Abstract
Introduction
There is little data currently available to guide surgical decision making regarding emergent surgical interventions in leukopenic patients. The purpose of this study was to investigate the impact of leukopenia among patients undergoing emergency abdominal operations in order to better guide preoperative decision making.
Methods
The 2005-2012 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database was queried to identify patients who underwent emergent laparotomy. Patients were stratified by pre-operative white blood cell (WBC) count (<4.0 × 109/L vs 4.0-12.0 × 109/L). Baseline demographics, comorbidities, and outcomes were compared. Multivariable logistic regression was performed to estimate the adjusted association between leukopenia and mortality taking into account the robust array of patient-related factors.
Results
Of the 20,443 patients who met study criteria, 2,057 (8.2%) were leukopenic (WBC <4.0) prior to surgery. Unadjusted comparison demonstrated significantly increased major morbidity (45.4% vs 26.9%, p<0.001) as well as mortality (24.4% vs 10.8%, p<0.001) for patients with leukopenia compared to patients with a normal preoperative WBC count. Only 46.0% (n=947) of patients with leukopenia prior to surgery were able to avoid major morbidity or mortality compared to 69.4% (n=15,974) of patients with a normal preoperative WBC count (p<0.001). After multivariable adjustment for patient-related factors, leukopenia was maintained as a significant predictor of mortality.
Conclusions
Although leukopenia remains associated with mortality in patients undergoing emergent laparotomy despite adjustment for other patient-related factors, it is not necessarily prohibitive. Understanding the risk of complications and mortality associated with these procedures is pertinent for pre-operative clinical decision making.
Level of Evidence
Level III, Prognostic and Epidemiological Study.
Keywords: Leukopenia, Emergent Abdominal Surgery, White Blood Cell Count
Background
Leukocytes have numerous essential functions, several of which become uniquely important to patients undergoing perioperative care. They play a critical role in promoting recovery after surgery by facilitating the healing of surgical incisions and anastomoses and by mediating the host-defense response against invading pathogens (1-4). As such, leukocyte depletion or dysfunction is associated with delayed surgical wound healing, increased rates of postoperative infection, and poor overall outcomes following surgery (5-7). For these reasons, surgeons may defer operating on leukopenic patients until the underlying cause of the leukopenia can be addressed and leukocyte counts can be restored. However, in the emergent setting, surgeons and patients must acutely decide whether to proceed with surgery or to instead opt for nonopertive treatment, which may be associated with eminent death.
The decision to proceed with surgery in such circumstances is a difficult one for surgeons and patients alike. However, there are currently little data available that quantitate the effect of leukopenia on outcomes in emergent general surgery. As a result, surgeons are limited in their ability to specifically counsel patients on the likelihood of survival or avoidance of major morbidity with operative intervention, which in turn limits the patient’s ability to make a well-informed choice on whether or not to proceed with a surgery that may not be in line with his or her goals of care. In order both to adequately determine whether surgery is a feasible option for these patients, and to accurately assess and convey the risk of surgery to patients and their families, we attempted to characterize the risks of emergent abdominal surgery associated with leukopenia after adjustment for other comorbid conditions.
Methods
Patient Population
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Participant user files from 2005-2012 were queried for patients undergoing an emergent laparotomy (defined by Current Procedural Terminology codes 49000, 44120, 44143, 44150, 44160, 44144, 44140, 44005, 44141, 44050, 44125, 44130, 44145, 44310, 44320, and 44615). Patients were excluded if they were under the age of 18, were pregnant, had known disseminated cancer, had an unknown pre-operative white blood cell (WBC) count or had an elevated WBC count prior to surgery (>12.0 × 109/L). Patients with known disseminated cancer were specifically excluded as these patients present with a very different clinical situation that is out of the scope of this study. Patients over the age of 89 were also excluded due to the inability to differentiate patients’ ages above 89 in the ACS-NSQIP database.
Variables
The primary predictor was pre-operative WBC count. The primary outcome was mortality and major morbidity (defined as organ space infection, abdominal wound dehiscence, failure to wean from ventilator for greater than 48 hours, cerebrovascular accident with neurologic deficit, cardiac arrest, myocardial infarction, reintubation, reoperation, or new dialysis requirement). A composite for a history of cardiac issues was created including a history of congestive heart failure within 30 days of the operation, a history of a myocardial infarction within 6 months of the operation, a previous percutaneous coronary intervention, previous cardiac surgery, or a history of angina within 1 month before surgery. Procedures were also classified as either not involving any resection of bowel (CPT: 44005, 44050, 49000), involving resection of small bowel (CPT: 44120, 44125, 44130, 44310, 44615), or involving resection of colon (CPT: 44140, 44141, 44143, 44144, 44145, 44150, 44160, 44320). Missing variables were assumed to be missing at random (MAR).
Statistical Analysis
Patients were initially grouped by pre-operative WBC (<4.0 and 4.0-12.0 × 109/L), and compared with regards to baseline demographics, comorbidities, operative characteristics, and outcomes. Comparison by WBC groups for continuous variables were conducted with the Wilcoxon Rank Sum test while categorical variables were compared with Fisher’s Exact test or the Chi-Square test.
Multivariable logistic regression was utilized in order to determine the adjusted association of pre-operative WBC count and mortality as well as major morbidity. For this analysis, patients were grouped a priori on the basis of their WBC count (0.0-0.9, 1.0-1.9, 2.0-2.9, 3.0-3.9, and 4.0-12.0 × 109/L). The increased number of categories were used in order to better discriminate between pre-operative WBC levels, in order to provide additional data for clinical decision making (a WBC count of 0.5 is likely very different from a WBC count of 1.5). Additional variables were also incorporated on the basis of their clinical significance and initially included age, sex, body mass index (BMI), previous cardiac history, diabetes, chronic obstructive pulmonary disorder (COPD), renal failure, dependent functional status, steroid use within 30 days of the operation, chemotherapy use within 30 days of the operation, current smoking status, sepsis, albumin level, presence of ascites, greater than 10% weight loss over the past 6 months, American Society of Anesthesiologists (ASA) physical status classification, procedure (involving either small or large bowel resection), and do not resuscitate (DNR) status. Interaction terms were initially included between WBC count and pre-operative steroid use, pre-operative chemotherapy use, and pre-operative sepsis in the mortality model in order to determine if the association of WBC count and outcomes were different among these subgroups than among the overall cohort. The final variables included in the model were selected via backwards stepwise variable selection. All variables were assessed for linearity prior to inclusion in the model, and categorized into groups or transformed if necessary. Models were evaluated with Receiver Operator Curves (ROC) and the Pearson Chi-square test for goodness-of-fit. All statistical analyses were performed using R version 3.1.0 (Vienna, Austria). A p-value of 0.05 was used to determine statistical significance.
Results
Following the exclusion of 1,785 patients who were less than 18 or greater than 89 years of age, 96 patients who were pregnant, 2,992 patients who presented with disseminated cancer, 860 patients with an unknown WBC count, and 20,443 patients who had an elevated WBC count pre-operatively, 25,089 patients remained. 2,057 (8.2%) had a pre-operative WBC count less than 4.0 × 109/L, while 23,032 (91.8%) had a normal pre-operative WBC count (4.0-12.0 × 109/L, Figure 1). Compared to patients with a normal pre-operative WBC count, patients who were leukopenic more often had pre-operative renal failure (10.4% vs 6.5%, p<0.001), a dependent functional status (32.6% vs 20.6%, p<0.001), pre-operative sepsis or SIRS (systemic inflammatory response syndrome, 67.1% vs 32.1%, p<0.001), ascites (11.1% vs 6.5%, p<0.001) and an ASA class of 4 or greater (44.9% vs 26.4%, p<0.001) than patients with a normal pre-operative WBC count (Table 1). Leukopenic patients were also more likely to have undergone chemotherapy (10.1% vs 1.4%, p<0.001) and to have received steroid treatment (12.9% vs 7.7%, p<0.001) within 30-days of surgery. Lastly, patients who were leukopenic had similar rates of small bowel resections (23.5% vs 24.5%, p=0.309), but were more likely to require a colon resection (48.5% vs 44.8%, p=0.001).
Figure 1.
Variation in pre-operative white blood cell count for patients undergoing emergent laparotomy.
Table 1.
Patient Demographics and Comorbidities
| Variable | Leukopenic | Normal WBC | p-value |
|---|---|---|---|
| N | 2,057 (8.2%) | 23,032 (91.8%) | |
| Age | 64 (53, 76) | 64 (52, 76) | 0.599 |
| Female | 1,123 (54.7%) | 12,586 (54.8%) | 0.982 |
| Body Mass Index (kg/m2) | 25 (21, 29) | 26 (22, 30) | <0.001 |
| Diabetes | 354 (17.2%) | 3,735 (16.2%) | 0.255 |
| COPD | 172 (8.4%) | 2,167 (9.4%) | 0.127 |
| Cardiac History | 275 (13.4%) | 3,083 (13.4%) | 0.999 |
| Renal Failure | 214 (10.4%) | 1,489 (6.5%) | <0.001 |
| Dependent Status | 666 (32.6%) | 4,718 (20.6%) | <0.001 |
| Steroid Use | 265 (12.9%) | 1,775 (7.7%) | <0.001 |
| Chemotherapy | 164 (10.1%) | 253 (1.4%) | <0.001 |
| Radiation | 56 (3.4%) | 98 (0.5%) | <0.001 |
| Smoking Status | 407 (19.8%) | 4,751 (20.6%) | 0.380 |
| Pre-Operative Sepsis | <0.001 | ||
| None | 676 (32.9%) | 15,577 (67.9%) | |
| Sepsis | 408 (19.9%) | 2,253 (9.8%) | |
| Septic Shock | 564 (27.5%) | 1,876 (8.2%) | |
| SIRS | 405 (19.7%) | 3,235 (14.1%) | |
| Albumin (g/dL) | 3 (2, 4) | 4 (3, 4) | <0.001 |
| Presence of Ascites | 228 (11.1%) | 1,491 (6.5%) | <0.001 |
| Recent Weight Loss | 147 (7.1%) | 1,157 (5.0%) | <0.001 |
| ASA Class | <0.001 | ||
| 1 or 2 | 312 (15.2%) | 6,847 (29.8%) | |
| 3 | 822 (40.0%) | 10,087 (43.9%) | |
| 4 or 5 | 923 (44.9%) | 6,064 (26.4%) | |
| DNR Status | 44 (2.7%) | 424 (2.3%) | 0.395 |
COPD – Chronic Obstructive Pulmonary Disorder. CHF – Congestive Heart Failure. ASA – American Society of Anesthesiologists. DNR – Do Not Resuscitate. Continuous variables are presented as median (interquartile range). Categorical variables are presented as frequency (percentage).
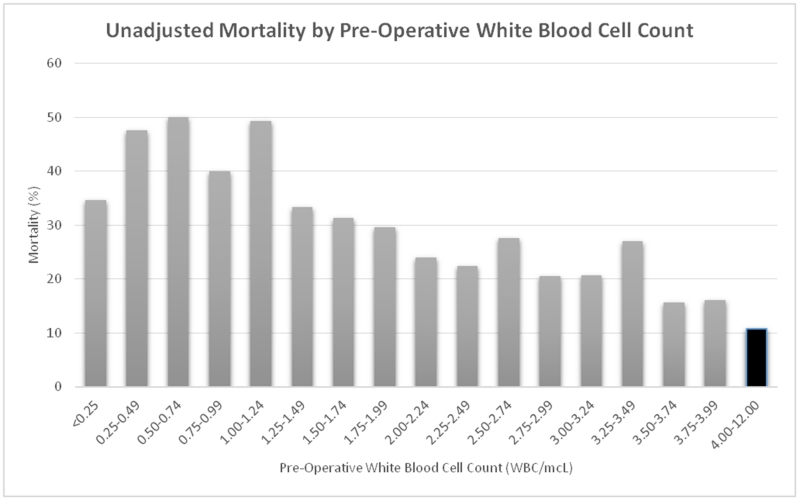
Patients who were leukopenic were more likely to have dirty/infected (class IV) surgical wounds (44.0% vs 28.1%, p<0.001) than patients with a normal WBC count, although median operative times were similar between the two groups (94 vs 95 minutes, p=0.786). On review of unadjusted outcomes, patients who were leukopenic prior to the operation were significantly more likely to suffer from mortality (24.4% vs 10.8%, p<0.001, Figure 2) as well as major morbidity (45.4% vs 26.9%, p<0.001) than patients with a normal WBC (Table 2). Leukopenic patients also had longer post-operative lengths of stay (median 10 vs 8 days, p<0.001). Only 46.0% (n=947) of leukopenic patients were able to avoid mortality or major morbidity following their procedure as compared to 69.4% (n=15,974) of patients with a normal WBC count (p<0.001).
Figure 2.
Unadjusted mortality by pre-operative white blood cell count among patients with leukopenia undergoing emergent laparotomy. The black bar denotes patients with a normal white blood cell count preoperatively.
Table 2.
Outcomes following emergent laparotomy.
| Variable | Leukopenic | Normal WBC | p-value |
|---|---|---|---|
| N | 2,057 (8.2%) | 23,032 (91.8%) | |
| Mortality | 502 (24.4%) | 2,478 (10.8%) | < 0.001 |
| Morbidity Composite | 934 (45.4%) | 6,203 (26.9%) | < 0.001 |
| Superficial Infection | 143 (7.0%) | 1,842 (8.0%) | 0.101 |
| Deep Wound Infection | 41 (2.0%) | 523 (2.3%) | 0.462 |
| Organ Space Infection | 150 (7.3%) | 1,065 (4.6%) | < 0.001 |
| Dehiscence | 57 (2.8%) | 672 (2.9%) | 0.756 |
| Urinary Tract Infection | 120 (5.8%) | 1,006 (4.4%) | 0.003 |
| Pneumonia | 227 (11.0%) | 1,798 (7.8%) | < 0.001 |
| Failure to Wean from Ventilator | 639 (31.1%) | 3,487 (15.1%) | < 0.001 |
| Renal Failure | 181 (8.8%) | 849 (3.7%) | < 0.001 |
| Sepsis | 468 (22.8%) | 3,233 (14.0%) | < 0.001 |
| Re-intubation | 206 (10.0%) | 1,434 (6.2%) | < 0.001 |
| Return to OR | 330 (16.0%) | 2,602 (11.3%) | < 0.001 |
| Readmission | 76 (11.5%) | 918 (12.0%) | 0.766 |
| Post-op LOS | 10 (6, 18) | 8 (5, 13) | < 0.001 |
CVA – Cerebrovascular Accident. MI – Myocardial Infarction. OR – Operating Room. LOS – Length of Stay. Continuous variables are presented as median (interquartile range). Categorical variables are presented as frequency (percentage).
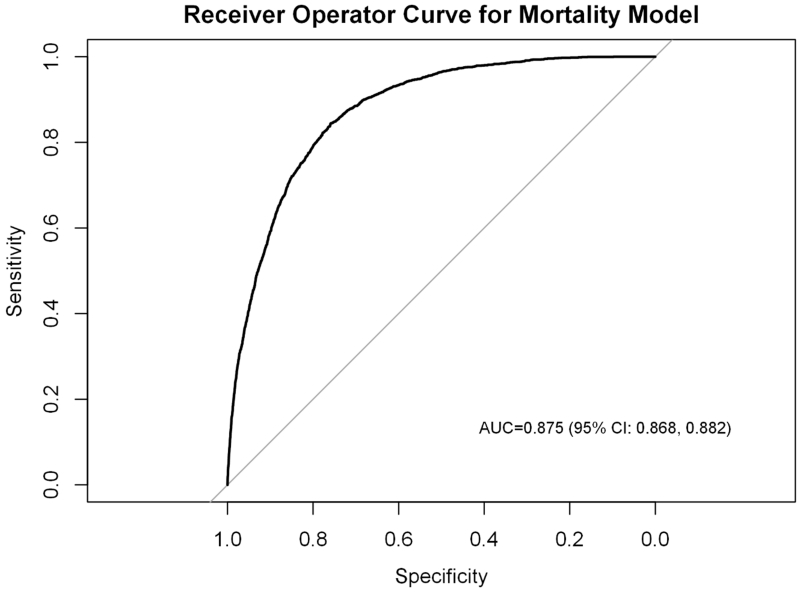
Following variable selection, the final logistic regression model for mortality included age, sex, BMI, a cardiac history, COPD, pre-operative renal failure, dependent functional status, preoperative steroid therapy, preoperative chemotherapy, current smoking status, preoperative sepsis, preoperative albumin <3.0, presence of ascites, ASA class, DNR status, and pre-operative WBC count (c-statistic: 0.875 [95% confidence interval (CI): 0.868, 0.882], Pearson chi-square test for goodness-of-fit: 0.785). There were no significant interactions between WBC count and chemotherapy, steroid use, or preoperative sepsis, indicating that the association between WBC count and mortality was not significantly different among these sub-populations as compared to the overall cohort. Despite adjustment for patient demographics and comorbidities, leukopenia continued to be significantly associated with reduced survival (Figure 3).
Figure 3.
Variables associated with mortality among patients undergoing emergent laparotomy. WBC-White Blood Cell count (× 109/L). BMI – Body Mass Index (kg/m2). COPD – Chronic Obstructive Pulmonary Disease. SIRS- Systemic Inflammatory Response Syndrome. ASA – American Society of Anesthesiologists physical status. DNR – Do Not Resuscitate status.
Following variable selection, the final model for major morbidity included sex, BMI, a cardiac history, a diagnosis of COPD, preoperative renal failure, dependent functional status, pre-operative steroid therapy, pre-operative chemotherapy, current smoking status, preoperative sepsis, preoperative albumin, presence of ascites, ASA class, DNR status, and preoperative WBC count (c-statistic: 0.778 [95% CI: 0.770, 0.785], Pearson Chi-Square test for goodness-of-fit: 0.294). Compared to a normal preoperative WBC count, a WBC count between 1.0 and 3.9 × 109/L was significantly associated with increased major morbidity (Figure 4). A WBC count <1.0 × 109/L was not significantly associated with increased morbidity.
Figure 4.
Variables associated with major morbidity among patients undergoing emergent laparotomy. WBC-White Blood Cell count (× 109/L). BMI – Body Mass Index (kg/m2). COPD – Chronic Obstructive Pulmonary Disease. SIRS- Systemic Inflammatory Response Syndrome. ASA – American Society of Anesthesiologists physical status. DNR – Do Not Resuscitate status.
Discussion
Prior to every operation, a surgeon must weigh the risks and benefits of the treatment he/she is performing. In patients presenting with emergent abdominal pathology requiring a laparotomy, it can be difficult to fully assess the risks of proceeding with surgery. It is especially difficult when a patient presents with leukopenia, as there is currently very little literature to describe the morbidity and mortality associated with laparotomy in these patients. In this study, we performed an analysis in the ACS-NSQIP database in order to better delineate how preoperative leukopenia affects postoperative mortality and morbidity in patients undergoing emergent laparotomy. We found that the incidence of postoperative mortality (24.4% vs 10.8%, p<0.001) and major morbidity (45.4% vs 26.9%, p<0.001) were both increased in leukopenic patients and were approximately double that of patients with a normal preoperative WBC. Furthermore, after adjustment for a number of patient factors by multivariable regression, leukopenia continued to be associated with increased odds of mortality and it generally appeared the risk of mortality increased with worsening leukopenia. Also after adjustment by multivariable regression, we continued to observe that preoperative leukopenia was generally associated with increased major morbidity (although this did not reach statistical significance for patients with a WBC <1.0 compared to a normal WBC likely secondary to either a small sample population or increased mortality among these patients). Altogether, the findings of this study demonstrate that nearly 54% of leukopenia patients will experience either mortality or major morbidity after emergent laparotomy.
Unlike elective surgery, in the emergent surgical setting there is limited opportunity to optimize a patient’s medical status. As a result, when proceeding with emergency surgery, patients and surgeons must ultimately decide to accept any added perioperative risks that may arise from unaddressed concomitant comorbidities. This decision can usually be justified since not operating for an emergent surgical condition often portends eminent death. Nevertheless, the availability of high-quality quantitative data on the added perioperative risks associated with a given comorbidity can be useful in assisting the patient in making a well-informed decision on whether or not to proceed with operative intervention. Although preoperative leukopenia has long been appreciated as a factor that portends poor wound healing and added risk of postoperative infection and other morbidity after surgery, the extent to which postoperative mortality and morbidity are affected by the presence of preoperative leukopenia has not previously been well defined (3, 4, 7). As such, the quantitative information on risks of mortality and major morbidity provided by this study may potentially be valuable to surgeons in effectively counseling patients on the risks and benefits of surgery in order to ensure that surgery is adherent with the patient’s goals of care.
This is not the first study to investigate factors associated with increased morbidity and mortality after emergent surgery. Sullivan et al demonstrated that chemotherapy within 30 days of emergency surgery was also associated with significantly increased mortality (8). This same group also demonstrated a similar association between radiation therapy within 90 days of emergency surgery and mortality (9). Other factors which have also been associated with increased complications following emergent surgery include cirrhosis, ASA grade, and obesity (10-12). Unfortunately, based on the definition of “emergency surgery,” there is no real alternative, and therefore the presence of these factors does not necessarily preclude operative intervention. However, understanding how these factors impact subsequent morbidity and mortality is important both to determine if an operation is feasible, and for patient and family discussions.
Some of the early work investigating the impact of leukopenia on outcomes following emergent abdominal surgery was in the HIV/AIDS (human immunodeficiency virus/acquired immune deficiency syndrome) population. These patients are at increased risk for many intra-abdominal emergencies including acute colitis, bowel obstructions, and intra-abdominal infections (13, 14). Early operative mortality after emergency surgery is extremely high in these patients; around 40-50% in the emergency setting as compared to roughly 10% in the elective setting (13). Furthermore, studies in this population have demonstrated that a CD4 cell count under 200 cells/μL is an important predictor of increased risk of morbidity and mortality (15). Although we could not differentiate CD4 counts in this study, it appears from our findings that leukopenia to such extreme levels is still a powerful predictor of operative mortality.
Another important finding in this study was our inability to demonstrate a significant interaction between pre-operative chemotherapy, pre-operative sepsis/SIRS, or pre-operative steroid treatment and leukopenia in either the major morbidity or mortality models. We had initially hypothesized that the association between leukopenia and mortality may be dependent on the etiology of the leukopenia, and that chemotherapy induced leukopenia may have a different association with mortality than other etiologies such as sepsis. By not being able to demonstrate this interaction, it can be postulated that the additional risk conferred by leukopenia is independent of etiology. However, as pre-operative chemotherapy and sepsis are both factors associated with increased mortality in abdominal surgery independent of leukopenia, their presence should be viewed as additive when assessing the risks of surgical intervention (8, 16).
As with all retrospective studies, there are limitations to our study. As a retrospective study of a national database, there is always an inherent risk of confounding by variables that are not accounted for in the database. However, the ACS-NSQIP database contains an extensive array of clinically important variables, including pre-operative sepsis, chemotherapy, and steroid treatment, and this database has been used previously to build validated risk adjusted models (17, 18). Second, although the risk of major morbidity and operative mortality are important for clinical decision-making, they may not adequately describe the risks related to surgery. Long-term outcomes as well as discharge location may also be very important, and unfortunately these are either not available in the ACS-NSQIP database or were not available for the majority of the years studied. Third, although the ACS-NSQIP database lists the total white count pre-operatively, the white count differential is not included, and we could therefore not determine how the differential was associated with outcomes. Lastly, we could not account for patients presenting with similar clinical scenarios who did not undergo an operation, either because they were not offered the operation or because they refused. It is likely that the sickest of patients did not undergo surgery, and therefore this may bias our results to make it appear that surgery leads to better outcomes than would be found in a real-world scenario.
In conclusion, we have demonstrated in this study not only that leukopenia is associated with increased mortality and major morbidity following emergent abdominal surgery, but more importantly, the magnitude of this effect. Roughly 46% of leukopenic patients can survive an emergent abdominal operation without major morbidity or mortality. Therefore, the presence of leukopenia should not necessarily prohibit an operation. Knowledge of these risks is necessary to guide clinical decision making for patients who present with abdominal emergencies requiring a laparotomy. This data will also aid in the discussion of the risks and benefits of surgical intervention with patients and their family members, and help to align the treatment plan with a patient’s individual goals of care.
Acknowledgements
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. The statistical methods were reviewed by Maragatha Kuchibhatla, PhD. In addition, Dr Gulack and Dr Englum are supported by the NIH funded Cardiothoracic Surgery Trials Network, 5U01HL088953-05.
Appendix
Figure.
Receiver Operator Curve (ROC) for mortality model.
Figure.
Receiver Operator Curve (ROC) for major morbidity model.
Footnotes
Meeting Information: This work will be presented at the 10th Annual Academic Surgical Congress, February 4, 2015, Las Vegas, NV
Conflicts of Interest: The authors do not have any conflicts of interest to declare.
Author Contribution: BCG, BRE, DDL, and MLS designed the study concept and design. DPN, JEK, and JES evaluated and critiqued the study design. BCG performed the statistical analysis. BCG wrote the initial manuscript. All authors were involved in editing and approval of the final manuscript.
References
- 1.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Archives of Surgery. 1997;132(9):991–6. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 2.Hunt TK, Hopf HW. Wound healing and wound infection. What surgeons and anesthesiologists can do. The Surgical clinics of North America. 1997;77(3):587–606. doi: 10.1016/s0039-6109(05)70570-3. [DOI] [PubMed] [Google Scholar]
- 3.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. American journal of surgery. 2004;187(5a):11s–6s. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury. 2006;37(Suppl 1):S5–12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Tone Y, Shibata F, Toma T, Yachie A. Delayed wound healing in leukocyte adhesion deficiency type 1. The Journal of pediatrics. 2011;158(2):342. doi: 10.1016/j.jpeds.2010.07.057. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DC, Schmalsteig FC, Finegold MJ, Hughes BJ, Rothlein R, Miller LJ, Kohl S, Tosi MF, Jacobs RL, Waldrop TC. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. Journal of Infectious Diseases. 1985;152(4):668–89. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- 7.Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. British journal of surgery. 1998;85(4):444–60. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MC, Roman SA, Sosa JA. Does chemotherapy prior to emergency surgery affect patient outcomes? Examination of 1912 patients. Annals of surgical oncology. 2012;19(1):11–8. doi: 10.1245/s10434-011-1844-7. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MC, Roman SA, Sosa JA. Emergency surgery in patients who have undergone recent radiotherapy is associated with increased complications and mortality: review of 536 patients. World journal of surgery. 2012;36(1):31–8. doi: 10.1007/s00268-011-1230-4. [DOI] [PubMed] [Google Scholar]
- 10.Arenal JJ, Bengoechea-Beeby M. Mortality associated with emergency abdominal surgery in the elderly. Canadian journal of surgery. 2003;46(2):111. [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122(4):730–6. doi: 10.1016/s0039-6060(97)90080-5. [DOI] [PubMed] [Google Scholar]
- 12.Serejo LGG, da Silva-Júnior FP, Bastos JPC, de Bruin GS, Mota RMS, de Bruin PFC. Risk factors for pulmonary complications after emergency abdominal surgery. Respiratory medicine. 2007;101(4):808–13. doi: 10.1016/j.rmed.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SE, Robinson G, Williams RA, Stabile BE, Cone L, Sarfeh IJ, Miller DR, Passaro E., Jr. Acquired immune deficiency syndrome (AIDS). Indications for abdominal surgery, pathology, and outcome. Annals of surgery. 1989;210(4):428–33. doi: 10.1097/00000658-198910000-00002. discussion 33-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson T, Allen-Mersh TG, Miles AJ, Gazzard B, Wastell C, Vipond M, Stotter A, Miller RF, Fieldman NR, Slack WW. Emergency laparotomy in patients with AIDS. The British journal of surgery. 1991;78(8):924–6. doi: 10.1002/bjs.1800780809. [DOI] [PubMed] [Google Scholar]
- 15.Albaran RG, Webber J, Steffes CP. CD4 cell counts as a prognostic factor of major abdominal surgery in patients infected with the human immunodeficiency virus. Archives of Surgery. 1998;133(6):626–31. doi: 10.1001/archsurg.133.6.626. [DOI] [PubMed] [Google Scholar]
- 16.Causey MW, Stoddard D, Johnson EK, Maykel JA, Martin MJ, Rivadeneira D, Steele SR. Laparoscopy impacts outcomes favorably following colectomy for ulcerative colitis: a critical analysis of the ACS-NSQIP database. Surgical endoscopy. 2013;27(2):603–9. doi: 10.1007/s00464-012-2498-7. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. Journal of the American College of Surgeons. 2013;217(5):833–42. e1–3. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. Journal of the American College of Surgeons. 2009;208(6):1009–16. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]