Abstract
Objective
: To investigate the effects of N-acetylcysteine (NAC) and pentoxifylline in a model of remote organ injury after hind-limb ischemia/reperfusion (I/R) in rats, the lungs being the remote organ system.
Methods
: Thirty-five male Wistar rats were assigned to one of five conditions (n = 7/group), as follows: sham operation (control group); hind-limb ischemia, induced by clamping the left femoral artery, for 2 h, followed by 24 h of reperfusion (I/R group); and hind-limb ischemia, as above, followed by intraperitoneal injection (prior to reperfusion) of 150 mg/kg of NAC (I/R+NAC group), 40 mg/kg of pentoxifylline (I/R+PTX group), or both (I/R+NAC+PTX group). At the end of the trial, lung tissues were removed for histological analysis and assessment of oxidative stress.
Results
: In comparison with the rats in the other groups, those in the I/R group showed lower superoxide dismutase activity and glutathione levels, together with higher malondialdehyde levels and lung injury scores (p < 0.05 for all). Interstitial inflammatory cell infiltration of the lungs was also markedly greater in the I/R group than in the other groups. In addition, I/R group rats showed various signs of interstitial edema and hemorrhage. In the I/R+NAC, I/R+PTX, and I/R+NAC+PTX groups, superoxide dismutase activity, glutathione levels, malondialdehyde levels, and lung injury scores were preserved (p < 0.05 for all). The differences between the administration of NAC or pentoxifylline alone and the administration of the two together were not significant for any of those parameters (p > 0.05 for all).
Conclusions
: Our results suggest that NAC and pentoxifylline both protect lung tissue from the effects of skeletal muscle I/R. However, their combined use does not appear to increase the level of that protection.
Keywords: Skeletal muscle, Ischemia, Reperfusion injury, Lung injury, Acetylcysteine, Pentoxifylline
INTRODUCTION
Re-establishing perfusion in a tissue after a period of ischemia worsens the initial ischemic injury. This process is known as ischemia/reperfusion (I/R) injury.( 1 ) Such injury constitutes an important clinical event and is common in the lower extremities. Although restoration of blood flow can save the extremity, it can also result in multiple organ dysfunction syndrome.( 2 ) For example, I/R of a lower limb leads to noncardiogenic pulmonary edema by means of pulmonary vasoconstriction, pulmonary hypertension, and increased alveolar membrane permeability.( 3 ) Pulmonary dysfunction after I/R injury of a lower extremity continues to be a major cause of morbidity and mortality.( 4 ) Previous studies have suggested that oxygen free radicals, inflammatory mediators, and, especially, neutrophils play an important role in the development of lung injury related to I/R in a lower limb.( 5 , 6 )
Various agents have been reported to reduce remote lung injury after hind-limb I/R in rats.( 7 - 9 ) N-acetylcysteine (NAC) is an antioxidant that acts by increasing intracellular levels of glutathione, as well as by direct scavenging of reactive oxygen species (ROS) such as hypochlorous acid, hydrogen peroxide, superoxide, and hydroxyl radical.( 10 ) Pentoxifylline, a nonspecific phosphodiesterase inhibitor, has been shown to improve tissue oxygenation and endothelial function as well as inhibiting pro-inflammatory cytokine production.( 11 ) Pentoxifylline also inhibits cell proliferation and extracellular matrix accumulation. ( 12 ) Therefore, in the present study, we evaluated the possible involvement of oxidative stress in skeletal muscle I/R-induced lung injury in rats by examining the effects of pentoxifylline, NAC, and the combination of the two.
METHODS
All experimental procedures were performed in accordance with established guidelines for the ethical treatment of experimental animals, and the study was approved by the Institutional Animal Care and Use Committee of the Islamic Azad University School of Veterinary Medicine, in the city of Tehran, Iran.
Thirty-five healthy adult male Wistar rats, 90-120 days of age and weighing 250-350 g, were purchased from the Pasteur Institute of Iran. The animals were housed in a temperature- and humidity-controlled environment (22 ± 1°C; relative humidity, 50 ± 5%), on a 12/12-h light/dark cycle, with ad libitum access to a commercial pellet diet and filtered tap water. The animals were randomly divided into five experimental groups of seven rats each: an I/R group, in which the animals were subjected to 2 h of hind-limb ischemia, induced by clamping the left femoral artery, followed by intraperitoneal administration of 2 mL of 0.9% saline solution and 24 h of reperfusion; a sham-operated (control) group, in which the animals were subjected to all surgical procedures except arterial occlusion and also received 0.9% saline (2 mL, i.p.); an I/R+NAC group, in which the animals were subjected to I/R, as described above, and received 150 mg/kg of NAC in 0.9% saline solution (i.p., in a total volume of 2 mL); an I/R+PTX group, in which the animals were subjected to I/R, as described above, and received 40 mg/kg of pentoxifylline in 0.9% saline solution (i.p., in a total volume of 2 mL); and an I/R+NAC+PTX group, in which the animals were subjected to I/R, as described above, and received a combination of NAC and pentoxifylline (at the doses listed above) in 0.9% saline solution (i.p., in a total volume of 2 mL).
The rats were weighed, after which they were anesthetized with a combination of ketamine hydrochloride 10% and xylazine hydrochloride 2% (i.m., 50 mg/kg and 10 mg/kg, respectively). The animals were placed on a warming pad, in dorsal recumbency, their thoraces and hind limbs immobilized with adhesive tape. After aseptic surgical preparation, 250 IU of heparin were administered via the jugular vein (in order to prevent clotting) and a skin incision was made on the medial surface of the left hind limb. After isolating the left femoral artery from the surrounding tissues, we induced ischemia by using a microvascular clamp to occlude the artery for 2 h. Throughout the period of ischemia, the animals were maintained in dorsal recumbency and remained anesthetized (additional doses given as necessary). Body temperature was monitored with a rectal thermometer. At the end of the period of ischemia, the clamp was removed and the surgical site was routinely closed with 4/0 polypropylene sutures. Animals in the control group underwent a similar surgical procedure, although without arterial occlusion. The rats subjected to ischemia then underwent 24 h of reperfusion. Throughout the periods of ischemia and reperfusion, the animals received noninvasive ventilatory support.
At the end of the experimental period, all of the rats were euthanized with an overdose of pentobarbital (300 mg/kg, i.p.) and the lungs were removed en bloc. The left lungs were immersed in 10% formalin solution, and the right lungs were stored at −20°C for subsequent biochemical analysis. The lung tissue homogenate and supernatant samples were prepared as described by Yildirim et al.( 13 ) Malondialdehyde levels, superoxide dismutase (SOD) activity, and glutathione levels were measured in the right lungs, whereas samples of the left lungs were submitted to histopathological evaluation under light microscopy.
Malondialdehyde levels were determined by thiobarbituric acid reaction, as described by Yagi.( 14 ) In the thiobarbituric acid reaction test, malondialdehyde (or malondialdehyde-like substances) react with thiobarbituric acid to produce a pink chromogen with peak absorbance at 532 nm on a spectrophotometer. The tissue levels of malondialdehyde are expressed as nmol/g of tissue.
The SOD activity was determined according to the method devised by Winterbourn et al.,( 15 ) assayed as inhibition of the photochemical reduction of nitroblue tetrazolium at 560 nm. The SOD activity is expressed as U/g of tissue.
Glutathione levels were determined by the method described by Ellman,( 16 ) in which the level of glutathione is considered directly proportional to the rate of formation of the reduced chromogen, 5,5′-dithiobis(2-nitrobenzoic acid), as determined by measuring its absorbance at 412 nm. The results are expressed as nmol/g of tissue.
The left lung specimens were fixed in 10% buffered formalin, processed by standard techniques, and embedded in paraffin. Cross-sectional slices (5-µm thick) were taken from the middle zones of the lungs and mounted on slides. The slides were stained with hematoxylin and eosin, after which they were examined under light microscopy by a pathologist who was blinded to the groups. Lung injury was evaluated semiquantitatively with the classification system established by Koksel et al.( 17 ): grade 0, normal appearance; grade 1, mild-to-moderate interstitial congestion and neutrophil infiltration; grade 2, perivascular edema, partial destruction of the lung architecture, and moderate neutrophil infiltration; and grade 3, complete destruction of the lung architecture and dense neutrophil infiltration. A total of five slides from each lung sample were randomly screened, and the mean was accepted as the representative value of the sample.
All results are shown as mean ± standard deviation. The analytical results were evaluated using the Statistical Package for the Social Sciences, version 16.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis was done by analysis of variance. Values of p < 0.05 were considered statistically significant.
RESULTS
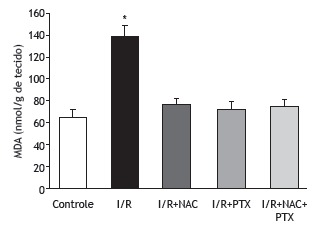
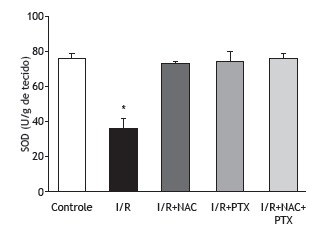
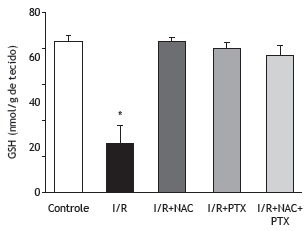
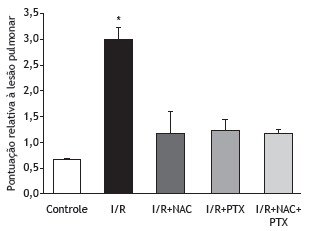
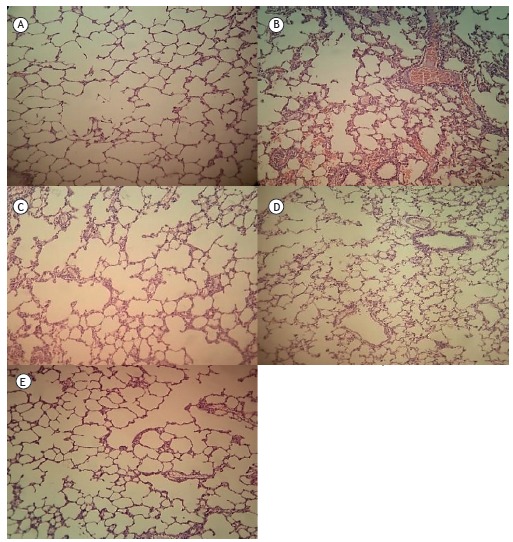
As can be seen in Figure 1, the malondialdehyde levels were significantly higher in the I/R group than in any of the other groups (p < 0.05). In addition, the malondialdehyde levels were lower in the I/R+NAC+PTX group than in the I/R+NAC and I/R+PTX groups, although the differences were not statistically significant. The SOD activity was significantly lower in the I/R group than in the control group (Figure 2), whereas it was significantly higher in the I/R+NAC, I/R+PTX, and I/R+NAC+PTX groups than in the I/R group (p < 0.05). Although SOD activity was highest in the I/R+NAC+PTX group, there were no significant differences among the I/R+PTX, I/R+NAC, and I/R+NAC+PTX groups in terms of SOD activity. Glutathione levels were also significantly lower in the I/R group than in the control group (Figure 3), whereas they were significantly higher in the I/R+NAC, I/R+PTX, and I/R+NAC+PTX groups than in the I/R group. Although glutathione levels were higher in the I/R+NAC+PTX group than in the I/R+NAC and I/R+PTX groups, the differences among those three groups were not significant. The mean histopathological lung scores are shown, by group, in Figure 4. The mean I/R group score was significantly lower than was that of the I/R+NAC, I/R+PTX, and I/R+NAC+PTX groups, although lung injury scores did not differ significantly among the three treated groups. As shown in Figure 5, interstitial inflammatory cell infiltration was markedly more pronounced in the lung samples from rats in the I/R group than in those from rats in the other groups. The lungs of I/R group rats also showed various signs of interstitial edema and hemorrhage.
Figure 1. Malondialdehyde (MDA) levels in lung tissue after 2 h of hind-limb ischemia and 24 h of reperfusion. I/R: ischemia/reperfusion; NAC: N-acetylcysteine; and PTX: pentoxifylline. *p < 0.05 vs. all other groups.
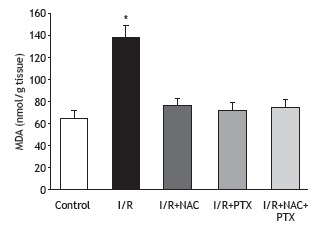
Figure 2. Superoxide dismutase (SOD) activity in lung tissue after 2 h of hind-limb ischemia and 24 h of reperfusion. I/R: ischemia/reperfusion; NAC: N-acetylcysteine; and PTX: pentoxifylline. *p < 0.05 vs. all other groups.
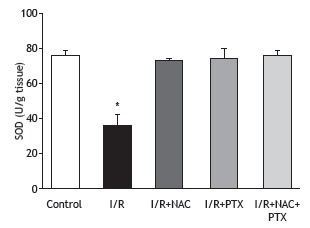
Figure 3. Glutathione (GSH) levels in lung tissue after 2 h of hind-limb ischemia and 24 h of reperfusion. I/R: ischemia/reperfusion; NAC: N-acetylcysteine; and PTX: pentoxifylline. *p < 0.05 vs. all other groups.
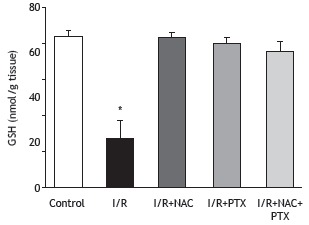
Figure 4. Histological lung injury scores after 2 h of hind-limb ischemia and 24 h of reperfusion. I/R: ischemia/reperfusion; NAC: N-acetylcysteine; and PTX: pentoxifylline. *p < 0.05 vs. all other groups.
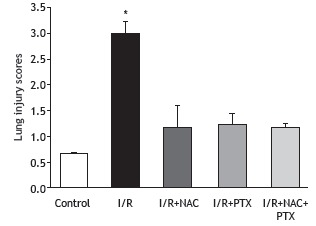
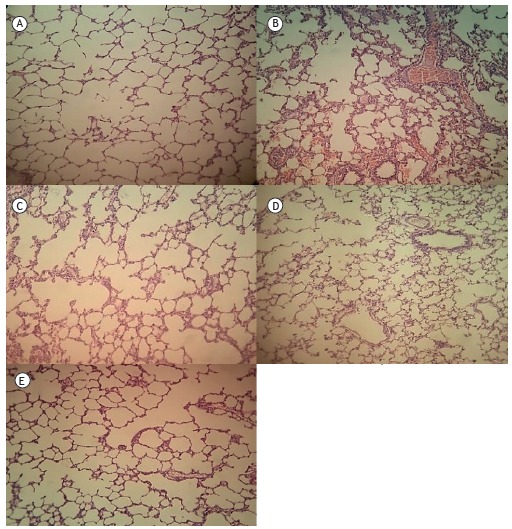
Figure 5. Lung tissue samples stained with hematoxylin and eosin (original magnification, ×100): (A) control group sample, showing no remarkable pathological changes; (B) ischemia/reperfusion (I/R) group sample, showing widespread histological changes such as edema, severe alveolar congestion, alveolar collapse, and inflammatory cell infiltration; and (C, D, and E, respectively) I/R+N-acetylcysteine, I/R+pentoxifylline, and I/R+N-acetylcysteine+pentoxifylline group samples, all showing fewer histological alterations (markedly less interstitial edema and inflammatory cell infiltration) in comparison with the I/R group sample.
DISCUSSION
Peripheral artery clamping is routinely used during orthopedic surgery or trauma, in elective and emergency procedures. Lung damage has been shown to occur following transient arterial occlusion. The ischemic damage results from a decrease in the blood flow to an organ. When blood flow is restored, more pronounced damage, known as reperfusion injury, occurs. It has been suggested that oxidative stress plays a role in the development of I/R injury.( 18 ) Various tissue markers of oxidative stress have been measured to evaluate the effects of I/R injury. It is known that ROS, which are potent oxidizing and reducing agents that can directly damage cellular membranes by lipid peroxidation, are overproduced during oxidative stress.( 19 ) Peroxidation of endogenous lipids leads to the conversion of reduced glutathione to glutathione-disulfide.( 20 ) Malondialdehyde is an end product derived from the peroxidation of polyunsaturated fatty acids and related esters. Therefore, tissue levels of malondialdehyde are a valid reflection of lipid peroxidation. Another line of cellular defense against free radicals is a system of three enzymes, SOD, catalase, and glutathione peroxidase. SOD catalyzes the conversion of superoxides to hydrogen peroxide, which is subsequently converted to water and oxygen by catalase or glutathione peroxidase. Because it plays such a key role in cellular defense against free radicals, SOD is also an important indicator of the oxidative state.( 21 )
The glutathione precursor NAC is a small molecule containing a thiol group, which has antioxidant properties, and is freely filterable with ready access to intracellular compartments.( 22 ) The diversity of pharmacological applications of NAC is due mainly to the chemical properties of the cysteinyl thiol group of its molecule, the ability of reduced thiol groups to scavenge oxygen free radicals having been well established.( 23 - 25 ) In addition, NAC has a variety of anti-inflammatory effects.( 26 , 27 ) In previous rat studies, the administration of NAC at doses of approximately 400 mg/kg has been shown to protect organs against oxidative damage.( 28 , 29 ) In the present study, we found that NAC administration after ischemia (prior to reperfusion) resulted in lower malondialdehyde levels, greater SOD activity, and higher glutathione levels in comparison with no treatment. In other words, NAC effectively attenuated the I/R-induced increase in the level of malondialdehyde.
Pentoxifylline is a methylxanthine derivative with multiple hemorheological properties. Pentoxifylline acts by increasing intracellular cyclic adenosine monophosphate on red blood cells, thus improving oxygen delivery to ischemic tissues, increasing cyclic adenosine monophosphate on polymorphonuclear leukocytes, and decreasing oxygen free radical production.( 30 - 33 ) Recent reports suggest that pentoxifylline can enhance the chemotactic response of neutrophils, as well as inhibiting phagocytosis and superoxide production by neutrophils and monocytes.( 34 ) Those findings have translated into clinical benefits, pentoxifylline having been used in order to attenuate I/R injury in patients with lung, intestinal, or kidney damage.( 31 ) Previous studies have shown that supplementation with 50 mg/kg of pentoxifylline has the beneficial effect of reducing oxidative stress and inflammatory indices in I/R-induced spinal cord injury and fatty liver disease. ( 35 , 36 ) In the present study, pentoxifylline administration after ischemia (prior to reperfusion) resulted in lower malondialdehyde levels, greater SOD activity, and higher glutathione levels in comparison with no treatment.
In conclusion, the significant I/R-induced increase in malondialdehyde levels, decrease in glutathione levels, and destructive appearance on histology of the lung suggest that skeletal muscle I/R-induced lung injury is mediated by oxidative reactions. The results of our study confirm that pentoxifylline and NAC are both protective against I/R injury. These effects might be, at least in part, due to the inhibition of ROS production. To our knowledge, this was the first study to compare the effects of these two substances on remote lung injury. We found that the antioxidant properties of pentoxifylline were comparable to those of NAC. However, we observed no additional effect when the two were administered in combination. Further studies are needed in order to determine the clinical importance of treatment with pentoxifylline and NAC, especially regarding the possible mechanisms other than ROS scavenging. Such treatments might prove effective for enhancing protection of the lungs after lower-limb I/R.
Footnotes
Study carried out at the Young Researchers and Elites Club, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Financial support: None.
REFERENCES
- 1.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282(2):C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 2.Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26(1):115–121. doi: 10.1007/s00268-001-0169-2. [DOI] [PubMed] [Google Scholar]
- 3.Groeneveld AB, Raijmakers PG, Rauwerda JA, Hack CE. The inflammatory response to vascular surgery-associated ischaemia and reperfusion in man: effect on postoperative pulmonary function. Eur J Vasc Endovasc Surg. 1997;14(5):351–359. doi: 10.1016/S1078-5884(97)80284-5. [DOI] [PubMed] [Google Scholar]
- 4.Paterson IS, Klausner JM, Pugatch R, Allen P, Mannick JA, Shepro D. Noncardiogenic pulmonary edema after abdominal aortic aneurysm surgery. Ann Surg. 1989;209(2):231–236. doi: 10.1097/00000658-198902000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991;78(6):651–655. doi: 10.1002/bjs.1800780607. [DOI] [PubMed] [Google Scholar]
- 6.Fantini GA, Conte MS. Pulmonary failure following lower torso ischemia: clinical evidence for a remote effect of reperfusion injury. Am Surg. 1995;61(4):316–319. [PubMed] [Google Scholar]
- 7.Takhtfooladi H, Takhtfooladi M, Moayer F, Mobarakeh S. Melatonin attenuates lung injury in a hind limb ischemia-reperfusion rat model. Rev Port Pneumol (2006) 2015;21(1):30–35. doi: 10.1016/j.rppnen.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Jahanshahi G, Takhtfooladi HA, Aslani K. Effect of tramadol on lung injury induced by skeletal muscle ischemia-reperfusion: an experimental study. J Bras Pneumol. 2013;39(4):434–439. doi: 10.1590/S1806-37132013000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotoudeh A, Takhtfooladi MA, Jahanshahi A, Asl AH, Takhtfooladi HA, Khansari M. Effect of N-acetylcysteine on lung injury induced by skeletal muscle ischemia-reperfusion. Histopathological study in rat model. Acta Cir Bras. 2012;27(2):168–171. doi: 10.1590/S0102-86502012000200012. [DOI] [PubMed] [Google Scholar]
- 10.Nitescu N, Ricksten SE, Marcussen N, Haraldsson B, Nilsson U, Basu S. N-acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia reperfusion. Nephrol Dial Transplant. 2006;21(5):1240–1247. doi: 10.1093/ndt/gfk032. [DOI] [PubMed] [Google Scholar]
- 11.Okumura AS, Rodrigues LE, Martinelli R. Pentoxifylline in ischemia-induced acute kidney injury in rats. Ren Fail. 2009;31(9):829–832. doi: 10.3109/08860220903137509. [DOI] [PubMed] [Google Scholar]
- 12.Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: A 12-month randomized trial. Am J Kidney Dis. 2008;52(3):464–474. doi: 10.1053/j.ajkd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim Z, Kotuk M, Erdogan H, Iraz M, Yagmurca M, Kuku I. Preventive effect of melatonin on bleomycin-induced lung fibrosis in rats. J Pineal Res. 2006;40(1):27–33. doi: 10.1111/j.1600-079X.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 14.Yagi K. Lipid peroxides and related radicals in clinical medicine. In: Armstrong D, editor. Free radicals in diagnostic medicine.A systems approach to laboratory technology, clinical correlations, and antioxidant therapy. New York: Plenum Press; 1994. pp. 1–15. [DOI] [Google Scholar]
- 15.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85(2):337–341. [PubMed] [Google Scholar]
- 16.ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Koksel O, Yildirim C, Cinel L, Tamer L, Ozdulger A, Bastürk M. Inhibition of poly(ADP-ribose) polymerase attenuates lung tissue damage after hind limb ischemia-reperfusion in rats. Pharmacol Res. 2005;51(5):453–462. doi: 10.1016/j.phrs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Schoenberg MH, Beger HG. Reperfusion injury after intestinal ischemia. Crit Care Med. 1993;21(9):1376–1386. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49(2):91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 20.Brivida K, Sies H. Non-enzymatic antioxidant defense system.In Frei B, editor. Natural antioxidants in human health and disease. San Diego: Academic Press; 1994. [Google Scholar]
- 21.de Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26(1-2):202–226. doi: 10.1016/S0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 22.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20(2):123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Daneshi MH, Khansari M, Takhtfooladi HA. The antioxidant role of N-acetylcysteine on the testicular remote injury after skeletal muscle ischemia and reperfusion in rats. Pol J Pathol. 2013;64(3):204–209. doi: 10.5114/pjp.2013.38140. [DOI] [PubMed] [Google Scholar]
- 24.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- 25.Cuzzocrea S, Mazzon E, Costantino G, Serraino I, De Sarro A, Caputi AP. Effects of n-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc Res. 2000;47(3):537–548. doi: 10.1016/S0008-6363(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 26.Sehirli AO, Sener G, Satiroglu H, Ayanoglu-Dülger G. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol. 2003;16(1):75–80. [PubMed] [Google Scholar]
- 27.DiMari J, Megyesi J, Udvarhelyi N, Price P, Davis R, Safirstein R. N-acetyl cysteine ameliorates ischemic renal failure. Pt 2Am J Physiol. 1997;272(3):292–298. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- 28.Da Silveira M, Yoshida WB. Trimetazidine and N-acetylcysteine in attenuating hind-limb ischemia and reperfusion injuries: experimental study in rats. Int Angiol. 2009;28(5):412–417. [PubMed] [Google Scholar]
- 29.Shimizu MH, Danilovic A, Andrade L, Volpini RA, Libório AB, Sanches TR. N-acetylcysteine protects against renal injury following bilateral ureteral obstruction. Nephrol Dial Transplant. 2008;23(10):3067–3073. doi: 10.1093/ndt/gfn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stafford-Smith M. Evidence-based renal protection in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9(1):65–76. doi: 10.1177/108925320500900107. [DOI] [PubMed] [Google Scholar]
- 31.Emrecan B, Tulukoglu E, Bozok S, Kestelli M, Onem G, Küpelioglu A. Effects of lloprost and pentoxifylline on renal ischemia-reperfusion in rabbit model. Eur J Med Res. 2006;11(7):295–299. [PubMed] [Google Scholar]
- 32.Dávila-Esqueda ME, Martinez-Morales F. Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res. 2004;5(4):245–251. doi: 10.1080/154386090897974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunduz Z, Canoz O, Per H, Dusunsel R, Poyrazoglu MH, Tez C. The effects of pentoxifylline on diabetic renal changes in streptozotocin-induced diabetes mellitus. Ren Fail. 2004;26(6):597–605. doi: 10.1081/JDI-200038329. [DOI] [PubMed] [Google Scholar]
- 34.Vadiei K, Brunner LJ, Luke DR. Effects of pentoxifylline in experimental acute renal failure. Kidney Int. 1989;36(3):466–470. doi: 10.1038/ki.1989.218. [DOI] [PubMed] [Google Scholar]
- 35.Savas S, Delibas N, Savas C, Sütçü R, Cindas A. Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord. 2002;40(5):224–229. doi: 10.1038/sj.sc.3101281. [DOI] [PubMed] [Google Scholar]
- 36.Zaitone S, Hassan N, El-Orabi N, El-Awady el-S. Pentoxifylline and melatonin in combination with pioglitazone ameliorate experimental non-alcoholic fatty liver disease. Eur J Pharmacol. 2011;662(1-3):70–77. doi: 10.1016/j.ejphar.2011.04.049. [DOI] [PubMed] [Google Scholar]