Abstract
Importance
Data regarding the contribution of red blood cell (RBC) transfusion and anemia to necrotizing enterocolitis (NEC) are conflicting. These associations have not been prospectively evaluated, accounting for repeated, time-varying exposures.
Objective
To determine the relationship between RBC transfusion, severe anemia, and NEC.
Design, Setting, and Participants
In a secondary, prospective, multicenter observational cohort study from January 2010 to February 2014, very low-birth-weight (VLBW, ≤1500 g) infants, within 5 days of birth, were enrolled at 3 level III neonatal intensive care units in Atlanta, Georgia. Two hospitals were academically affiliated and 1 was a community hospital. Infants received follow-up until 90 days, hospital discharge, transfer to a non–study-affiliated hospital, or death (whichever came first). Multivariable competing-risks Cox regression was used, including adjustment for birth weight, center, breastfeeding, illness severity, and duration of initial antibiotic treatment, to evaluate the association between RBC transfusion, severe anemia, and NEC.
Exposures
The primary exposure was RBC transfusion. The secondary exposure was severe anemia, defined a priori as a hemoglobin level of 8 g/dL or less. Both exposures were evaluated as time-varying covariates at weekly intervals.
Main Outcomes and Measures
Necrotizing enterocolitis, defined as Bell stage 2 or greater by preplanned adjudication. Mortality was evaluated as a competing risk.
Results
Of 600 VLBW infants enrolled, 598 were evaluated. Forty-four (7.4%) infants developed NEC. Thirty-two (5.4%) infants died (all cause). Fifty-three percent of infants (319) received a total of 1430 RBC transfusion exposures. The unadjusted cumulative incidence of NEC at week 8 among RBC transfusion-exposed infants was 9.9% (95% CI, 6.9%-14.2%) vs 4.6% (95% CI, 2.6%-8.0%) among those who were unexposed. In multivariable analysis, RBC transfusion in a given week was not significantly related to the rate of NEC (adjusted cause-specific hazard ratio, 0.44 [95% CI, 0.17-1.12]; P = .09). Based on evaluation of 4565 longitudinal measurements of hemoglobin (median, 7 per infant), the rate of NEC was significantly increased among VLBW infants with severe anemia in a given week compared with those who did not have severe anemia (adjusted cause-specific hazard ratio, 5.99 [95% CI, 2.00-18.0]; P = .001).
Conclusions and Relevance
Among VLBW infants, severe anemia, but not RBC transfusion, was associated with an increased risk of NEC. Further studies are needed to evaluate whether preventing severe anemia is more important than minimizing RBC transfusion.
Necrotizing enterocolitis (NEC) is a leading cause of mortality among preterm infants with case-fatality rates of 20% to 30%.1-3 The pathogenesis of NEC remains unclear with conflicting data regarding the role of 2 risk factors, red blood cell (RBC) transfusion and anemia, on the development of NEC. A meta-analysis of retrospective observational studies demonstrated an association between exposure to RBC transfusion and NEC, with receipt of RBC transfusion being associated with an increased risk of NEC (adjusted odds ratio, 2.0 [95% CI, 1.6-2.5]).4 However, more recent observational studies have found no association between RBC transfusion and NEC5,6 or have found RBC transfusion to be protective.7,8 In addition, a meta-analysis of randomized trials reported no difference in the incidence of NEC between infants receiving conservative vs liberal RBC transfusion approaches (pooled odds ratio, 1.67 [95% random-effects CI, 0.82-3.38]).9
Improving understanding of the role of RBC transfusion and anemia in the development of NEC is important because more than half of very low-birth-weight (VLBW, ≤1500 g) infants receive 1 or more transfusions during hospitalization.10 Some prior studies characterizing the associations between transfusion, anemia, and NEC were potentially limited by small sample size, case-control design, or lack of evaluation of time-varying exposures. As such, researchers have underscored the need for prospective study in which each RBC exposure, episode of anemia, and outcome of NEC can be systematically and consistently evaluated.4
This prospective study examined whether RBC transfusion and severe anemia were associated with the rate of NEC. The primary objective was to test whether NEC was increased in VLBW infants receiving RBC transfusion compared with non transfused VLBW infants. Further, exposure to severe neonatal anemia was examined as an independent risk factor for NEC.
Methods
Setting and Study Population
We performed a secondary analysis of a prospective, multi-center observational birth-cohort study investigating the transfusion-transmission of cytomegalovirus in preterm infants (TT-CMV study). The study design and results of the TT-CMV study have been previously published.11,12 Parents or guardians provided written informed consent before enrollment of their VLBW infant into the TT-CMV study. All enrolled VLBW infants received follow-up from birth to 90 days, hospital discharge, transfer to a non-study–affiliated hospital, or death.
All VLBW infants born at 3 level III neonatal intensive care units (NICUs) in Atlanta, Georgia, were assessed for eligibility. Two NICUs were academically affiliated (Grady Memorial Hospital and Emory University Hospital Midtown) and part of a single regional perinatal center. The other NICU was private and not academically affiliated (Northside Hospital). No standard transfusion practice guidelines were in place at the 3 institutions, allowing for evaluation of varying transfusion practices with differing tolerance of neonatal anemia, all within the standards of care. Inclusion criteria were VLBW (≤1500 g) and postnatal age of 5 days or less. Exclusion criteria were (1) infant not expected to survive beyond 7 days of life based on the assessment by the treating neonatologist; (2) severe congenital abnormality; (3) receipt of transfusion before enrollment; or (4) maternal decision not to participate. This study was approved by the institutional review board and/or research oversight committees at all participating centers.
Definitions
All patient, laboratory, and transfusion variables were collected in standardized case report forms using DataFax (Clinical DataFax Systems). The primary outcome was NEC, defined as Bell stage 2 or greater according to established criteria.13 The primary outcome was ascertained by active surveillance and systematic outcome assessment for all enrolled VLBW infants. The Bell staging of all cases of NEC was adjudicated by an independent, board-certified neonatologist with extensive expertise in the clinical management of NEC by review of clinical notes and abdominal radiographs (actual images and reports by pediatric radiologists) to minimize ascertainment bias. This person was not directly involved with the study. The primary defined epidemiological exposure was RBC transfusion. All RBC transfusion exposures were recorded by research nurses in case report forms. For patients who developed NEC, only transfusion exposures that occurred before the onset of clinical symptoms consistent with NEC were analyzed. In particular, timing of exposures and temporality between exposure and outcome were verified on the day of NEC onset through additional review of medical records to ascertain, with as much accuracy as possible, the sequence of the onset of clinical symptoms of NEC (eg, emesis, abdominal distention, bloody stools) and RBC transfusion.
The prespecified secondary exposure of interest was severe anemia, defined as a hemoglobin level of 8 g/dL or less. The cut point of 8 g/dL for severe anemia was determined by taking into consideration the lower thresholds of anemia tolerated in 2 randomized clinical trials comparing transfusion practices in preterm infants.14,15 We performed additional analysis to evaluate the sensitivity of the findings using severity of anemia specified as a continuous, instead of categorized, variable. Hemoglobin values, including the date of testing, were recorded at 8 scheduled assessments (birth, 3, 7, 14, 21, 40, 60, and 90 days or hospital discharge), with assessment windows becoming wider over time.
Baseline and clinical characteristics were reported for both exposure (RBC transfused and nontransfused) and outcome (NEC and non-NEC) groups. Baseline illness severity was determined using the Score for Neonatal Acute Physiology (SNAP), a validated measure of neonatal illness severity.16 Race and ethnicity, which are potential predictors of mortality, were determined by maternal report from options defined by federally funded study guidelines.
Missing Data
Missing data were minimized through regular quality assurance and quality control reports by the TT-CMV data coordinating center to ensure all key variables were collected for enrolled participants. If hemoglobin level was not measured in a given week, the prior value was carried forward and imputed.17
Sample Size and Power Calculations
A sample size estimation was performed in August of 2011 based on an interim analysis of infants completing the TT-CMV study, which reported 13 (7.5%) of 173 enrolled VLBW infants had been diagnosed with NEC. Assuming a NEC incidence of 7%, a final sample size of 535 enrolled VLBW infants was calculated to achieve 80% statistical power to detect a hazard ratio (HR) for NEC of approximately 2.5 for transfused VLBW infants compared with non-transfused VLBW infants (assuming proportional hazards), at a significance level of .05. Additional enrollment was continued until funds were exhausted from the TT-CMV study to allow retention of power after accounting for adjustment of additional covariates.
Statistical Analysis
The cumulative incidence of NEC and death was estimated using the cumulative incidence function for NEC and death.18 CIs were estimated using bootstrapping (1000 bootstrap samples), with the infant's mother as the clustering unit to account for multiple births. The Gray method (modified log-rank test) was used to compare NEC cumulative incidence according to baseline clinical characteristics.19 A competing risk analysis was performed to estimate the cause-specific HR and the subdistribution HR (SHR) for NEC and mortality using a Cox proportional-hazards regression model implemented with SAS PROC PHreg (version 9.4) using robust sandwich variance estimates to account for within-mother correlation. Time-dependent effects of RBC transfusion and severe anemia were used to investigate the temporal relationships between these covariates and the rate of NEC.
Because of the limited number of infants with NEC diagnosis and concern with model overfitting, covariates included in multivariable analyses were limited to main effects for baseline measures and time-dependent covariates for longitudinal measures. Tests for interactions between baseline covariates were not implemented. Covariate selection was driven by available knowledge and biological plausibility of potential confounders, taking into consideration the hypothesis of interest (eFigure 1 in the Supplement) and the reliability of the covariates as predictors of the outcome evaluated, using 1000 bootstrap samples20 (eMethods in the Supplement). Models were adjusted for birth weight, center, baseline illness severity, and duration of breastfeeding and antibiotic treatment (both evaluated in the first 10 days after birth). The addition of gestational age, in addition to birthweight, did not improve estimation or change point estimates for the 2 exposures of interest and was not included in the final model. Correlation between parameters in the Cox regression model was evaluated using the estimated correlation matrix of parameter estimates. The HRs and related 95% CIs were calculated for each risk factor in the presence of others in the final model.
Additional analyses evaluated the sensitivity of estimates of association in the primary model (model 1), with evaluation of possible model overfitting by specifying a parsimonious model limited to 4 covariates (model 2), additionally controlling for severity of early respiratory illness (model 3), and controlling for confounding by indication for RBC transfusion through the use of propensity scoring with covariate adjustment using the propensity score (model 4), and by inverse probability of treatment weighting (model 5; eMethods in the Supplement). The 8 independent variables for the propensity score model included the following baseline covariates potentially associated with RBC transfusion exposure, study outcomes, or both21: mechanical ventilation on the day of birth, receipt of 1 or more doses of antenatal steroids, birth weight, gestational age, SNAP, Apgar score at 5 minutes, hemoglobin value at birth, and clinical center. Standardized differences were used to assess the balance of baseline confounders between RBC transfusion exposed and unexposed patients after propensity scoring (eMethods in the Supplement). Additional analyses, limited only to patients exposed to RBC transfusion, were performed to evaluate the relationship between NEC and 2 specific donor RBC characteristics: storage age of transfused RBCs and the duration of RBC storage after γ-irradiation. Statistical significance was defined as a 2-sided P value of less than .05.
Results
Baseline Characteristics
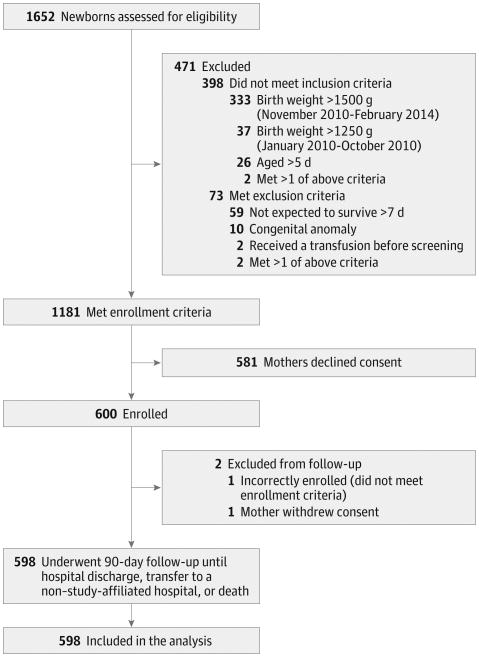
From January 2010 to February 2014, a total of 1652 infants were screened, of whom 600 VLBW infants met study criteria and were enrolled (Figure 1). Ninety-nine percent (598/600) of enrolled infants were included in the final analysis. The mean (SD) gestational age of the cohort was 27.9 (3.3) weeks and birthweight was 1015 g (273 g) (Table 1). Among the full cohort of 598 infants, 296 (49.5%) were female, 346 (57.9%) were black, 138 (23.1%) were small for gestational age, and 520 (87.0%) received at least some breast milk during the study. When comparing baseline characteristics by exposure to RBC transfusion, infants who received at least 1 RBC transfusion had a lower birthweight (864 g vs 1189 g) and gestational age (26.4 vs 29.5 weeks) compared with non–RBC-transfused infants (eTable 1 in the Supplement). In addition, infants who received RBC transfusions had a higher SNAP on the day of birth (12.4 vs 8.1) and more commonly received mechanical ventilation in the first week of life (72.1% vs 26.5%, eTable 2 in the Supplement) compared with those who did not receive RBC transfusions.
Figure 1. Flow of Participation for Very Low-Birth-Weight Infants With and Without Necrotizing Enterocolitis.
Of the 598 participants included in the analysis, 532 received follow-up until the end of the study (90 days or until hospital discharge) and 63 until the outcome event was reached (44 infants with necrotizing enterocolitis and 19 deaths among infants without necrotizing enterocolitis). Three (0.5%) participants were transferred to a non-study–affiliated hospital (1 after 45, 1 after 52, and 1 after 75 days in the study). The weight inclusion criterion threshold was changed from 1250 g to 1500 g in November 2010 to increase enrollment.
Table 1. Baseline and Clinical Characteristics for Birth Cohort (N = 598)a.
| Baseline Characteristics | No. (%) |
|---|---|
| Gestational age, mean (SD), wk | 27.9 (3.3) |
| Birth weight, mean (SD), g | 1015 (273) |
| Male sex | 302 (50.5) |
| Race | |
| Black | 346 (57.9) |
| White | 200 (33.4) |
| Asian | 25 (4.2) |
| More than 1 race | 23 (3.9) |
| Otherb | 4 (0.7) |
| Hispanic ethnicity | 50 (8.4) |
| Singleton birth | 411 (68.7) |
| Small for gestational agec | 138 (23.1) |
| Born outside study hospital | 7 (1.2) |
| 5-Minute Apgar score, median (IQR)d | 8 (7-9) |
| Score for Neonatal Acute Physiology, mean (SD)e | 10.4 (5.4) |
| Rupture of membranes >18 hours | 124 (20.7) |
| Chorioamnionitis, clinical or histological | 88 (14.7) |
| Cesarean delivery | 463 (77.4) |
| Received ≥1 dose of antenatal steroids | 501 (83.8) |
| Hemoglobin on day of birth, mean (SD), g/dL | 15.1 (2.6) |
| Highest level of respiratory support on day of birth | |
| Continuous positive airway pressure | 269 (45.0) |
| Mechanical ventilation | 263 (44.0) |
| None of the above | 66 (11.0) |
| Clinical characteristicsf | |
| Received any antibiotics in first 10 days of life, d | 551 (92.1) |
| Ever fed breast milk | 520 (87.0) |
| Mechanical ventilation in first week of life | 304 (50.8) |
| Received surfactant therapy in first week of life | 298 (49.8) |
| Patent ductus arteriosus | 154 (25.8) |
| Intraventricular hemorrhage, ≥grade II | 80 (13.4) |
| Positive blood culture | 62 (10.4) |
| Death | 32 (5.4) |
| Received erythropoiesis-stimulating agents | 2 (0.3) |
| Duration of antibiotics in first 10 days of life, median (IQR), d | 3 (3-8) |
| Age at first feeding, median (IQR), d | 2 (2-4) |
| Storage of transfused RBC, median (IQR), dg | 9 (7-13) |
| Storage after irradiation of RBC, median (IQR), dg | 1 (0-5) |
Abbreviations: IQR, interquartile range; RBC, red blood cell.
Values are reported as No. (%) unless otherwise indicated.
Indicates American Indian, Alaska Native, Native Hawaiian or other Pacific Islander, or other unidentified race.
Birth weight of less than the 10th percentile for gestational age using intrauterine growth curves by Olsen et al.22
The 5-minute Apgar score was unavailable for 2 infants born outside the study hospital.
Scores were measured on the day of birth (range, 0-42); higher scores indicate greater illness severity.
Assessed from birth to 90 days, hospital discharge, transfer to a non–study-affiliated hospital, or death, unless otherwise indicated.
Values indicate maximal duration of storage of transfused RBCs among 319 infants receiving at least 1 RBC transfusion (2 infants had 2 transfusions with missing storage duration after irradiation).
Description of Exposures and Outcomes
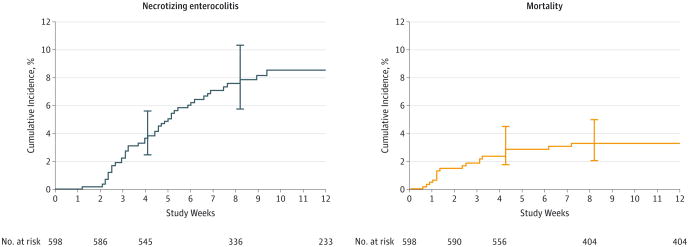
A total of 48 infants developed NEC and 44 infants were diagnosed with a Bell stage of 2 or greater after adjudication (eTable 3 in the Supplement). The overall cumulative incidence of NEC at 8 weeks was 7.7% (95% CI, 5.7%-10.3%), and the cumulative incidence of mortality was 3.3% (95% CI, 2.0%-5.0%) (Figure 2, eTable 4 in the Supplement). A total of 1430 RBC transfusions and 4565 hemoglobin measurements were recorded over the 12-week study period. Fifty-three percent (319/598) of infants received 1 or more RBC transfusions. Seventy-five percent (33/44) of infants with NEC received an RBC transfusion before diagnosis and 15.9% (7/44) received an RBC transfusion within 48 hours of diagnosis (eFigure 2 in the Supplement). The overall RBC transfusion rate was 1.20 (95% CI, 1.14-1.27) per 30 infant-days, although this varied by center (eTable 5 in the Supplement). Eighteen percent (109/598) of infants ever had severe anemia (hemoglobin ≤ 8 g/dL).
Figure 2. Cumulative Incidence of Necrotizing Enterocolitis and Mortality as Competing Risks Over Time.
The cumulative incidence and number of infants at risk for necrotizing enterocolitis (Bell stage ≥ 2) and mortality are reported from the time of the last event. Error bars indicate 95% CIs.
Risk Factors for NEC and Mortality
In a bivariable analysis, factors associated with both NEC and mortality included gestational age, birth weight, presence of severe anemia in a given week, and the number of days of antibiotic treatment in the first 10 days of life (Table 2). The unadjusted cumulative incidence of NEC at week 8 in RBC-exposed infants was 9.9% (95% CI, 6.9%-14.2%) vs 4.6% (95% CI, 2.6%-8.0%) among infants who were not exposed to RBC transfusions. The rate of NEC was increased in VLBW infants who received RBC transfusions compared with infants who did not (cause-specific HR, 2.33 [95% CI, 1.18-4.60]; P = .01). In addition, increases in the SNAP on the day of birth were associated with an increased risk of mortality, while more days of breastfeeding in the first 10 postnatal days were associated with decreased mortality.
Table 2. Risk Factors for Necrotizing Enterocolitis and Mortality Using Bivariable Competing-Risks Cox Regression Model (N = 598)a.
| Necrotizing Enterocolitis |
Mortality | |||
|---|---|---|---|---|
| Cause-Specific HR (95% CI)b |
P
Value |
Cause-Specific HR (95% CI)b |
P Value |
|
| Necrotizing Enterocolitis Risk Factors | ||||
| Gestational age, per 1-week increase | 0.82 (0.72-0.94) | .004 | 0.56 (0.44-0.72) | <.001 |
| Birth weight, per 100-g increase | 0.75 (0.66-0.85) | <.001 | 0.52 (0.42-0.64) | <.001 |
| Race, white (relative to black) | 0.61 (0.30-1.21) | .15 | 1.10 (0.43-2.83) | .85 |
| Race, other (relative to black) | 0.40 (0.10-1.62) | .21 | 0.59 (0.08-4.56) | .61 |
| 5-Minute Apgar, per 1-point decreasec | 1.16 (1.02-1.31) | .03 | 1.29 (1.10-1.52) | .002 |
| SNAP, per 1-point increase | 1.04 (0.98-1.09) | .19 | 1.22 (1.13-1.32) | <.001 |
| Hemoglobin at birth, per 1-g/dL decrease | 1.08 (0.98-1.20) | .12 | 1.30 (1.07,1.57) | .007 |
| Ever with severe anemia (hemoglobin ≤8 g/dL) | 0.93 (0.44-1.96) | .85 | 2.58 (1.03-6.44) | .04 |
| Severe anemiad | 1.92 (0.89-4.13) | .10 | 10.8 (3.95-29.5) | <.001 |
| Severe anemia in a given weeke | 4.13 (1.61-10.6) | .003 | 7.29 (2.01-26.5) | .003 |
| Nadir hemoglobin measure in a given weeke, per 1-g/dL decrease | 1.29 (1.05-1.58) | .02 | 1.69 (1.28-2.24) | <.001 |
| Ever received RBC transfusion | 2.33 (1.18-4.60) | .01 | f | |
| Received RBC transfusiond | 2.82 (1.44-5.53) | .003 | f | |
| Received RBC transfusion in a given weeke | 1.60 (0.78-3.27) | .20 | 8.81 (2.96-26.2) | <.001 |
| No. of RBC transfusions in a given weeke | 1.18 (0.85-1.64) | .33 | 1.98 (1.72-2.29) | <.001 |
| Cumulative No. of RBC transfusionse | 1.11 (1.05-1.18) | <.001 | 1.39 (1.25-1.54) | <.001 |
| Days of breast milk feeding in first 10 days, per 1-day increase | 0.98 (0.92-1.05) | .54 | 0.81 (0.73-0.91) | <.001 |
| Days of antibiotic treatment in first 10 days, per 1-day increase | 1.12 (1.02-1.22) | .01 | 1.22 (1.07-1.39) | .003 |
| Age started enteral feeding, per 1-day increase | 1.02 (0.98-1.07) | .31 | 0.97 (0.88-1.07) | .50 |
| Additional Mortality Risk Factors | ||||
| Male sex | 0.81 (0.45-1.46) | .48 | 0.87 (0.36-2.14) | .77 |
| Multiple birth | 0.60 (0.30-1.21) | .16 | 0.56 (0.19-1.68) | .30 |
| Received ≥1 dose of antenatal steroids | 0.56 (0.28-1.09) | .09 | 0.71 (0.24-2.14) | .54 |
| Received surfactant therapy in first week of life | 1.91 (1.02-3.63) | .05 | 2.76 (1.00-7.62) | .05 |
| Mechanical ventilation in first week of life | 1.25 (0.68-2.31) | .47 | 8.13 (1.89-35.0) | .005 |
Abbreviations: HR, hazard ratio; RBC, red blood cell; SNAP, Score for Neonatal Acute Physiology.
Competing risks were 44 infants with necrotizing enterocolitis and 32 deaths total; 13 infants with necrotizing enterocolitis died. A total of 1430 RBC transfusions and 4565 hemoglobin measurements over 12 weeks were evaluated (post-necrotizing enterocolitis measurements were excluded).
To estimate cause-specific HR for necrotizing enterocolitis, 44 events were used, and 19 deaths were used to estimate cause-specific HR for mortality.
The 5-minute Apgar score was unavailable for 2 infants born outside the study hospital.
Time-dependent covariate defined as a dichotomous variable that can change, at most, once from unexposed to exposed over 12 weeks of follow-up (ie, once exposed, always exposed).
Time-dependent covariate. Values for some infants may change from a given week to the subsequent week (eg, not severely anemic to severely anemic). For cumulative number of transfusions, the total number in a given week plus the number of prior transfusions was used. For nadir hemoglobin level, the lowest measure in a given week was used. If no hemoglobin measurement was obtained, the previous week's hemoglobin value was used (last observation carried forward).
All observed deaths occurred in the transfusion group; therefore, hazard could not be estimated.
In multivariable analysis, including adjustment for birth weight, duration of breastfeeding, illness severity, severity of anemia, duration of antibiotic treatment, and center, any RBC transfusion in a given week was not independently associated with an increased rate of NEC (cause-specific HR, 0.44 [95% CI, 0.17-1.12]; P = .09) or mortality (cause-specific HR, 1.36 [95% CI, 0.27-6.82]; P = .71). In a given week, VLBW infants with severe anemia had a higher estimated rate of NEC compared with VLBW infants without severe anemia (adjusted cause-specific HR, 5.99 [95% CI, 2.00-18.0]; P = .001). Similar results were found when controlling for the severity of early respiratory illness (model 3) and the propensity for RBC transfusion (models 4 and 5; Table 3). Severe anemia and birth weight were independent reliable predictors of NEC because they appeared as significant risk factors in more than 50% of 1000 bootstrap models.20 Given the presence of some correlation between RBC transfusion and severe anemia (coefficient of −0.43), we analyzed reduced multivariable models without 1 of the 2 correlated exposures and found similar HR estimates for both RBC transfusion and severe anemia as models with both variables included (eTable 6 in the Supplement). However, the reduced model provided better precision as indicated by the reduced width of the 95% CI.
Table 3. Risk Factors for NEC and Mortality Using Multivariable Competing-Risks Cox Regression Modela.
| Risk Factors | NEC | Mortality | ||||
|---|---|---|---|---|---|---|
| Cause-Specific HR (95% CI)b | P Value | % Reliabilityc | Cause-Specific HR (95% CI)b | P Value | % Reliabilityc | |
| Model 1—Primary Analysis (N = 598)d | ||||||
| Birth weight, per 100-g increase | 0.72 (0.62-0.84) | <.001 | 98 | 0.63 (0.48-0.82) | <.001 | 99 |
| Received RBC transfusion in a given weeke | 0.44 (0.17-1.12) | .09 | 45 | 1.36 (0.27-6.82) | .71 | 19 |
| Severe anemia in a given week (hemoglobin ≤8 g/dL)e | 5.99 (2.00-18.0) | .001 | 70 | 1.66 (0.40-6.85) | .48 | 25 |
| Days of breast milk feeding in first 10 days of life, per 1-day increase | 1.10 (1.01-1.21) | .04 | 37 | 0.87 (0.77-0.98) | .02 | 47 |
| SNAP on day of birth, per 1-point increase | 1.00 (0.93-1.07) | .99 | 8 | 1.12 (1.03-1.23) | .01 | 59 |
| Days of antibiotic treatment in first 10 days of life, per 1-day increase | 1.04 (0.93-1.16) | .50 | 8 | 1.01 (0.83-1.21) | .96 | 9 |
| Model 2—Study Exposures + Confounders With Reliability ≥50% (N = 598)d | ||||||
| Birth weight, per 100-g increase | 0.72 (0.62-0.84) | 99 | 0.57 (0.44-0.75) | 99 | ||
| Received RBC transfusion in a given week | 0.43 (0.18-1.04) | 47 | 2.02 (0.45-8.97) | 25 | ||
| Severe anemia in a given week | 5.49 (1.81-16.6) | 69 | 2.25 (0.52-9.79) | 35 | ||
| Model 3—Model 2 + Adjustment for Early Respiratory Illness Severity (N = 598)d | ||||||
| Birth weight, per 100-g increase | 0.72 (0.61-0.83) | 99 | 0.57 (0.43-0.75) | 100 | ||
| Received RBC transfusion in a given week | 0.45 (0.18-1.14) | 44 | 1.72 (0.30-9.72) | 26 | ||
| Severe anemia in a given week | 5.48 (1.78-16.8) | 68 | 2.36 (0.53-10.4) | 35 | ||
| Received surfactant therapy in first week of life | 1.95 (0.86-4.30) | 11 | 0.46 (0.16-1.38) | 14 | ||
| Mechanical ventilation in first week of life | 0.45 (0.22-0.94) | 28 | 3.23 (0.58-18.0) | 14 | ||
| Model 4—Propensity Score Adjusted With Propensity Score Specified as a Continuous Covariate (n = 596)f | ||||||
| Received RBC transfusion in a given week | 0.57 (0.23-1.40) | 25 | 1.92 (0.40-9.26) | 49 | ||
| Severe anemia in a given week | 4.52 (1.51-13.6) | 64 | 2.95 (0.77-11.2) | 40 | ||
| Model 5—Propensity Score Adjusted With Inverse Probability of Treatment Weighting (n = 596)f | ||||||
| Birth weight, per 100-g increase | 0.69 (0.62-0.77) | 100 | 0.55 (0.43-0.72) | 100 | ||
| Received RBC transfusion in a given week | 0.37 (0.11-1.16) | 60 | 3.35 (0.74-15.1) | 45 | ||
| Severe anemia in a given week | 3.42 (1.05-11.1) | 52 | 1.78 (0.41-7.78) | 23 | ||
Abbreviations: HR, hazard ratio; NEC, necrotizing enterocolitis; RBC, red blood cell; SNAP, Score for Neonatal Acute Physiology.
Competing risks were 44 infants with NEC and 32 deaths total; 13 infants with NEC died. All models include adjustment for center (not shown).
To estimate cause-specific HR for NEC, 44 events were used, and 19 deaths were used to estimate cause-specific HR for mortality.
Percentage of time risk factor appears in 1000 bootstrap models. Risk factors appearing in at least 50% of models are reliable.
Models 1, 2, and 3 include center as a covariate.
All models specify RBC transfusion and severe anemia as time-dependent covariates evaluated in 1-week intervals.
The following covariates were used to model the propensity score: mechanical ventilation on the day of birth, receipt of at least 1 dose of antenatal steroids, birth weight, gestational age, SNAP, 5-minute Apgar score, hemoglobin level at birth, and clinical center. Models exclude 2 infants due to missing data on 5-minute Apgar score (additional information on propensity score modeling reported in eMethods, eTables 11-14, and eFigures 3-4 in the Supplement). Models 4 and 5 include center in the propensity score model estimating probability of RBC exposure.
To estimate the hazard of NEC among a more homogenous population of infants, an analysis limited to a subset of VLBW infants who received at least 1 RBC transfusion (n = 319) was performed. In this analysis, 2 donor RBC storage characteristics were also evaluated. Overall, median storage age of transfused RBCs was 9 days (interquartile range, 7-13 days) and median duration of storage after gamma irradiation was 1 day (interquartile range, 0-5 days). No association was detected between NEC and the maximum storage age of RBCs transfused in a given week nor the maximum duration of storage after γ-irradiation (eTable 7 in the Supplement). In multivariable analysis, RBC transfusion was not associated with the rate of NEC, but severe anemia remained an independent risk factor for NEC (eTable 8 in the Supplement).
The sensitivity of the use of a hemoglobin cut point of 8 g/dL to categorize severe anemia was assessed by specifying the degree of anemia as a continuous variable (lowest nadir of hemoglobin). For each 1-g/dL decrease in nadir hemoglobin in any given week, the HR for NEC increased by 65% (cause-specific HR, 1.65 [95% CI, 1.23-2.12]; P < .001) with reliability of 92% (eTable 9 in the Supplement). Longitudinal modeling of hemoglobin values, adjusted for birth weight, demonstrated that hemoglobin levels among infants who developed NEC and those in non-NEC infants who received at least 1 RBC transfusion declined similarly during 90 days of follow-up (test for interaction between time on study and group, P = .25). No difference was detected in baseline mean hemoglobin level (mean difference at birth, 0.52 g/dL [95% CI, −0.02 to 1.05]; P = .06) or lower mean hemoglobin by 49 to 90 days of age between NEC and non–NEC-transfused infants (mean difference, −1.50 g/dL [95% CI, −3.05 to 0.04]; P = .06) (eTable 10 in the Supplement).
Discussion
In this secondary, prospective, multicenter study, RBC transfusion was not an independent risk factor for NEC. These findings are in contrast to the published systematic review and meta-analysis of 12 retrospective studies that demonstrated an association between RBC transfusion and NEC4 but a reconsistent with those of Sharma et al,6 which indicated that RBC transfusion was not associated with the cumulative incidence of NEC. By evaluating each RBC transfusion exposure during hospitalization using time-varying covariates, the results of this study may be less subject to the biases of prior studies in which each infant was categorized as exposed or unexposed to any RBC transfusion or in which temporality between exposure and outcome was not evaluated. The prospective design helped ensure the accuracy and completeness of the longitudinal data.
In addition, the results suggest that severe anemia may be an important potential risk factor for NEC. Similarly, Singh et al23 demonstrated that each percentage-point decrease in the lowest measured hematocrit level was associated with an increased risk of NEC in VLBW infants (odds ratio, 1.10 [95% CI, 1.02 to 1.18]). DeRienzo et al24 found the severity of pretransfusion anemia to be associated with NEC and suggested a possible interaction between transfusion and anemia on the outcome of NEC. The results of this study raise potential concern for the safety of conservative transfusion practices with transfusion thresholds that tolerate severe anemia, particularly since transfusion practices based on hemoglobin thresholds may not adequately factor in an individual patient's clinical status and illness severity.25 Taken in the context of recent observational studies identifying associations between anemia and NEC,23,24 our findings indicate that anemia, rather than RBC transfusion, may be a key risk factor for NEC.
Ascertaining temporality between exposure and outcome was important in determining the relationship between RBC transfusion and NEC because many infants received RBC transfusion on the day of diagnosis of NEC but after onset of clinical symptoms. It is possible that prior studies identifying an association between RBC transfusion and NEC may have been subjected to the effects of reverse causation, whereby clinical instability from evolving NEC leads a clinician to administer a RBC transfusion before the formal diagnosis of NEC is established.
An association between breastfeeding in the first 10 days after birth and a higher risk of NEC was found, which is in contrast to the published literature.26 The lack of a protective effect of breastmilk in this study may be due to the high incidence of breastfeeding (ie, small number of unexposed), the assessment limited to the first 10 days of age, or the favorable association of breastfeeding on a lower risk of the competing outcome of death.
The strengths of the study include the prospective and systematic collection of data. Evaluation of time-varying exposures allowed use of data from each of the numerous episodes of RBC transfusion and severe anemia that a VLBW infant may experience during hospitalization. Further, each transfusion exposure was systematically evaluated, donor RBC characteristics were identified, and the temporal relationship between exposure and outcome was determined. Only definite NEC cases (Bell stages 2-3) were included, determined by active surveillance by research nurses with adjudication of staging by a neonatologist not directly involved with the study to minimize ascertainment bias. Detection bias of NEC was minimized by implementing follow-up of all enrolled infants for the primary outcome. Attrition of infants from the study was minimal.
The study has several limitations. The observational design prohibits the establishment of causality between exposures to both RBC transfusion and anemia and the outcome of NEC. Reverse causation is possible for cases in which sub-clinical NEC may lead to severe anemia before clinical signs of NEC are obvious. However, no infants with severe anemia included in the analysis were diagnosed with bloody stool at the time of laboratory assessment of anemia, making this source of acute anemia in infants with NEC less likely. In addition, all potential confounders could not be accounted for, and residual confounding of the risk estimates by unmeasured variables is possible. The external validity of the study may be limited to centers with differing transfusion practices, including those that commonly use erythropoiesis-stimulating agents.
Because severe anemia, but not RBC transfusion, was a risk factor for NEC in this study, preventing severe anemia may be more clinically important than minimizing RBC transfusion exposure as a strategy to decrease the risk of NEC. However, the effect of such a strategy on other important neonatal outcomes is unclear, and further study is needed. Ongoing clinical trials comparing liberal vs conservative transfusion practices may provide additional experimental data regarding the risks of both severe anemia and RBC transfusion to NEC.
Conclusions
Among VLBW infants, severe anemia but not RBC transfusion was associated with an increased risk of NEC. Further studies are needed to evaluate whether preventing severe anemia is more important than minimizing RBC transfusion.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health. The National Heart, Lung, and Blood Institute provided funding for the parent study under award number P01 HL086773. The National Center for Advancing Translational Sciences provided support for this study and for Dr Patel under award numbers UL1 TR000454 and KL2 TR000455. Dr Patel reports receipt of research support from the Emory Children's Center for Clinical and Translational Research.
Role of the Sponsor/Funder: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Patel and Mr Easley had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Patel, Roback, Easley, Josephson.
Acquisition, analysis, or interpretation of data: Patel, Knezevic, Shenvi, Hinkes, Keene, Roback, Easley, Josephson.
Drafting of the manuscript: Patel, Shenvi, Roback, Easley, Josephson.
Critical revision of the manuscript for important intellectual content: Knezevic, Shenvi, Hinkes, Keene, Roback, Easley, Josephson.
Statistical analysis: Patel, Knezevic, Shenvi, Roback, Easley, Josephson.
Obtained funding: Patel, Roback, Josephson.
Administrative, technical, or material support: Josephson.
Study supervision: Roback, Easley, Josephson.
Additional Contributions: We would like to thank all of the families who volunteered to participate in this study; the following research nurses (all of whom received salary support for prospective data collection for this study): Jane Skvarich, BSN, MN (Department of Pathology, Emory University School of Medicine, Atlanta, Georgia), Katrina H. Grier, BSN (Northside Hospital, Atlanta, Georgia), and Janna M. Benston, BSN (Northside Hospital, Atlanta, Georgia); and the following individuals for their epidemiological and statistical input (no compensation received): Mitch Klein, PhD (Rollins School of Public Health, Emory University, Atlanta, Georgia), Amita Manatunga, PhD (Rollins School of Public Health, Emory University, Atlanta, Georgia), and Muna Qayed, MD, MSc (Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia).
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
Contributor Information
Ravi M. Patel, Department of Pediatrics, Emory University School of Medicine & Children's Healthcare of Atlanta, Atlanta, Georgia.
Andrea Knezevic, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Atlanta, Georgia.
Neeta Shenvi, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Atlanta, Georgia.
Michael Hinkes, Department of Neonatology, Northside Hospital, Atlanta, Georgia.
Sarah Keene, Department of Pediatrics, Emory University School of Medicine & Children's Healthcare of Atlanta, Atlanta, Georgia.
John D. Roback, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia; Center for Transfusion and Cellular Therapies, Emory University School of Medicine, Atlanta, Georgia.
Kirk A. Easley, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Atlanta, Georgia.
Cassandra D. Josephson, Department of Pediatrics, Emory University School of Medicine & Children's Healthcare of Atlanta, Atlanta, Georgia; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia; Center for Transfusion and Cellular Therapies, Emory University School of Medicine, Atlanta, Georgia.
References
- 1.Patel RM, Kandefer S, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129(3):529–540. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 5.Wallenstein MB, Arain YH, Birnie KL, et al. Red blood cell transfusion is not associated with necrotizing enterocolitis: a review of consecutive transfusions in a tertiary neonatal intensive care unit. J Pediatr. 2014;165(4):678–682. doi: 10.1016/j.jpeds.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Kraemer DF, Torrazza RM, et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J Perinatol. 2014;34(11):858–862. doi: 10.1038/jp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AlFaleh K, Al-Jebreen A, Baqays A, et al. Association of packed red blood cell transfusion and necrotizing enterocolitis in very low birth weight infants. J Neonatal Perinatal Med. 2014;7(3):193–198. doi: 10.3233/NPM-14814048. [DOI] [PubMed] [Google Scholar]
- 8.Sood BG, Rambhatla A, Thomas R, Chen X. Decreased hazard of necrotizing enterocolitis in preterm neonates receiving red cell transfusions. J Matern Fetal Neonatal Med. 2015;29(5):737–744. doi: 10.3109/14767058.2015.1016422. [DOI] [PubMed] [Google Scholar]
- 9.Kirpalani H, Zupancic JA. Do transfusions cause necrotizing enterocolitis? Semin Perinatol. 2012;36(4):269–276. doi: 10.1053/j.semperi.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Strauss RG. Practical issues in neonatal transfusion practice. Am J Clin Pathol. 1997;107(4) suppl 1:S57–S63. [PubMed] [Google Scholar]
- 11.Josephson CD, Castillejo MI, Caliendo AM, et al. Prevention of transfusion-transmitted cytomegalovirus in low-birth weight infants (≤1500g) using cytomegalovirus-seronegative and leukoreduced transfusions. Transfus Med Rev. 2011;25(2):125–132. doi: 10.1016/j.tmrv.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168(11):1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion(PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91(3):617–623. [PubMed] [Google Scholar]
- 17.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 20.Blackstone EH. Breaking down barriers: helpful breakthrough statistical methods you need to understand better. J Thorac Cardiovasc Surg. 2001;122(3):430–439. doi: 10.1067/mtc.2001.117536. [DOI] [PubMed] [Google Scholar]
- 21.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Visintainer PF, Frantz ID, 3rd, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31(3):176–182. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derienzo C, Smith PB, Tanaka D, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90(5):237–240. doi: 10.1016/j.earlhumdev.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein HG, Flegel WA, Natanson C. Red blood cell transfusion: precision vs imprecision medicine. JAMA. 2015;314(15):1557–1558. doi: 10.1001/jama.2015.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramani M, Ambalavanan N. Feeding practices and necrotizing enterocolitis. Clin Perinatol. 2013;40(1):1–10. doi: 10.1016/j.clp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.