Abstract
Objective:
To examine the efficacy, safety, and tolerability of vilazodone for subjects (aged 18–75 years) with generalized social anxiety disorder.
Method:
Forty-four subjects with generalized social anxiety disorder (DSM-IV-TR criteria) were randomized to vilazodone or placebo in a 12-week double-blind, flexible-dose trial. Change from baseline to endpoint on the Liebowitz Social Anxiety Scale (LSAS) was the primary outcome measure. Secondary outcome measures included response and remission rates and changes in depression and anxiety. Data were collected between November 2012 and April 2014. The study was conducted at a private clinical trials facility in New York, New York.
Results:
The mean baseline LSAS score was 91.9 (SD = 17.5) and the mean Clinical Global Impressions–Severity scale score was 5.3 (SD = 0.56), indicating marked to severe illness. There were no significant baseline differences in severity of social anxiety between the treatment groups. At the end of treatment, in the intent-to-treat sample (n = 39), the vilazodone group had improved significantly more than the placebo group by 14.3 points on the LSAS (t = 1.80, P = .04, one-tail test) (Cohen d = 0.58).
Conclusions:
The findings suggest that vilazodone may be a promising treatment for social anxiety disorder. Further study is needed given the limited sample size.
Trial Registration:
ClinicalTrials.gov identifier: NCT01712321
Clinical Points
■ Vilazodone should be considered for further study in social anxiety disorder
■ Screening and treating patients with social anxiety in the primary care setting may limit the overall impact and subsequent health risks that the disorder can impose.
Social anxiety disorder is a prevalent, chronic, and disabling condition.1 The DSM-IV recognized a generalized form, characterized by severe anxiety and avoidance in both interpersonal and performance situations. The age at onset of generalized social anxiety disorder is early; academic, vocational, and social impairment are often severe; and depression and alcohol abuse are common sequelae.2
More therapeutic agents for social anxiety disorder are needed. Only 45%–55% of any given sample significantly improve with any of the 4 current marketed drugs for social anxiety disorder,3–6 and many responders are still clinically symptomatic.
Vilazodone was chosen for study in social anxiety disorder because it differed in several ways from already approved selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors. In addition to having SSRI properties, vilazodone is a partial 5-HT1A agonist.7,8 There exists some overlap for the treatment of social anxiety disorder and generalized anxiety disorder. Recently, vilazodone was found to be effective in treating generalized anxiety disorder.9 Khan et al7 and Robinson et al10 found vilazodone to have a good tolerability and safety profile.
METHOD
The study was a 12-week double-blind, placebo-controlled, flexible-dose trial; daily doses of vilazodone 20 mg/d to 40 mg/d or matching placebo were administered in a 1:1 ratio. Data were collected between November 2012 and April 2014.
Subjects
Enrollment was planned for 30 subjects who achieved the prospectively determined minimum adequate treatment of at least 6 consecutive weeks on ≥ 20 mg/d of vilazodone or the placebo equivalent. Subjects included men and women, aged 18–75 years, who met DSM-IV-TR criteria for generalized social anxiety disorder and had a minimum total Liebowitz Social Anxiety Scale (LSAS)11 score at screening and baseline of 70 and a minimum Clinical Global Impressions–Severity scale (CGI-S)12 score of 4 (moderately ill). Subjects also had to agree to practice effective contraception methods.
Exclusion criteria included lifetime bipolar disorder, schizophrenia, and body dysmorphic disorder, as well as posttraumatic stress disorder, obsessive-compulsive disorder, panic disorder, and substance dependence within the past 24 weeks. Comorbid major depression, dysthymia, generalized anxiety disorder, and specific phobias were allowed if generalized social anxiety disorder was the primary disorder (the major clinical problem for which the subjects sought treatment). Subjects who were suicidal, who were medically unstable, who had a history of cancer or treatment-refractory generalized social anxiety disorder (failure to respond to adequate trials of 2 effective agents), or who were in active cognitive-behavioral therapy or were currently pregnant or lactating were excluded. Zolpidem as needed was allowed for insomnia if not taken more than 3 times per week. Other psychotropic drugs had to be discontinued at least 2 weeks before the baseline visit.
Dosing
Subjects started at baseline on vilazodone 10 mg/d or placebo, taken in the morning with food, and increased to 20 mg/d or placebo after 1 week and to 40 mg/d or placebo after the second week. Dose increases could be delayed or reversed for problems of tolerability; however, attempts were made to raise all subjects to 40 mg/d. Noncompliance was defined as < 80% or > 120% of prescribed drug taken during any evaluation period. Subjects who were noncompliant at more than 2 consecutive study visits could be terminated.
Procedures
This trial was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Asentral Institutional Review Board (IRB) and Forest. Approval for the study was obtained from the Asentral IRB. Subjects were recruited via IRB-approved advertising and from the site’s database for this single-site study. The study was conducted at The Medical Research Network, LLC, a private clinical trials facility in New York, New York (ClinicalTrials.gov identifier: NCT01712321).
The subjects were screened by an in-person psychiatric interview with one of the investigators (J.M.C. or M.R.L.). Subjects deemed appropriate and interested in the study signed the IRB-approved informed consent after full explanation of the study procedures and having all questions asked and answered. Subjects were specifically informed that vilazodone was approved by the US Food and Drug Administration only for the treatment of major depressive disorder13 and use for the treatment of social anxiety disorder was considered experimental. Subjects were then administered the LSAS, 17-item Hamilton Depression Rating Scale (HDRS-17),14 Hamilton Anxiety Rating Scale (HARS),15 CGI-S, and Mini-International Neuropsychiatric Interview (MINI)16; underwent routine blood and urine tests; and were screened for drugs of abuse and pregnancy. A physical examination and electrocardiogram (ECG) were also completed. If present, psychotropic medications were tapered.
Subjects returned for the baseline visit 1–2 weeks later, and the LSAS, CGI-S, HARS, and HDRS-17 were repeated. Those eligible were randomized to vilazodone or placebo in cohorts of 6.
After the baseline visit, subjects were seen at weeks 1, 2, 4, 6, 8, 10, and 12 and were assessed for clinical progress and adverse events. Ratings on the LSAS, CGI-S, Clinical Global Impressions–Improvement scale (CGI-I), and subject-rated Patient Global Impression of Change (PGIC)17 were obtained. A follow-up visit at week 14 was also completed.
Measures
The MINI was used for diagnostic assessment of DSM-IV disorders. The CGI-S, CGI-I, PGIC, and LSAS were administered to assess social anxiety disorder severity and change and global improvement. The LSAS is a 24-item instrument developed by Liebowitz11 that assesses anxiety and avoidance in a variety of commonly encountered performance and social situations and was found by Heimberg et al18 to be reliable and valid. The HDRS-17 and HARS were used to quantify depressive and anxiety symptoms at baseline and endpoint. Safety measures included routine laboratory tests, ECGs, and physical examinations. Subjects were asked about adverse events and concomitant medications at each study visit.
Data Analyses
Primary efficacy analyses were done in the intent-to-treat (ITT) sample, defined as all subjects who took at least 1 dose of drug or placebo with at least 1 postrandomization LSAS rating. The primary outcome measure was change in total LSAS score from baseline to endpoint in the ITT sample. Secondary outcome measures included CGI- and PGIC-based categorical ratings and HDRS and HARS symptom ratings in the ITT sample. Outcomes before week 12 also were examined on an exploratory basis. Secondary efficacy analyses also were conducted in subjects who achieved the prospectively determined minimal adequate treatment of at least 6 weeks on ≥ 20 mg/d of vilazodone or the placebo equivalent.
Pretreatment and posttreatment comparisons on dimensional ratings were done using last observation carried forward (LOCF). In addition, subjects rated as 1 (very much improved) or 2 (much improved) on the CGI-I at endpoint were considered treatment responders. Subjects whose endpoint total LSAS score was ≤ 30 were considered treatment remitters. Subjects who rated themselves as 1 (very much improved) or 2 (much improved) on the PGIC at endpoint were considered self-rated responders.
Group differences in treatment outcome were evaluated using a 1-tail t test for independent samples, while baseline comparisons utilized 2-tail tests. For the bivariate responder analyses (CGI-I, PGIC, and LSAS remitters), a test of 2 binary proportions and hypothesized difference of greater than zero (1-tail tests) between the active and placebo groups was conducted. Effect sizes were estimated using Cohen d (the difference between group means divided by pooled standard deviation). Odds ratios (ORs) were calculated by dividing the ratio of responders to nonresponders in the active group by the ratio of responders to nonresponders in the placebo group.
RESULTS
Baseline
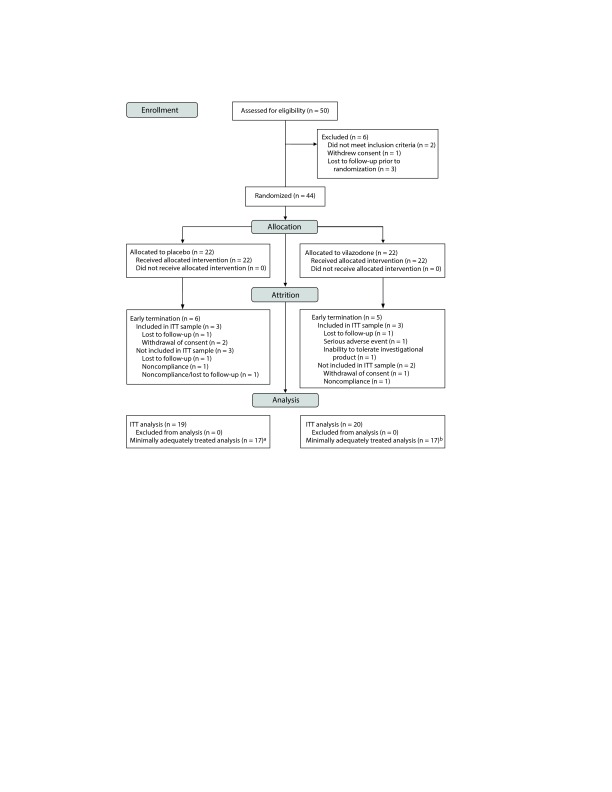
Fifty subjects signed informed consent (Figure 1). Six subsequently screen failed (3 lost to follow-up, 2 failed to meet inclusion criteria, and 1 withdrew consent), while 44 were randomized to study drug. Of these 44 subjects, 33 (75%) completed the trial (17 drug, 16 placebo), and 6 others (3 drug, 3 placebo) provided sufficient data to be included in the ITT sample of 39 (20 drug, 19 placebo). Five other randomized subjects (2 drug, 3 placebo) were excluded from the ITT sample because of noncompliance (1 drug, 1 placebo), withdrawal of consent (1 drug), lost to follow-up (1 placebo), and noncompliance/lost to follow-up (1 placebo). The prospectively defined minimum adequately treated sample (at least 6 weeks of 20 mg/d of vilazodone) included 34 subjects, consisting of the 33 completers (17 drug, 16 placebo) and 1 other subject (placebo).
Figure 1.
Consort Diagram for Vilazodone Clinical Study
aExcludes 2 ITT subjects with fewer than 6 weeks at minimum dose level; includes 16 completers and 1 early termination subject who achieved minimally adequate treatment.
bExcludes 3 ITT subjects with fewer than 6 weeks at minimum dose level; includes 17 completers. Abbreviation: ITT = intent-to-treat.
Treatment groups in the ITT sample did not differ on age or sex (Table 1). While most subjects reported an onset of illness during childhood or adolescence, the mean age at onset was lower and duration of illness was longer for those randomized to vilazodone than placebo (P = .0001 and P = .005, respectively) (Table 1). Drug and placebo groups did not differ in baseline total LSAS or CGI-S scores (Table 1). The mean baseline LSAS score was 91.9 (SD = 17.5) and the mean CGI-S score was 5.3 (SD = 0.56), indicating marked to severe illness.
Table 1.
Baseline Summary for the Intent-to-Treat Sample
| Variable | Active Drug (n = 20) | Placebo (n = 19) | t or Z | P (2-tail) |
| Age, mean (SD), y | 42.4 (13.4) | 35.5 (15.6) | t = 1.50 | .142 |
| Sex, n (%)a | ||||
| Male | 14 (70) | 12 (63) | Z = 0.45 | .325 |
| Female | 6 (30) | 7 (37) | ||
| Age at social anxiety disorder onset, mean (SD), y | 7.0 (3.7) | 14.1 (5.8) | t = 4.58 | .0001 |
| No. of years with social anxiety disorder, mean (SD) | 35.5 (14.8) | 21.4 (14.8) | t = 2.97 | .005 |
| Baseline LSAS total score, mean (SD) | 88.0 (12.7) | 96.0 (20.9) | t = 1.46 | .153 |
| Baseline CGI-S score, mean (SD) | 5.3 (0.4) | 5.3 (0.7) | t = 0.07 | .942 |
| HDRS-17 total score at visit 2, mean (SD) | 5.1 (3.6) | 6.9 (2.3) | t = 1.87 | .069 |
| HARS total score at visit 2, mean (SD) | 6.0 (3.6) | 9.1 (2.9) | t = 2.89 | .006 |
Male: n = 26, 67%; female: n = 13, 33%.
Abbreviations: CGI-S = Clinical Global Impressions–Severity, HARS = Hamilton Anxiety Rating Scale, HDRS-17 = 17-item Hamilton Depression Rating Scale, LSAS = Liebowitz Social Anxiety Scale.
Effects of Treatment
At the end of the treatment phase, 70% (14/20) of subjects receiving the active drug were on the highest dose of 40 mg/day compared to 89.5% (17/19) of subjects receiving placebo. The mean endpoint vilazodone dose was 33.5 mg/day, and the mean placebo dose was the equivalent of 37.9 mg/day.
Primary outcome.
At the end of treatment, in the ITT sample (n = 39), the vilazodone group (n = 20) had improved significantly more (LSAS score mean change from baseline) than the placebo group (n = 19) by 14.3 points (95% lower-bound CI = 0.92) on the LSAS (t = 1.80, P = .04, 1-tail test) (Table 2). The effect size (Cohen d) of vilazodone was 0.58 (95% CI, −0.06 to 1.22) (medium size effect) on the LSAS.
Table 2.
Endpoint Data for the Intent-to-Treat Sample
| Variable | Active Drug (n = 20) | Placebo (n = 19) | t | P (1-tail) |
| Endpoint LSAS total score, mean (SD) | 57.0 (26.0) | 79.4 (29.8) | 2.50 | .008 |
| LSAS score change from baseline, mean (SD)a | 30.9 (26.1) | 16.6 (23.4) | 1.80 | .040 |
| HDRS-17 total score at week 9/early termination, mean (SD) | 4.2 (3.8) | 6.1 (4.0) | 1.49 | .073 |
| HDRS-17 score change from baseline, mean (SD)a | 1.1 (4.2) | 0.9 (4.4) | 0.08 | .461 |
| HARS total score at week 9/early termination, mean (SD) | 5.0 (3.2) | 7.6 (4.3) | 2.02 | .026 |
| HARS score change from baseline, mean (SD)a | 1.2 (4.5) | 1.5 (4.3) | 0.19 | .574 |
Final mean change scores reflect cumulative rounding of individual change scores.
Abbreviations: CGI-S = Clinical Global Impressions–Severity, HARS = Hamilton Anxiety Rating Scale, HDRS-17 = 17-item Hamilton Depression Rating Scale, LSAS = Liebowitz Social Anxiety Scale.
Secondary outcomes.
For the minimally adequately treated sample (n = 34), the mean reduction in total LSAS score from baseline to endpoint was 33.4 (SD = 27.0) in the vilazodone group (n = 17) and 17.8 (SD = 24.5) in the placebo group (n = 17), for a difference of 15.6 points (95% lower-bound CI, 0.61). The difference between groups was significant (t32 = 1.76, P = .044, 1-tail). The Cohen d was 0.61 (95% CI, −0.08 to 1.29), indicating a moderate/large effect size.
The response rate (subjects having CGI-I scores of 1 or 2 at endpoint) for the ITT sample was significantly greater for the vilazodone group (11/20, 55%) than the placebo group (5/19, 26%) (Z = 1.91, P = .028, 1-tail test, OR = 3.42, 95% CI, 0.9–13.2). For the minimally adequately treated sample, response rates were 10/17 (58.8%) for the vilazodone group and 5/17 (29.4%) for the placebo group (Z = 1.81, P = .035, 1-tail test, OR = 3.43, 95% CI, 0.83–14.2).
Treatment groups did not differ significantly with regard to remission rates. In the ITT sample, 3/20 (15%) subjects randomized to vilazodone and 1/19 (5.3%) randomized to placebo had endpoint LSAS scores ≤ 30 (Z = 1.03, P = .152, 1-tail test, OR = 3.18, 95% CI, 0.30–33.58). Among the minimally adequately treated, 3/17 (17.6%) of the vilazodone group and 1/17 (5.9%) of the placebo group were LSAS remitters (Z = 1.08, P = .139, 1-tail test, OR = 3.43, 95% CI, 0.32–36.83).
On the subject-rated global outcome scale (the PGIC), in the ITT sample, 9/19 (47.4%) of subjects randomized to vilazodone and 5/18 (27.8%) randomized to placebo rated themselves as responders at the study endpoint, a nonsignificant difference (Z = 1.26, P = .104, OR = 2.34, 95% CI, 0.60–9.20). Among the minimally adequately treated, 8/17 (47.1%) of the vilazodone and 5/17 (29.4%) of the placebo subjects rated themselves as responders, also not a significant difference (Z = 1.08, P = .141, OR=2.13, 95% CI, 0.52–8.76).
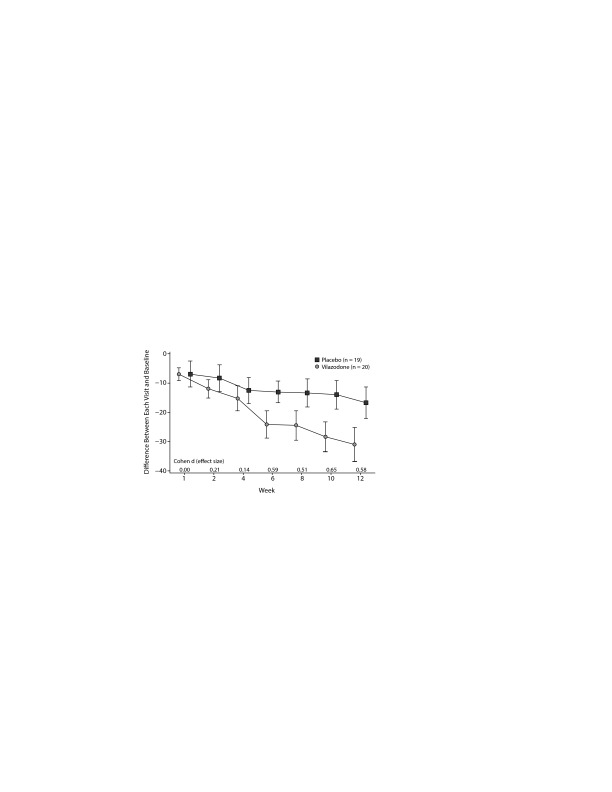
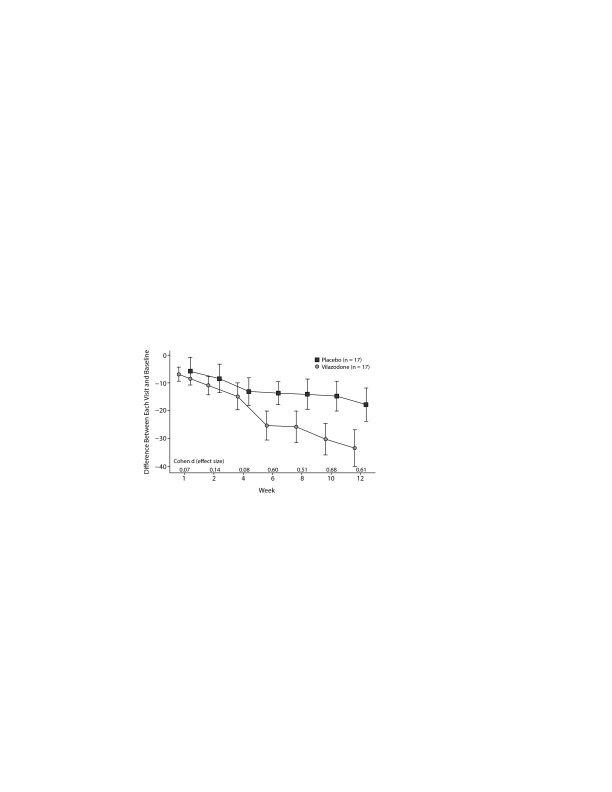
In the ITT sample and the minimally adequately treated sample, the mean reduction on the HARS and the HDRS-17 was not significant. Figures 2 and 3 show the mean total LSAS scores and effect sizes for group differences at each visit for the ITT sample and minimally adequately treated subset. In both samples, clinically significant differences between drug and placebo are first evident after 6 weeks of treatment and then persist for the rest of the study.
Figure 2.
Liebowitz Social Anxiety Scale and Effect Size at Each Visit (intent-to-treat)a
aBars are 1 standard error from the mean.
Figure 3.
Liebowitz Social Anxiety Scale and Effect Size at Each Visit (minimally adequately treated)a
aBars are 1 standard error from the mean.
Attrition
Eleven subjects (5 vilazodone, 6 placebo) were early terminators: 3 (1 vilazodone, 2 placebo) due to withdrawal of consent, 3 (1 vilazodone, 2 placebo) were lost to follow-up, 2 (1 vilazodone, 1 placebo) due to noncompliance, 1 (vilazodone) due to inability to tolerate investigational product, 1 (vilazodone) due to a serious adverse event (submandibular abcess), and 1 (placebo) due to both noncompliance and being lost to follow-up.
Adverse Events
There was 1 serious adverse event; a patient taking vilazodone was hospitalized for a submandibular abscess that was judged by the investigator as not related to study medication. Table 3 lists the adverse events that occurred in at least 2 vilazodone or 2 placebo subjects.
Table 3.
| Adverse Event | Active Drug (n = 20) | Placebo (n = 19) |
| Cramping | 2 (10) | 2 (11) |
| Nausea | 5 (25) | 1 (5) |
| Diarrhea | 4 (20) | 1 (5) |
| Gas | 2 (10) | 0 (0) |
| Dizziness | 3 (15) | 3 (16) |
| Drowsiness | 5 (25) | 3 (16) |
| Dry mouth | 1 (5) | 2 (11) |
| Fatigue | 3 (15) | 3 (16) |
| Headache | 3 (15) | 3 (16) |
| Insomnia | 2 (10) | 3 (16) |
| Tremor | 0 (0) | 2 (11) |
Values are presented as n (%).
Adverse events did not differ significantly between groups (Fisher exact P values > .05).
All adverse events were assessed as mild or moderate in severity and did not differ significantly in frequency between drug and placebo; however, we lacked the statistical power to examine this definitively. There were no clinically significant abnormalities found on routine laboratory tests, ECGs, physical examinations, or changes in blood pressure, heart rate, or body weight over the course of the study.
DISCUSSION
Despite the small sample size, comparison of our current findings for vilazodone to those of other medications found effective for social anxiety disorder encourage further work with vilazodone for this condition. There are several caveats to our findings. The small sample size renders the results somewhat imprecise and widens the 95% CIs. Use of 1-tail tests to compare outcome, although specified prospectively, differs from the other cited social anxiety disorder studies. Advantages of the 1-tail test include greater specificity of the answer to the research question and the need for fewer research subjects. Potential disadvantages include having a drug that turned out to be significantly worse than placebo fall into the null category of ≤ placebo. The small sample size also may have contributed to an imperfect randomization. However, the demographic features of the study sample are typical of those seen in social anxiety disorder trials, and the between-group differences found should not have rendered the drug group significantly more treatment responsive.
Further study is needed to conclude if vilazodone may be a viable alternative for patients with generalized social anxiety disorder (including sertraline, fluvoxamine, venlafaxine extended release, and paroxetine). However, the mean reduction in total LSAS scores seen with vilazodone was clinically meaningful. Subjects treated with vilazodone had a baseline mean LSAS score of 88 and a CGI-S score of 5.3, usually indicative of significant impairment in social or work function or both. Their endpoint mean LSAS score was 57, indicating some social and performance anxiety symptoms with no significant impairment in functioning and below the minimum required for entry into this study.
Several other study features are noteworthy. The placebo response rate of 26% is low for studies of social anxiety disorder, which tend to show placebo response rates in the 30%–35% range. One contributing factor might have been the requirement that subjects coming into the study have an LSAS total score ≥ 70, a higher score than most prior trials that required LSAS score minimums of 60–68.4–7,9,10 Another interesting feature is that the active drug group appears to be showing continuing improvement through week 12 of treatment (Figure 2), suggesting that patients may improve even more with continued treatment, as was demonstrated by Stein et al3 for fluvoxamine controlled release.
Vilazodone also was well tolerated in this limited sample, with 1 serious adverse event considered unrelated to study drug and 1 other subject who terminated early due to problems tolerating the active medication. The most common adverse events reported for vilazodone in this trial were nausea, drowsiness, and diarrhea. The data encourage further work with vilazodone in social anxiety disorder.
Drug names:
fluvoxamine (Luvox and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), vilazodone (Viibryd), zolpidem (Ambien, Edluar, and others).
Potential conflicts of interest:
Dr Liebowitz has received grant/research support from the Forest Research Institute, is the copyright holder of the Liebowitz Social Anxiety Scale, and is the managing director of The Medical Research Network, LLC. Dr Careri and Ms Draine are employees of and Dr Hanover has served as a consultant to The Medical Research Network, LLC.
Funding/support:
This study was supported by an investigator-initiated grant from Forest Research Institute, Inc, Jersey City, New Jersey, to The Medical Research Network, LLC.
Role of the sponsor:
Forest is the manufacturer of vilazodone and provided both financial and material support (study drug and placebo) for the study. Forest also provided a review of the first draft of the manuscript with no right of approval or disapproval.
References
- 1.Ruscio AM, Brown TA, Chiu WT, et al. Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med. 2008;38(1):15–28. doi: 10.1017/S0033291707001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneier FR, Foose TE, Hasin DS, et al. Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2010;40(6):977–988. doi: 10.1017/S0033291709991231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- 4.Van Ameringen MA, Lane RM, Walker JR, et al. Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry. 2001;158(2):275–281. doi: 10.1176/appi.ajp.158.2.275. [DOI] [PubMed] [Google Scholar]
- 5.Davidson J, Yaryura-Tobias J, DuPont R, et al. Fluvoxamine-controlled release formulation for the treatment of generalized social anxiety disorder. J Clin Psychopharmacol. 2004;24(2):118–125. doi: 10.1097/01.jcp.0000106222.36344.96. [DOI] [PubMed] [Google Scholar]
- 6.Liebowitz MR, Mangano RM, Bradwejn J, et al. SAD Study Group. A randomized controlled trial of venlafaxine extended release in generalized social anxiety disorder. J Clin Psychiatry. 2005;66(2):238–247. doi: 10.4088/jcp.v66n0213. [DOI] [PubMed] [Google Scholar]
- 7.Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441–447. doi: 10.4088/JCP.10m06596. [DOI] [PubMed] [Google Scholar]
- 8.Rickels K, Athanasiou M, Robinson DS, et al. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(3):326–333. doi: 10.4088/jcp.08m04637. [DOI] [PubMed] [Google Scholar]
- 9.Gommoll C, Durgam S, Mathews M, et al. A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32(6):451–459. doi: 10.1002/da.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643–646. doi: 10.1097/JCP.0b013e31822c6741. [DOI] [PubMed] [Google Scholar]
- 11.Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 12.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare Public Health Service Alcohol, Drug Abuse and Mental Health Administration; 1976. [Google Scholar]
- 13.Cincinnati, OH: Forest Pharmaceuticals, Inc; 2015. USPI Viibryd. (Vilazodone) [package insert] [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and. ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 17.Wyrwich KW, Kawata AK, Thompson C, et al. Validation of the Self-assessment of Treatment Questionnaire among patients with postherpetic neuralgia. Pain Research and Treatment. 2012 doi: 10.1155/2012/621619. vol 2012: Article ID 621619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimberg RG, Horner KJ, Juster HR, et al. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29(1):199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]