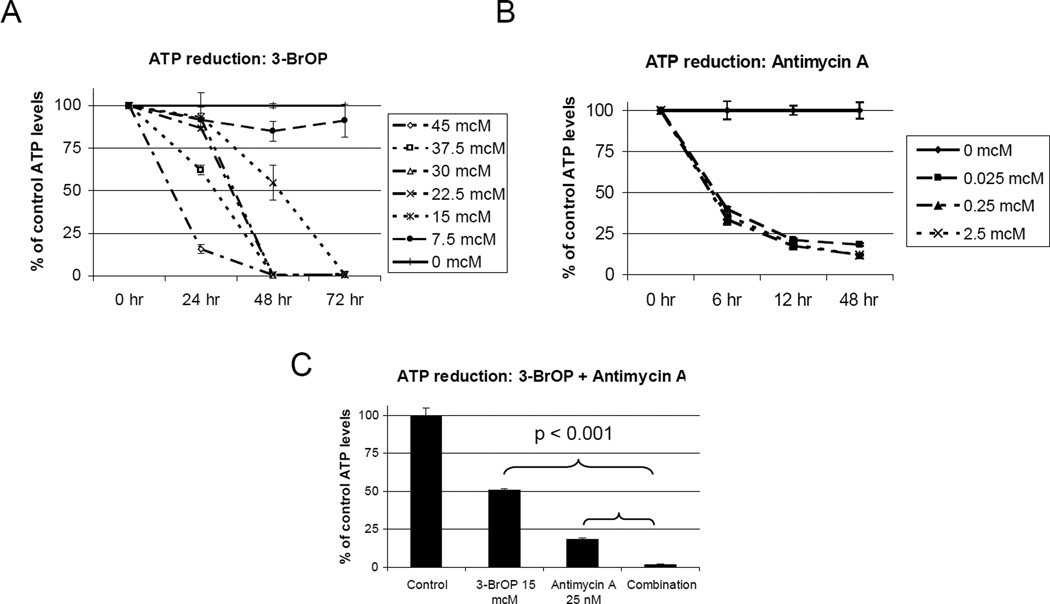
Figure 3. ATP reduction by 3-BrOP and Antimycin A.
ATP levels measured in Nalm6 (pre-B ALL) by the ATPlite assay. Average +/− SD of three samples shown for each. A, Time and dose dependent decrease in ATP levels after treatment with 3-BrOP. B, Time dependent decrease in ATP levels after treatment with antimycin A. C, Depletion in ATP with the combination of 3-BrOP and antimycin A at 72 hours (3-BrOP alone vs. combination, 2-tailed student’s T-test p<0.001)(Antimycin A vs, combination, 2-tailed student’s T-test p<0.001).
As expected, exposure to 3-BrOP reduced intracellular ATP concentrations in a dose and time dependent manner (Figure 3A). In Nalm6 cells (pre-B ALL), by 48 hours of exposure, the ATP levels were 50% of control at a dose of 15 mcM, the IC50 for this cell line. At a dose of 22.5 mcM, the ATP level was 13% of control, and by 30 mcM there was no detectable ATP (Figure 3A). Importantly, at higher doses (>30mcM) ATP levels were reduced significantly within 24 hours, however with lower doses (<15mcM) ATP levels did not decrease until 48–72 hours (Figure 3A). This effect on ATP levels correlates with dose and time dependent viability shown in Figures 1+2.
In contrast, when these cells were treated with antimycin A, a significant decrease in ATP concentration was seen as early as 6 hours (Figure 3B). The ATP level was approximately 40% of control, at a range of 25 nM to 2.5 mcM. By 48 hours, ATP concentrations had decreased to approximately 12% for the higher doses, and 19% for the lowest dose of 25 nM. The effects on ATP levels were not significantly dose dependent, suggesting that lower doses, below 25nM, may still be effective. Despite its rapid depletion of intracellular ATP levels, even at higher doses (20mcM) of antimycin A, there was never a complete loss of ATP, suggesting perhaps that ATP production through glycolysis maintained an moderate ATP level.
When 3-BrOP and antimycin A were combined, there was a dramatic decrease in ATP levels (Figure 3C). At 48 hours of treatment, ATP was completely depleted at 3-BrOP doses of 30 mcM and 22.5 mcM, with any concentration of antimycin A. At 15 mcM, the ATP level of 2% of control with all doses of antimycin A, and at 7.5 mcM, the ATP level was only 3.5% of control with the addition of any antimycin A. Importantly at 12 hours of treatment, ATP levels were <6% for all doses of 3-BrOP when combined with any dose of antimycin A. These results demonstrate significant decrease in ATP levels with the combination of 3-BrOP and antimycin A (p<0.001).
The combination of 3-BrOP and antimycin A lead to enhanced cell death
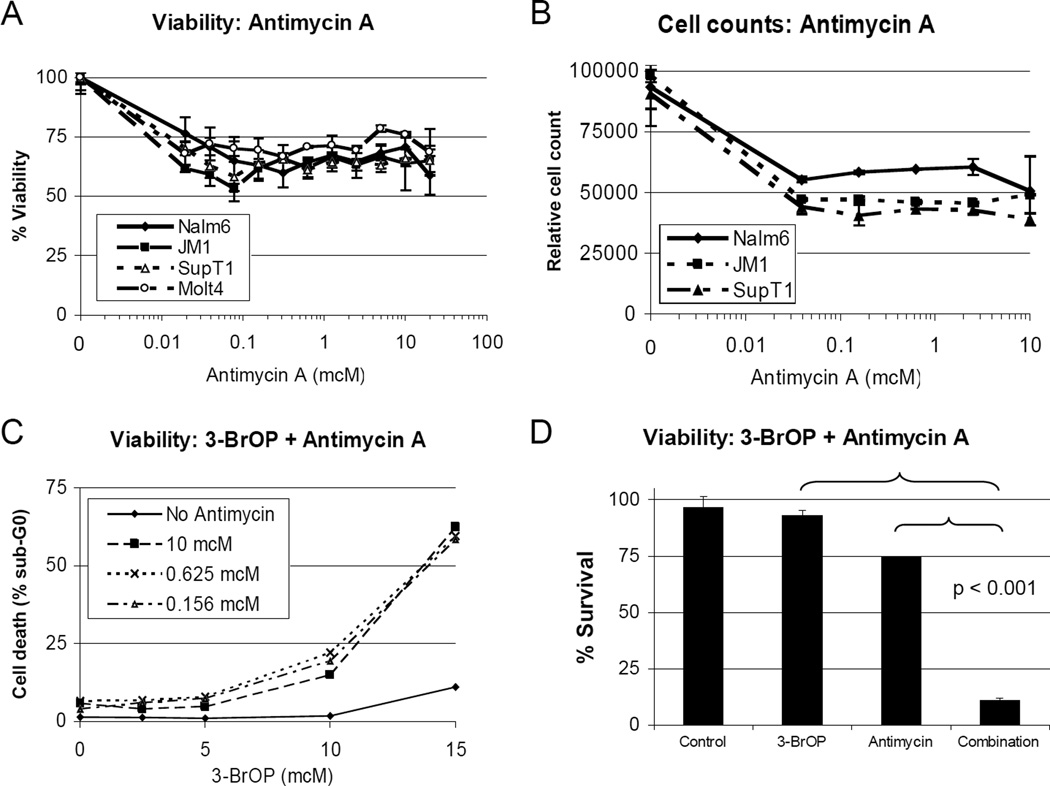
To evaluate whether the profound decrease in ATP levels seen with 3-BrOP and antimycin A would potentiate 3-BrOP effects in leukemia cells, we tested this combination in pre-B ALL (Nalm6 and JM1) and T-ALL cell lines (Molt4 and SupT1). Treatment with antimycin A alone with a dose range from 20 nM to 20 mcM did not achieve an IC50 (Figure 4A), with 48-hour viability between 55 and 80%. When relative cell counts were measured by flow cytometry, the treated cell number ranged from 40 to 60% of controls (Figure 4B). Furthermore, there was minimal increase in apoptosis when sub-G0 percentages were assessed with flow cytometry using propidium iodide staining. The sub-G0 population increased from a background of 2–4% in the untreated cells to 4–9% in the antimycin A treated cells after 48 hours of exposure (Figure 4C, 3-BrOP at 0).
Figure 4. Synergistic growth arrest and apoptosis with antimycin A and 3-BrOP.
Average +/− SD of three samples shown in each graph. A, Viability of pre-B (Nalm6 and JM1) and T-ALL (SupT1 and Molt4) lines treated with antimycin A for 48 hours measured by AlamarBlue assay. B, Viable cell count in pre-B (Nalm6) and T-ALL (Molt4) cells treated with Antimycin A for 48 hours. C, Sub-G0 content measured by flow cytometry following TPI staining in pre-B ALL (Nalm6) cells treated with antimycin A and 3-BrOP for 48 hours. D, Viability in pre-B ALL (Nalm6) following combined treatment with 1mcM Antimycin A and/or 15mcM 3-BrOP for 48 hours, measured by AlamarBlue assay.
In the combination assay with antimycin A and 3-BrOP, there was a significant increase in the percentage of sub-G0 cells when measured by propidium iodide staining and flow cytometry. For example, in Nalm6 (pre-B ALL) cells treated for 72 hours, the percent of sub-G0 cells was 10% with 15 mcM 3-BrOP alone, 6.5% with antimycin A alone (at any dose), but increased dramatically to 60% with the combination (p<0.001, Figure 4C). Highly potentiated effects were seen using a wide range of dose combinations. At low doses of 3-BrOP with 2.5 mcM and 5 mcM in Nalm6 cells, the addition of antimycin A from a dose of 150 nM to 2.5 mcM yielded a combination index of 0.1 to 0.4. At effective doses of 3-BrOP with 10 mcM the CI values ranged from 0.008 to 0.138, and at 15 mcM, the CI values were all less than 0.001 (Figure 4D). Similar effects were seen in the other pre-B ALL (JM1) and T-ALL lines (SupT1 and Molt4) tested. The T-ALL cells had increased apoptosis when treated with antimycin A alone, with 40–50% of the cells in sub-G0 compared with a background of 8–14% in untreated cells. When treated with 3-BrOP alone, at a dose of 15 mcM, the percentage of cells in sub-G0 was 25–29%. When combined with antimycin A however, sub-G0 percentages increased to 80–90% for SupT1 and 78–85% for Molt4. In these cases the combination indices ranged from 0.02 to 0.21.
These data demonstrate that acute leukemia cells appear to be dependent on glycolysis for survival, as blockade of oxidative respiration has only modest effects on viability. However, the combined inhibition of glycolysis and oxidative phosphorylation lead to significant cell death. It is not yet clear whether this will lead to excessive toxicity in nonmalignant cells.
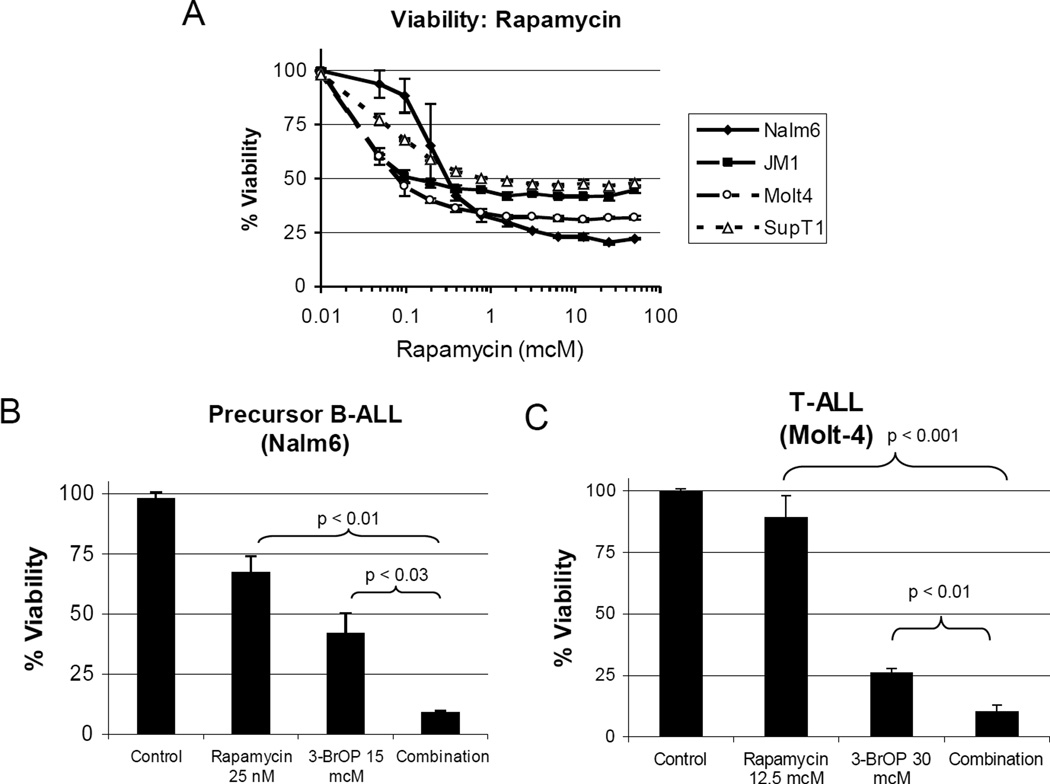
3-BrOP is potentiated by Rapamycin
An alternative approach to potentiate 3-BrOP effects is inhibition of the mammalian target of rapamycin (mTOR) pathway. The mTOR pathway senses metabolic stress, such as lowered glucose metabolite levels and ATP concentrations, and compensates for these stresses, allowing cells to be resistant to glycolysis inhibition. Therefore we decided to evaluate the combination of 3-BrOP with rapamycin and hypothesized that ATP depletion caused by 3-BrOP combined with inhibition of the mTOR pathway and altered cellular metabolism may increase cell death.
Rapamycin has previously been shown to be effective against human ALL in vitro and in vivo (27). In our hands, rapamycin alone was effective, inducing 50–80% loss of viability at higher doses (Figure 5A). However, when combined with sub-therapeutic doses of 3-BrOP, we observed significant potentiation in both AlamarBlue viability and cell cycle assays. This potentiation was observed at multiple doses of 3-BrOP (2.5 to 40 mcM) and rapamycin (6.25 to 50 nM). For example, Nalm6 treated with 15 mcM of 3-BrOP and 25 nM of rapamycin had a statistically significant decrease in viability compared to either agent alone (p<0.03) (Figure 5B). Similar effects were seen in T-ALL p<0.01 (Figure 5C). These results demonstrate potentiation between glycolysis inhibition via 3-BrOP and mTOR inhibition by rapamycin.
Figure 5. Synergy with 3-BrOP and Rapamycin in pre-B and T-ALLs.
Average +/− SD of three samples shown in each graph. A, Decreased viability with rapamycin treatment in pre-B ALL (Nalm6 and JM1) and T-ALL (Molt4 and SupT1) lines after treatment for 72 hours. Cytotoxicity measured with AlamarBlue assay. B+C, Synergistic decrease in survival of pre-B ALL (B: Nalm6) and T-ALL (C: Molt4) cells following treatment with 3-BrOP and/or rapamycin (p=0.001–0.03) for 72 hours.
Discussion
We were surprised to see that in conditions of normoxia all fifteen cell lines and patient samples were sensitive to modest doses of 3-BrOP. This suggests that there is more dependence on glycolysis in the subtypes of acute leukemia than had previously been realized. As acute leukemias often have high proliferation rates, an increased dependence on ATP production and glycolysis is not unexpected. However, the apparent lack of ability to compensate for complete inhibition of glycolysis suggests that there may be additional mechanisms involved, as has been observed in several solid tumors (3). For example, expression of hypoxia inducible factors (HIF) have been shown to enhance expression of glycolytic genes, while inhibiting proteins in the oxidative phosphorylation. In renal cell carcinoma the loss of von Hippel Lindau (VHL) gene expression leads to inappropriate HIF stabilization and resulting enhanced glycolysis with decreased oxidative phosphorylation in normoxia (29). Of note, the enhanced dependence on glycolysis in ALL cells previously reported revealed patterns of metabolic dysregulation similar to those seen with HIF expression, including high glucose transporter GLUT1 expression (11).
Given these data, glycolysis inhibition provides a promising new approach for acute leukemia treatment, but malignant cells have an intrinsic ability to adapt and it is unlikely that blocking glycolysis alone would be adequate therapy (30). For example, even though a majority of ATP production may occur through glycolysis, leukemia cells may still utilize residual oxidative phosphorylation to maintain minimal ATP levels or use stress-induced pathways to compensate for ATP depletion (8, 9, 31).
Our data demonstrated that although the oxidative phosphorylation inhibitor antimycin A was potent at reducing ATP levels and inhibiting proliferation, even large doses were unable to induce cell death. In contrast, glycolysis inhibition with 3-BrOP induced slower depletion of ATP, but at higher doses and/or longer incubations, 3-BrOP alone was able to induce dramatic cell death and pro-caspase-3 cleavage. These results suggest that glycolysis can compensate for inhibition of oxidative phosphorylation, but complete inhibition of glycolysis cannot be compensated by oxidative respiration in acute leukemias. This suggests a compensatory defect in these leukemia subtypes. Importantly, the combination of 3-BrOP and antimycin A led to nearly complete depletion of ATP and enhanced cell death. Although glycolysis inhibitors and antimycin A derivatives have been used clinically, the potential toxicities of this combination may complicate clinical use of this combination.
As a potentially less toxic approach to target cancer cells, simultaneous inhibition of glycolysis and the mTOR pathway provides a rational therapeutic option. When ATP is depleted by glycolysis inhibition, blocking the mTOR pathway may further limit nutrient uptake, cell proliferation, and cell survival. The combination of 3-BrOP in both precursor-B ALL and T-ALL cell lines did demonstrate enhanced cell death, both by cytotoxicity assays and cell cycle analysis.
The sensitivity of these leukemia cells to the combinations of 3-BrOP and antimycin A or rapamycin suggests that multiple therapeutic combinations which alter the consequences and adaptation to cellular stress may also be effective. Indeed, glycolysis inhibition can cooperate with multiple anti-neoplastic agents (32, 33). In addition, the depletion of ATP during chemotherapeutic regimens may inhibit resistance mechanisms such as efflux pumps (MRP/MDR) and/or DNA repair mechanisms (34).
In summary, we report the broad and potent activity of a novel glycolysis inhibitor, 3-BrOP, in precursor-B, T and myeloid subtypes of acute leukemia. The effects of 3-BrOP were potentiated when used in combination with either the oxidative phosphorylation inhibitor antimycin A or the mTOR inhibitor rapamycin. Additional studies to address potential mechanisms of this dependence on glycolysis, and effects on chemotherapy resistance are needed. Based on these results, we believe that further pre-clinical development of this combinatorial approach is warranted.
Acknowledgments
I’d like to thank the members of the Zweidler-McKay lab, especially Sankar Kannan, PhD and Robert Sutphin, MD. From Dr. Peng Huang’s laboratory, Zhao Chen, PhD was of great assistance. From the Division of Pediatrics at the Children’s Cancer Hospital at UT M. D. Anderson, Anna Franklin, MD, Laura Worth, MD, PhD, Eugenie Kleinerman, MD, and Nadya Koshkina, PhD have been very supportive of this research. No writing assistance was used.
Role of the funding source
These studies were supported through UT M. D. Anderson Cancer Center Startup Funds. No grant or commercial interest was involved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contributions
L.J.A. designed, performed and analyzed the majority of the experiments, and wrote the manuscript; A.R.F. designed and interpreted the studies; W.F. and A.G.L. performed critical aspects of these studies; P.H. provided a critical reagent (3-BrOP), and revised the manuscript; P.A.Z-M. conceived, designed, analyzed, interpreted the studies, and revised the manuscript.
Conflict of Interest
No authors report a conflict of interest. No commercial funds or input contributed to this work.
References
- 1.McNeil DE, Cote TR, Clegg L, Mauer A. SEER update of incidence and trends in pediatric malignancies: acute lymphoblastic leukemia. Med Pediatr Oncol. 2002 Dec;39(6):554–557. doi: 10.1002/mpo.10161. discussion 2–3. [DOI] [PubMed] [Google Scholar]
- 2.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005 Nov 1;23(31):7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008 Dec;22(12):2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009 Jun;37(6):649–658. doi: 10.1016/j.exphem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Pediatr Clin North Am. 2008 Feb;55(1):21–51. ix. doi: 10.1016/j.pcl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005 Jan 15;65(2):613–621. [PubMed] [Google Scholar]
- 7.Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005 Dec;19(12):2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007 Jun;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007 Jun;39(3):267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 10.Hickey FB, Cotter TG. Identification of transcriptional targets associated with the expression of p210 Bcr-Abl. Eur J Haematol. 2006 May;76(5):369–383. doi: 10.1111/j.1600-0609.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 11.Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Hoffmann K, et al. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia. 2006 Oct;20(10):1731–1737. doi: 10.1038/sj.leu.2404365. [DOI] [PubMed] [Google Scholar]
- 12.Herst PM, Hesketh EL, Ritchie DS, Berridge MV. Glycolytic metabolism confers resistance to combined all-trans retinoic acid and arsenic trioxide-induced apoptosis in HL60rho0 cells. Leuk Res. 2008 Feb;32(2):327–333. doi: 10.1016/j.leukres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Hulleman E, Kazemier KM, Holleman A, VanderWeele DJ, Rudin CM, Broekhuis MJ, et al. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009 Feb 26;113(9):2014–2021. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, et al. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. Mar 18;29(11):1641–1652. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- 15.Suganuma K, Miwa H, Imai N, Shikami M, Gotou M, Goto M, et al. Energy metabolism of leukemia cells: glycolysis versus oxidative phosphorylation. Leuk Lymphoma. Nov;51(11):2112–2119. doi: 10.3109/10428194.2010.512966. [DOI] [PubMed] [Google Scholar]
- 16.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006 Aug 7;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 17.Kurtoglu M, Maher JC, Lampidis TJ. Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal. 2007 Sep;9(9):1383–1390. doi: 10.1089/ars.2007.1714. [DOI] [PubMed] [Google Scholar]
- 18.Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002 Jul 15;62(14):3909–3913. [PubMed] [Google Scholar]
- 19.Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004 Nov 5;324(1):269–275. doi: 10.1016/j.bbrc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, et al. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007 Mar;59(4):495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 21.Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001 Feb;3(2):183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 22.Park WH, Han YW, Kim SW, Kim SH, Cho KW, Kim SZ. Antimycin A induces apoptosis in As4.1 juxtaglomerular cells. Cancer Lett. 2007 Jun 18;251(1):68–77. doi: 10.1016/j.canlet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Loffler M. Towards a further understanding of the growth-inhibiting action of oxygen deficiency. Evaluation of the effect of antimycin on proliferating Ehrlich ascites tumour cells. Exp Cell Res. 1985 Mar;157(1):195–206. doi: 10.1016/0014-4827(85)90162-4. [DOI] [PubMed] [Google Scholar]
- 24.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001 Nov 2;294(5544):1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 25.Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004 Feb 15;100(4):657–666. doi: 10.1002/cncr.20026. [DOI] [PubMed] [Google Scholar]
- 26.Dancey JE. Inhibitors of the mammalian target of rapamycin. Expert Opin Investig Drugs. 2005 Mar;14(3):313–328. doi: 10.1517/13543784.14.3.313. [DOI] [PubMed] [Google Scholar]
- 27.Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R, et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maniatis T, Fritsch E, Sambrook J. Molecular cloning: a laboratory manual. 2nd. Cold Spring Harbor, MA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Hervouet E, Cizkova A, Demont J, Vojtiskova A, Pecina P, Franssen-van Hal NL, et al. HIF and reactive oxygen species regulate oxidative phosphorylation in cancer. Carcinogenesis. 2008 Aug;29(8):1528–1537. doi: 10.1093/carcin/bgn125. [DOI] [PubMed] [Google Scholar]
- 30.Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE. 2007 Apr 10;2007(381):e14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen PL. The cancer cell's "power plants" as promising therapeutic targets: an overview. J Bioenerg Biomembr. 2007 Feb;39(1):1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 32.Ihrlund LS, Hernlund E, Khan O, Shoshan MC. 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol. 2008 Jun;2(1):94–101. doi: 10.1016/j.molonc.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernlund E, Ihrlund LS, Khan O, Ates YO, Linder S, Panaretakis T, et al. Potentiation of chemotherapeutic drugs by energy metabolism inhibitors 2-deoxyglucose and etomoxir. Int J Cancer. 2008 Jul 15;123(2):476–483. doi: 10.1002/ijc.23525. [DOI] [PubMed] [Google Scholar]
- 34.Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003 Nov 1;63(21):7284–7290. [PubMed] [Google Scholar]