Abstract
Adult stem cells across diverse organs self-renew and differentiate to maintain tissue homeostasis. How stem cells receive input to preserve tissue structure and function largely relies on their communication with surrounding cellular and non-cellular elements. As such, how tissues are organized and patterned not only reflects organ function but also inherently hardwires networks of communication between stem cells and their environment to direct tissue homeostasis and injury repair. This review highlights how different methods of stem cell communication reflect the unique organization and function of diverse tissues.
Introduction
Tissues are maintained and restored by stem cells, a specialized population of cells capable of self-renewal and differentiating into cells that comprise functional tissue. Although key molecular pathways that regulate tissue stem cells and their progeny have been identified, comparatively less is known about how constituent cells of a tissue communicate and translate these signals to maintain large-scale organ structure and function. In particular, how are individual cell behaviors and cell fates coordinated to maintain tissue structure and function during homeostasis or to restore them following injury? Ultimately, elucidating the mechanisms by which stem cells communicate with their environment will lay the groundwork for understanding how perturbations to these interactions can promote disease states such as cancer, as well as how these interactions may be re-established after damage.
Classically, tissue stem cells are defined as a distinct population of cells capable of long-term self-renewal and differentiation, allowing them to durably provide specialized cells upon demand. The stem cell niche was first conceptualized and proposed by Schofield to be the cellular environment that anchors stem cells and confers long-term self-renewing capacity not only to undifferentiated stem cells, but also to the progeny that occupy it (Schofield, 1978). Accordingly, studies in at least some mammalian tissues suggest that stem cells are composed of a heterogeneous population of cells that show different transcriptional profiles and self-renewing ability, but are functionally equivalent with respect to their capacity to maintain tissue during homeostasis and restore tissue upon injury (Goodell et al., 2015; Krieger and Simons, 2015; Wabik and Jones, 2015). Therefore for the purpose of this review, we will use this original proposed definition of tissue stem cells to discuss the interaction of these cells with their environment and to highlight work that underlines the central role of communication in regulating stem cell behavior and function. As we learn more about what stem cell interactions look like in vivo, the concept of the niche is also evolving to encompass a more comprehensive understanding of how stem cells communicate through both chemical and physical factors to regulate tissue homeostasis. For instance, niche components are not comprised exclusively of cells, but also include extracellular matrix (ECM) and other non-cellular material (Gattazzo et al., 2014; Wang and Wagers, 2011). Cellular niche components have also expanded to include stem cell daughters as well as other heterologous stem cells, thereby providing additional tissue context that coordinates stem cell fate with the behaviors of their neighbors (Hsu and Fuchs, 2012; Nishimura et al., 2010; Rabbani et al., 2011).
How is stem cell fate and behavior regulated by the niche? Ultimately, the architecture and organization of the tissue in question must frame our understanding, as stem cell behaviors and functions are coordinated with the needs of the tissue at large. In virtually every organ, stem cells respond to input received from their environment, permitting them to meet homeostatic set points and provide tissue stability. Some fundamental features appear to be shared across tissues and species; for instance, how environmental input is relayed to tissue stem cells is thought to be largely a function of the relative position of stem cells within a tissue. However, different tissues and organisms use divergent modes of communication that reflect their unique tissue structures and functions. This review discusses different methods of stem cell communication across diverse tissues and organisms, highlighting the influence of tissue organization and function in constructing robust communication networks.
Stem cell communication networks established by tissue architecture
How stem cells communicate with their environment is a function of their position within a tissue, the architecture of that tissue, and the type of signal transmitted. While the identity and location of stem cells have been largely characterized in adult tissue, providing insight into the niche components that regulate their maintenance and differentiation, the concomitant discovery of markers to identify and track tissue-specific stem cells has also presented an opportunity to examine if and how tissue morphogenesis is coordinated with stem cell specification and organization. Emerging work is now beginning to uncover the origins of adult stem cells and how communication networks are imprinted early on.
Fixed stem cell position in a stable tissue structure
In tissues with stable architectures, polarized stem cell communication networks that are established during development are often maintained in adults, thereby ensuring the asymmetric fate determination of stem cells and their progeny during homeostasis. Although the niche theory was originally proposed for the mammalian hematopoietic system, it was first demonstrated in vivo in invertebrates, with the germ stem cell (GSC) niches of C. elegans and Drosophila (Kimble and White, 1981; Xie and Spradling, 2000). These relatively simple models not only provide a historical reference point for understanding some of the principles governing stem cell regulation and fate, but are useful for investigating different modes of communication between cells.
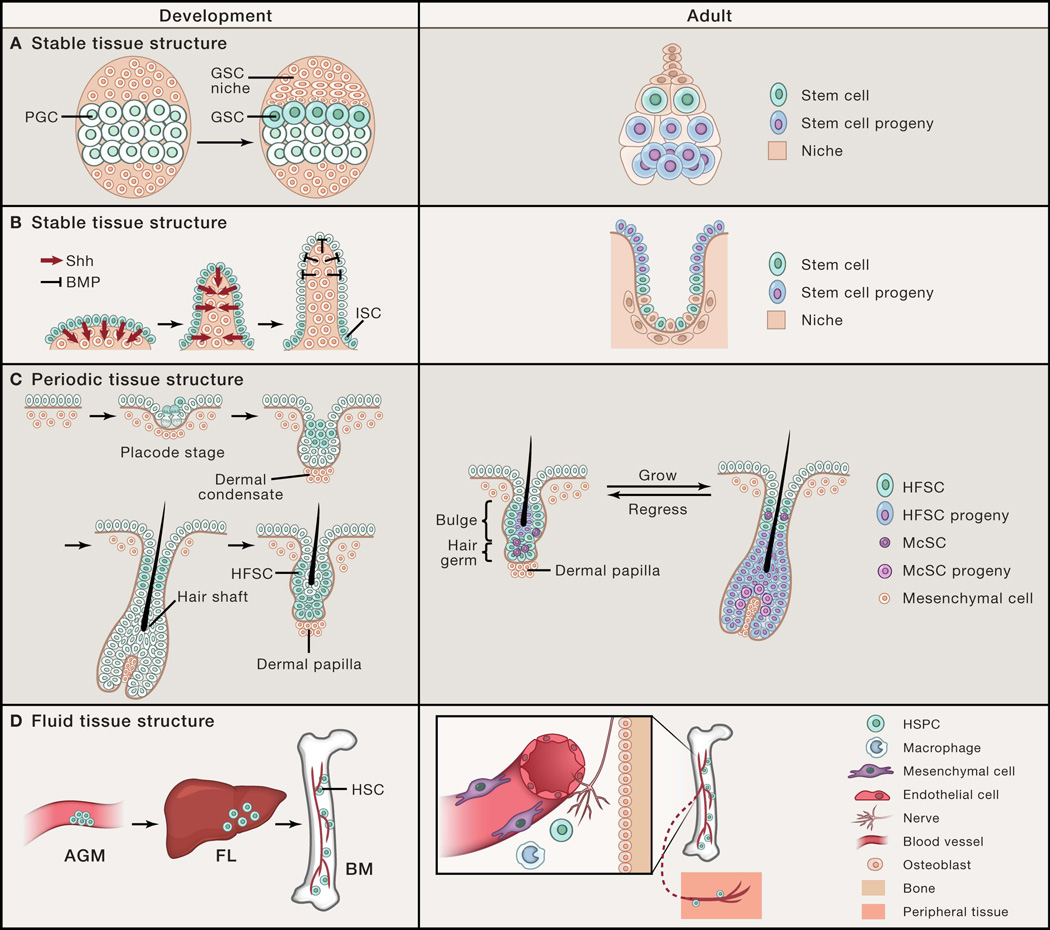
During fly gonad development, stem cells are allocated based on asymmetric position. In the female fly, GSC specification is coordinated with formation of a polarized niche, which is regulated by hormonal signals (Gancz et al., 2011). The larval gonad is formed during embryogenesis by the coalescence of mesodermal cells with GSC progenitors, called primordial germ cells (PGCs) (Dansereau and Lasko, 2008). It is likely that all PGCs initially have the potential to become adult GSCs, as they all show high BMP signaling and can function as adult GSCs when placed into ectopic niches (Gilboa and Lehmann, 2004; Song et al., 2007). However, in late larval gonads, only a subset of these PGCs is selected to become adult GSCs, a process that is directed by sequential steroid hormone ecdysone signaling, which establishes a polarized niche (Figure 1A) (Gancz et al., 2011). Although it is still unclear how niche formation and GSC selection is spatially controlled, stem cell allocation in the fly ovary is niche-dependent.
Figure 1. Stem cell communication networks established by tissue architecture.
(A) Stable polarized stem cell niche structure in the fly ovary directs asymmetric cell fates between stem cells and their progeny. Drosophila female germline stem cells (GSCs) are specified from primordial germ cells (PGCs) that are adjacent to the newly formed GSC niche during ovary development. In the adult, GSCs are maintained by signals provided by the niche, while displacement of the daughter progeny outside of the niche induces their differentiation. Here, stem cell position is polarized relative to their differentiated progeny and the tissue structure remains stable over time. (B) During intestinal development, intestinal stem cells (ISCs) are restricted to the base of the villi by morphogenesis-induced changes in signaling territories. Similar to the fly ovary, ISC position is polarized relative to their differentiated enterocytes that mobilize upward out of the crypt niche base to villus tip. (C) Hair follicle stem cells (HFSCs) are specified during hair follicle morphogenesis by asymmetric cell divisions and displaced suprabasally. In the adult hair follicle, periodic self-renewal and differentiation of HFSCs and melanocyte stem cells (McSCs) are coordinated with the oscillating hair follicle structure. In this example, the stem cell position is also polarized and fixed; however, the tissue structure periodically changes. (D) Definitive hematopoietic stem cells (HSCs) form in the aorta-gonad-mesonephros (AGM) and migrate to the fetal liver (FL) before populating the bone marrow (BM) during embryogenesis. In the adult, hematopoietic stem and progenitor cells (HSPCs) are centralized in the BM niche, but due to their fluid tissue architecture, can migrate into the blood circulation and travel between peripheral tissues. Here, stem cell position is flexible and mobile and dependent upon dynamic niche interactions.
In vertebrates, the development of many epithelial tissues similarly relies upon heterotypic epithelial-mesenchymal communication. One remarkable example of orchestrated morphogenesis and stem cell compartmentalization was recently demonstrated in the developing mouse and chick intestine (Shyer et al., 2015). Prior to intestinal villus formation, stem cell markers such as Lgr5 are expressed uniformly across the gut endoderm. As intestinal villi form through mechanical buckling of the gut endoderm, Sonic hedgehog (Shh)-expressing epithelial cells go from being in a flat sheet that secretes Shh protein uniformly to being curved. This curvature physically concentrates Shh protein at the tips of the villi, inducing formation of the villus cluster, a mesenchymal signaling center that secretes BMP to antagonize Wnt activation in the overlying tip epithelium. This results in restriction of the Lgr5+ territory to the villus base, thereby defining the location of stem cells (Figure 1B). Thus, physical deformation of tissue transforms a uniform planar morphogenetic field into patterned signaling centers, thereby coupling organogenesis and tissue deformation with signal and cellular compartmentalization. How this cellular organization is maintained in adulthood may, in part, reflect what has been engraved into the extrinsic tissue environment during development, as this BMP gradient is stable in the adult. Whether this applies to other developmental contexts is currently unknown, and may be topologically specific to tissues that are bent or folded.
For both fly GSCs and vertebrate intestinal stem cells (ISCs), the polarized structure of the stem cell niche that is built during development is maintained in adults. In adult female fly GSCs, the stem cell niche is located in the anterior tip of each ovariole, the basic unit of the fly ovary (Figure 1A) (Losick et al., 2011).. The anterior somatic cells that comprise the niche maintain GSC fate by sending and restricting bone morphogenetic protein (BMP) signals required for self-renewal to neighboring GSCs (Harris and Ashe, 2011). Meanwhile the posterior displacement of GSC progeny generated by GSC oriented cell divisions, together with directional movement facilitated by escort cells, expels GSC progeny from the niche, leading to the differentiation of the displaced progeny (Morris and Spradling, 2011).
The mammalian ISC niche is similar to the fly GSC microenvironment in that both are stable in location, are polarized with respect to their differentiated progeny, and undergo relatively continuous differentiation during homeostasis. Although the precise identity of ISCs is debatable, they are localized to the lower region of each intestinal crypt, which is the basic germinative unit for intestinal cell regeneration and conceptually resembles the polarized localization of fly GSCs (Figure 1B) (Barker, 2014). Analogous to fly GSC progeny, ISC progeny move upwards along the crypt epithelium and differentiate after leaving the niche at the crypt base (Figure 1B). The mammalian intestine displays polarized signaling in which high Wnt activation occurs in the lower half of the crypt where ISCs reside. Previous studies have demonstrated the critical role of Wnt signaling in maintaining ISC self-renewal, as disruption of the Wnt pathway by deletion of β-catenin or TCF4 blocks proliferation in crypts and leads to ISC loss (Fevr et al., 2007; van Es et al., 2012a). However, it is still not fully understood how Wnt activation in ISCs is regulated. Paneth cells, which are derived from ISCs and interspersed between ISCs within crypts, are considered to be key niche cells that express multiple signals including Wnt3a (Sato et al., 2011). However this view has been challenged by recent Paneth cell ablation studies (Durand et al., 2012). Further, other studies have demonstrated that the pericryptal stroma is another source of Wnt ligands, suggesting that multiple sources of Wnt ligands exist (Kabiri et al., 2014; San Roman et al., 2014). This functional redundancy in niche elements has also been shown in the fly GSC niche, as BMP is also produced by several different somatic niche cells. Yet despite the similarity between mammalian ISC and fly GSC niches, ISCs undergo symmetric division and neutral competition to generate committed progeny which contrasts the asymmetric divisions of fly GSCs, perhaps partly due to an unpolarized microenvironment along the axis of daughter cell displacement (Lopez-Garcia et al., 2010; Snippert et al., 2010). The precise mechanisms by which ISC fate is regulated to maintain tissue homeostasis is still unclear.
In summary, both fly GSCs and mammalian ISCs use polarized communication to set stem cells apart from their differentiated progeny. This likely represents a common principle shared by most tissues that rely on a stem cell population to maintain homeostasis, as asymmetric fate segregation typically requires asymmetric extrinsic regulation.
Fixed stem cell position in a periodic tissue structure
In contrast to the fixed tissue structures of the fly GSC and ISC niches, the skin hair follicle has a tissue structure that periodically changes. The adult hair follicle contains both an epithelial component, which includes hair follicle stem cells (HFSCs), and a mesenchymal component called the dermal papilla (DP). As a result of epithelial-mesenchymal interactions, the hair follicle cyclically regenerates through predictable phases of growth and differentiation, followed by phases of regression and rest (Figure 1C) (Sennett and Rendl, 2012) such that HFSCs encounter an oscillating microenvironment with each hair cycle. In the resting state, the stem cell population and its niche elements appear morphologically defined and compartmentalized. HFSCs reside in the bulge and hair germ (HG) regions of the resting hair follicle and are responsible for hair growth (Greco et al., 2009). The bulge population forms a two-layered epithelial sac in which the inner layer is composed of post-mitotic cells and the outer layer is composed of undifferentiated HFSCs (Cotsarelis et al., 1990; Hsu et al., 2011). Since the original bulge activation hypothesis, which showed that slow-cycling label-retaining cells (LRCs) reside in the bulge region of the hair follicle and are long-lived, subsequent studies have shed light onto the heterogeneity within the hair follicle stem cell population. In particular, the HG population, located just beneath the bulge and above the DP,is activated first during the onset of a new growth phase to give rise to differentiating cells of the inner layers of the hair follicle, suggesting that they represent a primed stem cell population that serves to initiate hair follicle growth (Greco et al., 2009; Ito et al., 2004; Rompolas et al., 2012).
Putative HFSCs have been shown to originate during embryonic hair follicle morphogenesis (Nowak et al., 2008). During development, hair follicles are specified within a relatively flat tissue sheet, through mechanisms involving lateral inhibition (Sick et al., 2006). Cells specified to become hair follicles first form placodes, which appear as patterned focal thickenings in the epidermis from which future hair follicles grow. Placode cells subsequently induce formation of an underlying dermal condensate, which remains in contact with the hair follicle epithelium and matures into the DP. HFSCs first appear within early placodes above the basal layer, and then in the upper outer layer of the hair follicle as the follicle continues to grow downward with the DP (Figure 1C) (Nowak et al., 2008). These cells are slow cycling LRCs that express HFSC markers, and can later be traced to the follicular bulge/HG region postnatally, suggesting that slow-cycling LRCs labeled in the embryo give rise to adult HFSCs. More recent work indicates that HFSCs are specified by asymmetric cell divisions that occur perpendicular to the basement membrane of hair follicle placodes, leaving the basal daughter cell with a high level of Wnt signaling, while the suprabasal daughter cell shows low levels of Wnt activation (Ouspenskaia et al., 2016). This suppression of Wnt signaling in the suprabasal cell is essential for its Sox9 expression and stem cell fate. At the same time, Shh produced by basal follicular cells signals to the suprabasal cells, resulting in symmetric divisions of the daughter stem cells. How these incipient HFSCs cells are eventually restricted to the upper region of the developing hair follicle is unknown. Their identification during early hair follicle morphogenesis suggests that their specification occurs independent of the morphologic bulge structure that originally defined the HFSC region in adult skin, as this structural bulge is only formed postnatally. This contrasts with embryonic ISC and fly GSC selection, which are dependent upon formation of a structural niche.
Nevertheless, the lack of a morphologic bulge does not necessarily preclude the presence of a functional niche that is currently not fully defined. It remains unclear how asymmetric divisions are established in the early placode and how the mesenchyme influences these events. Further, it is unknown if this same signaling apparatus and mode of communication are recapitulated in the adult hair follicle during the initiation of hair regeneration. How signals are propagated but compartmentalized to establish organized signaling territories is important not only for understanding how normal tissue growth occurs, but to also explain how these territories may become aberrantly expanded or disorganized during tumorigenesis. Currently, the lack of tools to visualize where specific ligands diffuse and accumulate has limited our understanding of how signals are concentrated or localized to influence signal territories and cell fate. Determining how HFSCs are first allocated holds important implications for understanding how they are durably maintained and how they communicate with their environment to drive cyclical and directional hair follicle regeneration in adult skin.
After embryonic development, the hair follicle cyclically regenerates through phases of growth, regression, and quiescence, and recent work suggests that the oscillating nature of the adult hair follicle structure imparts tight temporal control of HFSC interactions with its environment. The proximity of DP cells to HG cells and the fact that ablation of DP cells during the resting phase precludes hair follicle growth suggests close range epithelial-mesenchymal signals trigger HG activation (Chi et al., 2013; Rompolas et al., 2012). Similar to embryonic hair follicle development, Wnt signaling is essential to initiate HG activation and growth (Choi et al., 2013; Hsu et al., 2014; Myung et al., 2013). Subsequently, differentiating HG cells, also termed transit-amplifying cells, produce Shh during the early growth phase, resulting in bidirectional Smoothened (Smo) activation in both underlying DP cells as well as hair follicle epithelial cells, including differentiating HG cells themselves and overlying bulge cells (Hsu et al., 2014). This Shh-dependent bulge cell proliferation is required for generating a new HG population that can fuel subsequent hair cycles. Here, a model of sequential communication is suggested in which DP cells signal primed cells of the HG to initiate a program of proliferation and differentiation. Engaged HG cells subsequently provide Shh to both DP and bulge cells to promote hair follicle growth and as well as stem cell self-renewal, respectively. This mode of communication could provide a mechanism that coordinately regulates the timing and duration of stem cell self-renewal with the cyclical and oscillating regeneration of the hair follicle, as Shh-producing cells are only transiently in close proximity to bulge cells and migrate away from the bulge region of the upper hair follicle during hair growth. How the range of Shh activation in the hair follicle is regulated is unclear, but may involve communication modalities beyond simple ligand diffusion.
Although a self-renewing cell within the HG has not been demonstrated, these cells can repopulate the bulge region upon bulge stem cell depletion and concomitantly acquire bulge stem cell characteristics, including multipotency and self-renewal (Ito et al., 2004). The lack of genetic models to trace this population wholly and specifically has precluded definitive evaluation of their self-renewing capacity. Regardless, although the DP plays an essential role in regulating HFSC maintenance directly or indirectly, it is evident that the current model of what constitutes the HFSC niche, and therefore the mechanisms of communication, is evolving and not fully defined. For instance, some studies suggest that the basement membrane may be an important niche component for HFSCs that regulates their quiescence and activation (Morgner et al., 2015; Tanimura et al., 2011). Although it is unclear how specific basement membrane components regulate signaling in HFSCs, mechanisms resembling those of ligand concentration in the fly GSC niche may apply. Over the past several years, additional HFSC niche components have also been characterized, including adipocytes and immune cells (Castellana et al., 2014; Festa et al., 2011). However, further studies are needed to clarify how these different components are collectively integrated and communicated to HFSCs to regulate periodic hair follicle growth and maintenance.
Notably, the hair follicle not only houses epithelial stem cells but also harbors melanocyte stem cells (McSCs), which proliferate and differentiate coordinately with epithelial cells to provide pigment to the hair shaft during the growth phase (Nishimura et al., 2002). The majority of McSCs resides in the HG region of the resting follicle and become activated at the onset of a new hair follicle growth phase to give rise to pigment-producing melanocytes that populate the bulb region of the growing hair follicle as they deliver melanin to the hair shaft (Figure 1C). Previous work done to understand how McSCs are coordinately regulated with the hair cycle showed that epithelial HFSCs serve as a niche for McSCs by secreting TGF-b to neighboring McSCs, a signal required for their maintenance and quiescence (Nishimura et al., 2010). McSC activation and differentiation is also directly coupled to neighboring epithelial stem cells through Wnt signaling, a common signal that is essential for hair follicle growth (Deschene et al., 2014; Myung et al., 2013; Rabbani et al., 2011). Specifically, Wnt ligands secreted by epithelial HFSCs at the onset of hair follicle growth dually activate Wnt signaling in HG epithelial cells themselves and juxtaposed McSCs. How do follicular McSCs self-renew if they predominantly occupy the same space as that occupied by HG epithelial cells and coordinately differentiate with HG epithelial cells. Do the minority of McSCs in the bulge region behave similarly to their bulge epithelial counterparts to provide HG McSCs for the next hair cycle?
Knowing the answer to this question will be important for understanding how heterologous stem cells can differentially integrate signals to cohabit a shared space in a way that is not mutually exclusive. Nonetheless, some clues may be offered by the Drosophila male GSC niche, in which the apical tip of the fly testis hosts two different stem cell populations: GSCs and flanking cyst stem cells (CySCs) (de Cuevas and Matunis, 2011). The latter produce cyst cells that encase GSC progeny as they leave the niche, such that CySC and GSC behaviors must be coordinated. GSCs and CySCs share a common niche, and both CySCs and GSCs receive JAK/STAT signal from the niche but exhibit different consequences due to their distinct intrinsic differences imprinted from development, reminiscent of the interaction between epithelial HFSCs and McSCs. STAT signaling maintains CySC self-renewal by activating downstream targets Zfh1 and Chinmo, while in GSCs STAT activation promotes their adhesion to hub cells rather than self-renewal (Flaherty et al., 2010; Leatherman and Dinardo, 2008). Signals relayed between these two stem cell populations are required to coordinate their behaviors, and both niche signals and consequent signals communicated between the two stem cell populations may be important to ensure coordinated tissue regeneration and stem cell maintenance. (Kawase et al., 2004; Shivdasani and Ingham, 2003). These studies underline the importance of mutual regulation of diverse stem cells during complex tissue regeneration while also properly engaging the unique functions of constituent stem cell populations.
Similar to the fly GSC and mammalian ISC niches, the hair follicle features a polarized structure. However, the hair follicle model exhibits a mode of communication provided by a tissue architecture that fluctuates over time. The communication of resident HFSCs and McSCs not only requires spatial regulation, but also temporal regulation, and is reminiscent of the regenerative process of tissues with very slow turnover such as skeletal muscle. Muscle stem cells are typically quiescent during homeostasis, and the timing of stem cell activation and quiescence requires coordination with repair after injury.
Flexible stem cell position in a fluid tissue structure
Tissue morphogenesis is typically compartmentalized, resulting in formation of an organ that is fixed in location and size relative to the organism. In this context, specification of tissue stem cells or their niche elements could occur coordinately with the tissue as it is being shaped and assembled. By contrast, the hematopoietic system is structurally divergent from other organs in that it is fluid and its development occurs distant from its ultimate adult stem cell niche in the bone marrow (Mazo et al., 2011; Mikkola and Orkin, 2006). Significant work has been done to characterize and interrogate specific bone marrow components, including perivascular, endothelial, and neuronal cells, that can serve as a functional niche for maintaining adult hematopoietic stem cells (HSCs) (Figure 1D) (Morrison and Scadden, 2014). However, how HSCs are established during development is unclear partly due to the temporal and spatial variation in marker expression by HSCs throughout embryogenesis and the mobile and multifocal nature of their development.
Research in mice has shown that definitive HSCs can be identified in the aorta-gonad-mesonephros (AGM) region, yolk sac, and placenta, which eventually travel through the developing vascular network to seed the liver with HSCs and blood progenitors (Figure 1D) (Mikkola and Orkin, 2006). The migration of HSCs throughout the developing embryo is largely mediated by integrin adhesion molecules (e.g. αIIb integrin, β1 integrin) and cytokines (e.g. c-KitL) expressed on HSCs that bind to the ECM and cellular substrates to promote their retention in various embryonic niches, including the fetal liver (Christensen et al., 2004; Emambokus and Frampton, 2003; Ferkowicz et al., 2003; Hirsch et al., 1996; Kinashi and Springer, 1994; Kovach et al., 1995; Levesque et al., 1995). Once skeletal bones become vascularized, HSCs migrate from the liver to their final centralized niche where they reside in a relatively quiescent state (Figure 1D). The bone marrow chemoattractant CXCL12 (also known as SDF-1) is a key niche factor for adult HSC maintenance in the bone marrow, but is not a guidance cue for HSCs until later stages in development, after liver HSCs acquire the ability to respond to this signal (Christensen et al., 2004). In addition, reflective of the of the bone marrow endosteal niche composition, HSCs express calcium-sensing receptors that are important for the homing of HSCs from the liver to the bone marrow (Adams et al., 2006). Although the precise repertoire of niche elements that promote HSC development within each depot are still undefined, a recent study has begun to dissect the cellular mechanisms that regulate HSC colonization and function in intermediate hematopoietic tissues during zebrafish embryogenesis (Tamplin et al., 2015). High resolution live imaging of zebrafish embryos revealed that the arrival of HSCs in the intermediate hematopoietic tissue, which is analogous to the mammalian fetal liver, triggers endothelial cells to remodel and envelope the HSCs. Zebrafish perivascular mesenchymal stromal cells, also shown to be key niche components in the mammalian bone marrow niche, were shown to attach to HSCs and orient their cell divisions, possibly for asymmetric fate determination. Similar interactions between HSCs and endothelial cells were also observed in mouse fetal liver explants, suggesting a conserved mechanism for HSC colonization. Although many questions remain, this work may provide a common principle that explains the dynamic interactions between mobile HSCs and their multifocal niches.
The migration of HSCs through different niches also traces their coordinate development and maturation with the circulatory system and their inherent interaction with vessels (Mikkola and Orkin, 2006). After reaching the BM during development, HSCs and hematopoietic progenitor cells can continue to mobilize under steady-state conditions to travel through the blood and peripheral tissues, allowing them to patrol and respond to inflammatory signals throughout the body (Figure 1D) (Bhattacharya et al., 2009; Massberg et al., 2007; Wright et al., 2001). Recent studies show that HSCs are heterogeneous with respect to their self-renewing capacity and multipotency, while hematopoietic progenitors that had been previously considered lineage restricted can differentiate into mature blood cells of different hematopoietic lineages long-term (Goodell et al., 2015; Sun et al., 2014). Thus hereafter, we will use the term hematopoietic stem and progenitor cells (HSPCs) to refer to this heterogeneous population of cells.
How adult HSPCs interact with distant depots is still under investigation, but relies on their ability to enter and exit the vasculature and the ability of distant tissues to express HSPC chemoattractant and retention signals (Mazo et al., 2011). HSPCs can home back to their niche in the BM from the blood by their adhesion to specific endothelial selectins that are specifically expressed within the BM, including P- and E-selectin and VCAM-1 (Mazo et al., 1998; Mazo and von Andrian, 1999). These endothelial adhesion molecules can bind directly to ligands expressed on HSPCs that allow for dynamic tethering, rolling, and eventual firm adhesion to HSPCs as they are carried within a high-flow environment of the vasculature. Within the BM, HSPCs are retained through adhesion (e.g. α4β1, VCAM-1) and chemokine/cytokine (e.g. CXCL12-CXCR4, cKitL-cKit) interactions with the BM stroma, including the perivascular/endothelial stroma (Mazo et al., 2011). HSPCs can continue to migrate within the BM, and some progeny appear to have distinct compartments within the BM that support their own proliferation and further differentiation, perhaps allowing for compartmentalization of differentiation programs (Morrison and Scadden, 2014). In addition, CXCL12 can be upregulated in various peripheral tissues under conditions of stress allowing them to home to other sites (Kollet et al., 2003; Stumm et al., 2002), suggesting that different tissues can employ many of the same signals that retain HSPCs within the BM to promote HSPC population of peripheral sites and to function as distant and transient facultative niches.
Here, a principle of multifocal stem cell communication is illustrated. Due to the unique fluid tissue architecture and the dispersed arrangement of potential niche structures, the hematopoietic system holds stem cells at multiple positions, where they dynamically interact with local niche elements to fulfill their functions in immune regulation and erythropoiesis. It is currently unclear how the kinetics of HSPC entrance into and exit from a niche is controlled during homeostasis or in response to a local requirement for hematopoiesis.
Conserved and distinct principles of stem cell communication
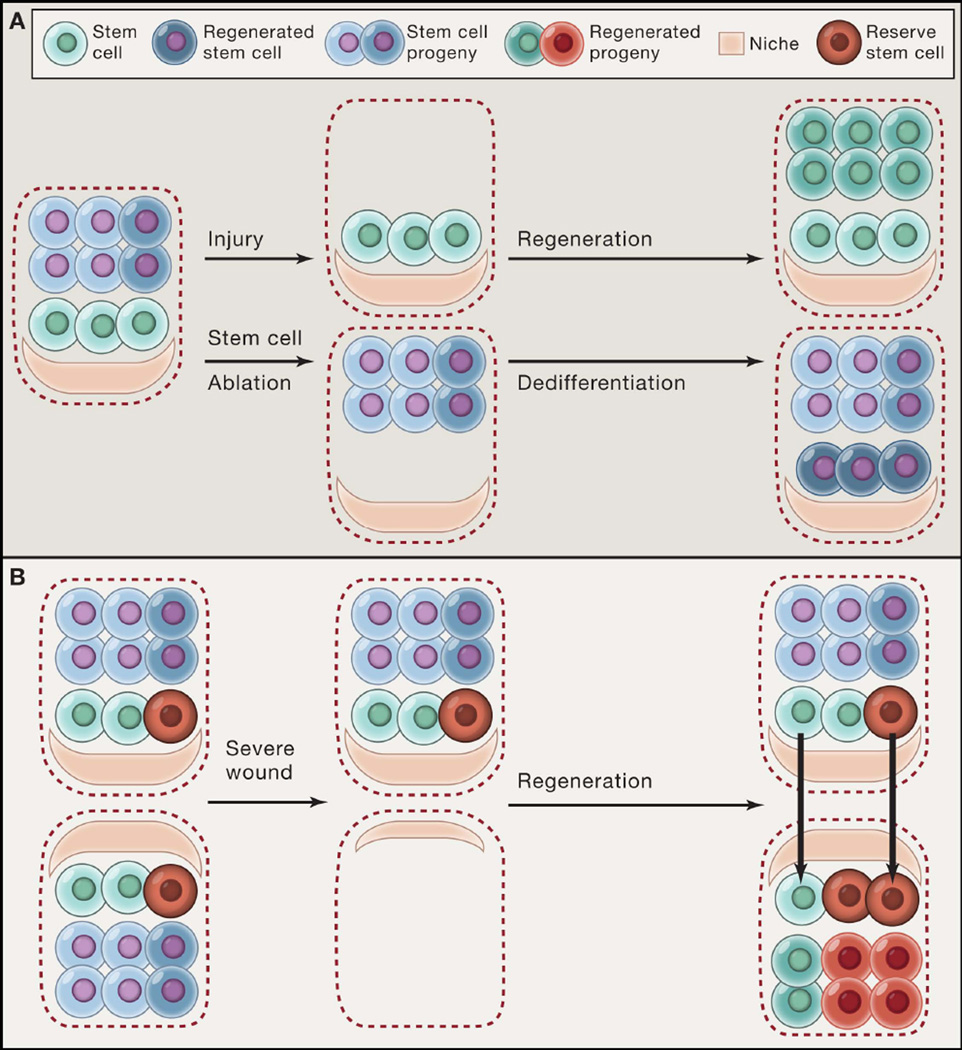
Although different tissue structures confer distinct communication modes between stem cells and their environment, there are common principles shared by various tissues. For example, stem cells can be heterogeneous and are not necessarily intrinsically unique, underscoring the central role of the niche in determining cell fate. Several examples show that when stem cells are experimentally depleted, other undifferentiated or even committed stem cell progeny can mobilize to the niche to re-establish the stem cell population and uphold normal tissue organization and function. The deterministic role of the niche in controlling stem cell fate was first demonstrated for fly GSCs, in which temporarily forcing the differentiation of cells by reversible genetic methods resulted in their later reversion to stem cells (Brawley and Matunis, 2004; Kai and Spradling, 2004). In mammals, a similar reversibility has been described. For example, in the mouse upper airway, in vivo ablation of basal stem cells induces committed secretory cell progeny to proliferate and dedifferentiate into functional basal stem cells capable of supporting homeostasis and repair (Figure 3A) (Tata et al., 2013). In vitro work suggests that the dedifferentiation of secretory cells may be induced by the loss of a contact-dependent signal supplied by basal stem cells, underlining the importance of cell contact in sensing perturbations to homeostasis in some tissues. In intestinal crypts, various committed cell populations capable of replenishing the stem cell pool have also been discovered. One study found that intestinal secretory precursors expressing high Dll1 can convert into ISCs after radiation damage, while another study identified a label-retaining Lgr5+ cell population, which is also committed to a secretory fate, contributes to the ISC pool following stem cell loss caused by chemotherapeutic agents or γ-irradiation (Buczacki et al., 2013; van Es et al., 2012b).
Figure 3. Maintaining robust stem cell communication networks after tissue injury.
(A) Stem cells regenerate differentiated lineages after injury (e.g. basal stem cells of the upper airway following SO2-mediated damage). However, when stem cells themselves are ablated, their progeny can also dedifferentiate into functional stem cells (e.g. Dll1+ intestinal secretory precursors following irradiation damage). (B) A subset of reserve stem cells (red) that do not normally contribute to homeostasis can be engaged along with other resident stem cells (green) to reconstitute tissue following injury (e.g. K5+p63+ basal stem cells in the distal airway). A recent study suggests that stem cells might regenerate tissue architecture by using the residual niche structures left as a template after severe injury.
Recent work using intravital microscopy has further illustrated this cell fate plasticity (Rompolas et al., 2013). Here, laser ablation of HFSCs located in either the bulge or hair germ in live mice resulted in the recruitment of surrounding epithelial cells within and outside of the hair follicle to replace these lost stem cells and that could function as HFSCs. However, if physical contact between the hair follicle epithelium and DP was disrupted, HFSC replenishment was impaired, suggesting that mobilization of cells to restore the stem cell population requires a close-range or contact-dependent signal from the DP. Despite the wealth of data showing that stem cells are not necessarily intrinsically unique, how cells communicate to acquire a stem cell state is unclear in many tissues. The observation that committed cells can assume a stem cell state upon stem cell loss suggests that the niche plays a chief role in determining cell state. In a number of invertebrate tissues, the niche components and signals that confer “stemness” have been well-characterized. For example, the C. elegans distal tip cell (DTC) utilizes Notch signaling to maintain GSC fate (Byrd and Kimble, 2009), and BMP signals sent from fly anterior somatic niche cells play a central role in GSC maintenance (Kai and Spradling, 2004; Xie and Spradling, 1998). By contrast, in many mammalian tissues, what constitutes the stem cell niche is incompletely understood. Pursuits to harness the plasticity of committed cells to promote tissue regeneration will require the daunting task of acquiring a detailed characterization of the biochemical, physical, and cellular components of the stem cell niche within each tissue.
Another generalized principle of stem cell communication across most tissue types is feedback control. Feedback regulation to parental stem cells by their progeny provides an internal means to ensure that stem cells proliferate and provide differentiated cells according to the tissue’s needs. In this context, stem cell progeny can constitute a functional niche for stem cells. As discussed above, Paneth cells in the intestine and transit-amplifying cells in the hair follicle act as important niche components to control the self-renewal of their respective parent stem cells. In addition to transit-amplifying progeny of HG cells, the inner bulge layer of hair follicle is also derived from differentiated HFSC progeny generated from the previous growth phase, which have been shown to secrete inhibitory signals such as FGF18 and BMP to the outer bulge cell layer and HG to promote stem cell quiescence during the resting phase (Hsu et al., 2011). This theme of feedback control also applies to the hematopoietic system, as macrophages and megakaryocytes can serve to retain HSCs in the BM niche (Bruns et al., 2014; Chow et al., 2011; Winkler et al., 2010; Zhao et al., 2014).
The reverse scenario in which parent stem cells communicate with their progeny to ensure a differentiated state is not common, suggesting it is distinct to certain specialized tissue architectures. In the fly midgut, for example, ISCs supply a Notch signal to their enterocyte progeny to sustain their differentiation through contact-dependent Notch communication (Ohlstein and Spradling, 2007). In the mammalian upper airway epithelium, basal cells serve as stem cells to their adjacent differentiated secretory and ciliated cells (Rock et al., 2009). Here, parental basal stem cells provide a Notch signal to their secretory progenitors to maintain their differentiated state, suggesting that airway epithelial architecture and composition are set by forward signals provided by parental stem cells (Pardo-Saganta et al., 2015). Direct interaction between Notch ligands on basal cells and Notch receptors on secretory cells maintains differentiated secretory cells, the latter of which can further differentiate into ciliated cells. How differentiated cell proportions are set may be a function of the relative levels of Notch ligand expressed by basal cells. This peculiar mechanism of communication may be partly due to the pseudo-stratified organization of the fly intestine and mammalian upper airway, in which stem cells and their progeny are essentially at an equivalent position.
Modes of signal transmission in stem cell communication
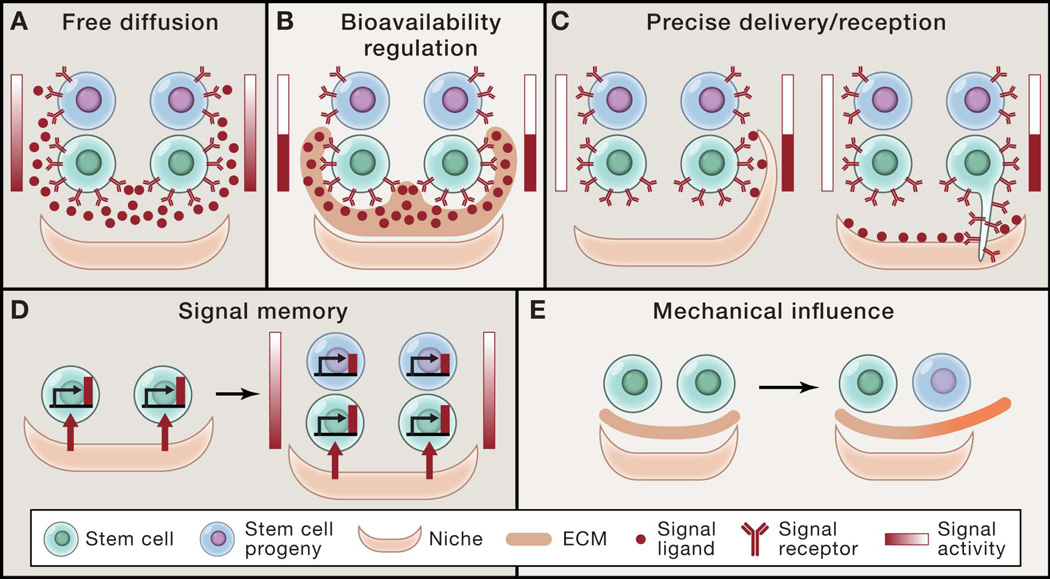
Despite growing data that has uncovered many of the cellular and molecular components that regulate tissue stem cells, how precisely these signals are communicated between cells over time and space during homeostasis and repair is still unclear. In fact, much of the work done to investigate how signals are transmitted, received, and translated across a population of cells in vivo has been done in invertebrate models that are experimentally more accessible than many mammalian systems. For example, niche cells of the female fly ovary maintain GSC fate by sending BMP signals to adjacent GSCs, while committed progeny are physically displaced away from the source of BMP. However, the high level of BMP signal experienced by GSCs is not solely attributed to regulation by free diffusion of BMP from the niche source, but is also due to distinctive niche ECM components, glypican (dally) and collagen IV, that stabilize and restrain BMP ligands, respectively (Figure 2A and 2B) (Guo and Wang, 2009; Hayashi et al., 2009; Wang et al., 2008). Knockdown of dally in somatic niche cells causes GSC loss, while loss of collagen IV or ectopic dally expression results in GSC expansion, illustrating how selective signal responses can be modulated by both cell position as well as the bioavailability (Figure 2B) of ligands (Guo and Wang, 2009; Hayashi et al., 2009; Wang et al., 2008). These studies also underscore the importance of non-cellular components such as the ECM in stem cell fate regulation.
Figure 2. Modes of signal transmission in stem cell communication.
(A) Free diffusion of signal ligand from a polarized source generates a signal gradient across stem cells and their progeny that determines asymmetric cell fates. (B) Regulation of the bioavailability of signal ligand by extracellular matrix (ECM) components can concentrate signal ligand to stem cells, while restricting ligand from differentiated progeny. (C) Specialized sub-cellular structures of either niche cells or stem cells precisely control signal activation in specific stem cells. (D) Differential signal activity between stem cells and their progeny can be generated by signal memory and decay once they lose exposure to the signal source. (E) Changes in mechanical properties such as matrix stiffness can influence stem cell self-renewal and differentiation.
Even beyond position, stem cells and their niche can also employ specialized sub-cellular structures to precisely deliver or receive signals within the niche (Figure 2C). One example of this is cap cells, which extend actin-rich filopodia called cytonemes to locally deliver Hedgehog (Hh) ligands to adjacent escort cells (Rojas-Rios et al., 2012). In a similar fashion, in the male fly GSC niche, BMP signals provided by the niche induce GSCs to form microtubule-based nanotubes that extend into the somatic niche to promote, and perhaps restrict, BMP signal transduction to GSCs (Inaba et al., 2015). It is perplexing that these nanotubes were only recently discovered, but the fact that these structures are only transiently formed during the cell cycle and sensitive to fixation techniques not only highlights why they may have escaped discovery in the past, but also underscores how in vivo studies are still limited by current technologies for visualizing and tracking cellular interactions and behaviors. Accumulating evidence in vertebrate models, including chick and zebrafish, have also demonstrated that other tissues employ such focal contact-dependent methods to deliver either short- or long-range morphogens to specific target cells, suggesting that these signaling conduits may be common to other organisms and tissues (Sanders et al., 2013; Stanganello et al., 2015). This intriguing form of signal modulation may function to deliver signals with more precise spatial and temporal control compared to simple ligand diffusion and may provide another level of differential signal transmission beyond cell location (Figure 2C). Additionally, these subcellular structures may also yield differences in intracellular signaling dynamics that go beyond the absolute quantity of ligand delivered.
In all these above modes of communication, the reception of different levels of a signal or morphogen generates differential signaling activity between cells. However, a recent study suggests that signal territories may also be patterned by signal memory (Figure 2D). In this study, a membrane-tethered form of Wnt ligand was sufficient to induce the effects of the wild type diffusible Wnt ligand, resulting in patterning of fly tissues such as the imaginal disc (Alexandre et al., 2014), raising the possibility that cells retain memory of earlier local signaling interactions despite cell divisions and dispersal of cells. Although some of these modes of communication may not be universal across tissues or species, these provocative studies have provided important insight into the complexity and diversity of how cells communicate to pattern tissues.
Over the past decade, emerging data on how stem cells are regulated to control tissue growth and maintenance has brought new recognition and insight into the complexity of the environment that cannot be accounted for by biochemical signals alone. Research into the mechanical and physical forces that influence cell fate and behaviors have emerged partly out of the fundamental observation that cell morphology frequently reflects the behavior and function of their constituent tissue. For instance, myofibers are formed from fusion of multiple myoblasts that together form a syncytial multinucleated myotube in which actin and myosin filaments are arranged in contractile sarcomere units. These units contract across the length of the myofiber to yield large-scale contraction and movement. Likewise, the apical constriction of a localized group of cells can result in invaginations and tubes or channels that are important for the development and function of many tissues such as secretory glands.
At heart, how tissue is organized is largely a function of changes in cell shape and polarity and the ability of cells to migrate and generate contractile forces. It is therefore no surprise that environmental forces influence stem cell fate decisions. The finding that cell spreading and matrix tension can directly affect cell proliferation and survival as well as direct the fate of mesenchymal stem cells (MSCs) to assume either osteogenic or adipogenic fates, both morphologically and molecularly, highlighted the importance of mechanotransduction in cell biology (Chen et al., 1997; Engler et al., 2006; Folkman and Moscona, 1978; Ingber, 1991; McBeath et al., 2004). In addition, activation of cytoskeletal contractility through non-muscle myosin II is essential for matrix elasticity-directed lineage specification (Engler et al., 2006). Since then, other mechanical properties of tissue that affect cell fate and function have been under investigation, including matrix topography and stiffness/elasticity transmitted to cells through integrins in focal adhesions and through lamin-A of the nucleoskeleton, as well as through cell-cell adhesion mediated by cadherins (Bellas and Chen, 2014; Li et al., 2011).
However, what are the forces that support stem cell identity? One study showed that maintaining skeletal muscle stem cells on a relatively soft matrix that matched their in vivo niche improved their engraftment and ability to regenerate muscle (Gilbert et al., 2010). Similarly, shear stress was also found to increase hematopoietic colony-forming potential in the aorta-gonad-mesonephros (AGM) of mouse embryos (Adamo et al., 2009). Mouse embryonic stem cells (mESCs) cultured on soft substrates that matched the intrinsic stiffness of mESCs were able to maintain pluripotency markers and long-term self-renewal in the absence of Leukemia inhibitory factor (LIF), which is normally required to maintain ESC pluripotency in culture (Chowdhury et al., 2010a). Conversely, cell spreading of mESCs was accompanied by differentiation and downregulation of pluripotency markers (Chowdhury et al., 2010b; Evans et al., 2009). Collectively, these data suggest that stem cells are sensitive to mechanical forces in their environment (Figure 2E). Interestingly, how mESCs respond to substrate stiffness was also influenced by their neighbors through E-cadherin, which can mechanically couple apical stiffness with basal traction when grown in cellular aggregates and may have implications for cell sorting and propagation of forces over a population during tissue morphogenesis and regeneration (Chowdhury et al., 2010a).
Despite the growth of tools available to study mechanotransduction in cells, a major challenge in understanding how these forces can have such a powerful influence on cell fate and behavior is connecting these forces to molecular machinery that controls cellular functions. Gene expression analysis of signaling pathways that are regulated by matrix stiffness or cell shape have focused interest on YAP/TAZ transcriptional factors that localize to the nucleus in response to matrix stiffness and also contribute to matrix stiffness (Dupont et al., 2011). Serum response factor (SRF) is another transcriptional factor that appears to function as a mechanosensor of cell spreading (Wozniak et al., 2012). How external forces regulate these transcriptional factors may be related to their effect on the nucleoskeleton and its intermediate filament, lamin-A, which could interact with nuclear actin to regulate nuclear localization of YAP and SRF (Bellas and Chen, 2014; Swift et al., 2013). Altogether, it is becoming clear that understanding tissue and cellular mechanics is integral to elucidating how stem cells communicate with their environment to maintain tissue function. Nevertheless, the field of mechanobiology is still in its infancy, and most studies have been performed in vitro and in two dimensions. Current efforts are geared toward generating the tools that can translate these studies to in vivo and three-dimensional in vitro platforms.
Reshaping stem cell connections in tissue repair
Development provides a template for tissue architecture that is sustained throughout adulthood in a manner that frequently echoes these same embryonic programs. By contrast, tissue repair following injury often involves deployment of new programs that restore order to tissue whose previously established connections have been disrupted. Wound repair and regeneration have been studied across diverse tissues for decades, and the role of specific stem cell populations in this process is still ambiguous in many organs. The observation that many committed populations have the ability to mobilize and serve as reserve stem cells raises the possibility that stem cells that maintain normal homeostasis may not necessarily be required to regenerate tissue following injury. By using genetic lineage tracing, recent studies have started to elucidate the mechanisms that reshape cell communication networks during wound healing.
As skin constitutes the outermost layer of the body, it is constantly exposed to the external environment and is the most susceptible to injury and toxins. As such, skin has become one of the most well-studied and versatile models to examine the mechanisms regulating wound healing. Following full-thickness wounds, a series of complex and overlapping phases is initiated with a hemostatic fibrin clot. The wound is subsequently resolved through the orchestrated proliferation and recruitment of inflammatory cells, fibroblasts, endothelial cells, and epithelial cells, which eventually results in closure of the wound (Zielins et al., 2014). During epidermal wound repair, epithelial cells proliferate and migrate toward the center of the wound following injury (Raja et al., 2007). However, whether or not specific epithelial populations are selected as primary contributors for healing the wound is still unclear. One study suggested that committed basal progenitor cells contribute less than their putative uncommitted counterparts to re-epithelialization with respect to clone size and sustainability, though both can be recruited to the wound area (Mascre et al., 2012). However, another study failed to reveal an ostensibly slow-cycling population that preferentially contributes to wound healing (Lim et al., 2013). Further studies are required to determine if a select subpopulation of uncommitted epidermal cells more durably contributes to wound healing and how different populations are coordinated to achieve that.
In contrast to the skin epidermis, where it is still unclear if a specialized stem cell population is reserved for wound repair, in other tissues a select stem cell population has been shown to be essential for injury repair (Figure 3B). In the mouse lung, influenza virus infection results in inflammation and massive damage to the lung parenchyma, including alveoli. Remarkably, the lung can heal and recover from this infection through a process of regeneration that restores lung tissue and air exchange. Two recent independent studies revealed the existence of a rare population of distal airway basal cells, which express both Keratin 5 and p63 (K5+p63+) and are functionally and molecularly distinct from upper airway basal cells (Vaughan et al., 2015; Zuo et al., 2015). Under homeostasis and following minor injury, differentiated secretory cells serve to maintain the lung epithelium. However, following massive lung injury induced by influenza virus infection, the rare population of bronchiolar K5+p63+ cells expands and migrates to the lung interstitium and gives rise to regenerated type I and type II alveolar pneumocytes as well as bronchiolar secretory cells. Moreover, ablation of these cells results in failure to regenerate lung tissue and function following influenza virus infection. In this case, a specific population of stem cells appears to be reserved for responding to major lung damage. How these cells respond to injury to migrate far and form functional alveolar sacs capable of reconnecting gas exchange between the airway and the capillary bed is perplexing, but likely entails carefully coordinated communication between distal airway stem cells, neighboring epithelial cells, and the stroma.
This concept of a specialized population of cells capable of responding to tissue damage but distinct from those that maintain tissue during homeostasis is illustrated in other systems. HSCs show significant heterogeneity as a population and are generally quiescent, but a subpopulation of dormant HSCs in the bone marrow that divides very infrequently and is unlikely to contribute significantly to hematopoiesis during normal homeostasis (Wilson et al., 2008) are multipotent and robustly divide in response to bone marrow injury. After homeostasis is recovered, the dormant HSC population is re-established, suggesting that the transition between dormancy and activation is reversible. In the liver, there is also evidence of “facultative” stem/progenitor cells that emerge only after liver damage and that show the capacity to differentiate into hepatocytes and cholangiocytes (Miyajima et al., 2014). How these select populations of cells are transiently deployed to respond to injury is a subject of active investigation, and in vivo models to directly examine their contributions to tissue repair are necessary.
In addition to resident stem cells contributing to tissue repair, stem cells from other tissue compartments can be plastic and contribute to wound healing. As discussed above, epidermal cells contribute to skin wound repair. However, other epithelial populations can also be recruited to participate in cutaneous wound healing. Using a Keratin-15-CrePR based lineage-tracing system, one study showed that HFSCs do not contribute significantly to normal epidermal homeostasis, but following wounding, file out of the hair follicle toward the center of the wound where they express interfollicular epidermal differentiation markers (Ito et al., 2005). However, these labeled cells are largely lost after a few weeks, showing that they do not durably contribute to the epidermis. Other groups showed that other hair follicle-derived cells, including those in the isthmus, also contribute to epidermal wound healing and remain in the re-epithelialized epidermis long-term (Brownell et al., 2011). The variable ability of follicular cells to contribute to wound healing suggests that cell intrinsic factors may play a role in determining cell fate following injury. Similar to hair follicle epithelial cells, follicular melanocyte stem cells also exit hair follicles at the wound periphery to populate the epidermis (Chou et al., 2013). However, in contrast to epithelial HFSCs, melanocyte migration occurs at the expense of the follicular melanocyte stem cell pool, resulting in a net loss of follicular melanocytes and hair depigmentation. It is still unknown what role HFSCs play in wound healing and what signals prompt them to migrate out of their normal niche. Do HFSCs exit as a response to changes in cellular behaviors or signals produced by interfollicular keratinocytes or are both populations coordinately regulated by common signals? How do epithelial cells interact with each other, as well as mesenchymal and inflammatory cells, to collectively mobilize and expand to close a wound? What is the functional heterogeneity of different epithelial populations that contribute to skin wound healing? Further work to define the cellular behaviors that promote wound healing are necessary to get a closer view of how cells communicate within a disrupted architecture to promote wound healing and restoration of tissue structure and function.
Altogether, evidence suggests that tissue stem cells, or subsets of stem cells, can respond and mobilize in response to injury. There is less evidence to show how these cells contribute functionally to wound repair and how the wound environment communicates to these cells to provoke their activation. A recent study on muscle regeneration has begun to interrogate how stem cells act to restore tissue organization when it is severely disrupted (Webster et al., 2015). Muscle stem cells rest in a quiescent and immobile state during homeostasis, but become activated upon injury such as that caused by cardiotoxin-induced muscle cell death, after which only stem cells remain. Intravital imaging of mouse muscle regeneration revealed that the regenerative behaviors of muscle stem/progenitor cells are governed by ECM remnants called “ghost fibers”. These ECM remnants control the directionality of myogenic progenitor divisions and their migration and act as a template to guide organization of the newly regenerated muscle. Myogenic progenitors that lose this guidance from “ghost fibers” show misoriented cell divisions and migration, resulting in disorganized regenerated myofibers. This study highlights the importance of maintaining clues or remnants of tissue structure after severe injury in order to re-establish tissue architecture, even when most of the cell connection networks are disrupted. The same mechanism may also apply to wounds in which stem cells are also locally depleted. In those cases (e.g. skin epidermis and lung injury), stem cells close to the wound might be recruited to regenerate the stem cell pool by following remnant niche structures (Figure 3B).
In other cases, tissue regeneration may not necessarily require clues from residual tissue structure. One example is de novo hair follicle neogenesis, a process that had been historically dismissed until one study definitively showed that neogenic hair follicles can form within the center of re-epithelialized large, full-thickness skin wounds of adult mice (Ito et al., 2007). These de novo hair follicles form in a manner that recapitulates many of the molecular and morphological events that chronicle embryonic hair follicle development, including the formation of new HFSC niches capable of driving cyclical hair follicle growth by resident HFSCs. Notably, HFSCs that were lineage labeled prior to wounding did not contribute significantly to the neogenic hair follicles, showing that other keratinocytes hold plasticity to differentiate into follicular keratinocytes (Ito et al., 2007). As epithelial-mesenchymal communication is essential for hair follicle development, the regenerating mesenchyme that underlies the epidermis likely plays an important role in orchestrating the specification and morphogenesis of de novo hair follicles (Gay et al., 2013). Recent data suggest that inflammatory cells, as well as inflammatory cytokines released during wound healing, play a critical role in resurrecting key signals required for hair follicle development (Gay et al., 2013; Nelson et al., 2015). Thus, this study implies that wound healing may co-opt immune responses to deploy hair follicle developmental programs.
Conclusion
Over the last decade, stem cell research has evolved tremendously and is beginning to provide a broader view of how stem cells function to maintain and repair tissue. Pioneering work that established the stem cell and niche concepts continue to serve as an anchor of principles that frame our understanding of stem cell regulation and tissue maintenance across virtually all organisms and tissues. These principles have led to a stratified hierarchical relationship of cells within a tissue, while revealing that stem cells are also heterogeneous. Moreover, there is a broad diversity in the ways in which stem cells are regulated, which largely reflects the diversity in tissue structure, architecture, and function. Concurrently, studies examining how cells communicate biochemically and physically with other cells and their ECM have demonstrated the interdependence of these aspects of biology in determining cell fate and function.
The field of stem cell biology has begun to recently integrate these global aspects of biology to bring a more comprehensive view of how stem cells behave and communicate in situ rather than in isolation, in an attempt to break down former rigid models of stem cell regulation. To uncover stem cell communication networks within each tissue, the development of new markers for non-cellular components of the niche as well as new approaches for more precisely characterizing cellular identities in situ are required. Meanwhile, establishing advanced high-resolution imaging techniques together with reporters both for faithfully reflecting the chemical and physical signal activity and for capturing the processes of signal delivery will further our understanding of the molecular principles of stem cell communication. Systems for manipulating specific niche elements with high spatiotemporal control, such as optogenetics, will also need to be used to test how these different aspects of biology influence stem cells. Altogether, such efforts will broaden our perspective of how stem cells are regulated according to their specific tissue organization to maintain homeostasis, which also lays the groundwork for understanding how alteration of stem cell communication modes contributes to wound healing or neoplasia. Ultimately, this knowledge may give rise to new strategies for promoting tissue repair and cancer treatment.
Acknowledgments
We thank members of the Greco laboratory for their priceless input and review of this manuscript. This work is supported by The New York Stem Cell Foundation and grants to V.G. by the American Cancer Society, grant no. RSG-12-059-02; the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and an Edward Mallinckrodt Jr. Foundation Grant, NIH, grant no. 1R01AR063663-01. P.M. is supported by NIAMS 1K08AR066790-01 and the Global Fibrosis Foundation Grant. T.X. is supported by the James Hudson Brown-Alexander Brown Coxe Postdoctoral Fellowship. V.G. is a New York Stem Cell Foundation Robertson Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews Molecular cell biology. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Bellas E, Chen CS. Forms, forces, and stem cell fate. Curr Opin Cell Biol. 2014;31:92–97. doi: 10.1016/j.ceb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. The Journal of experimental medicine. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo . Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell stem cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature medicine. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Byrd DT, Kimble J. Scratching the niche that controls Caenorhabditis elegans germline stem cells. Seminars in cell & developmental biology. 2009;20:1107–1113. doi: 10.1016/j.semcdb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS biology. 2014;12:e1002002. doi: 10.1371/journal.pbio.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell stem cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nature medicine. 2013;19:924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010a;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010b;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS biology. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dansereau DA, Lasko P. The development of germline stem cells in Drosophila . Methods in molecular biology. 2008;450:3–26. doi: 10.1007/978-1-60327-214-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschene ER, Myung P, Rompolas P, Zito G, Sun TY, Taketo MM, Saotome I, Greco V. beta-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science. 2014;343:1353–1356. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emambokus NR, Frampton J. The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity. 2003;19:33–45. doi: 10.1016/s1074-7613(03)00173-0. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–14. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Molecular and cellular biology. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila . Developmental cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gancz D, Lengil T, Gilboa L. Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS biology. 2011;9:e1001202. doi: 10.1371/journal.pbio.1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochimica et biophysica acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature medicine. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Current biology : CB. 2004;14:981–986. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Nguyen H, Shroyer N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nature reviews Molecular cell biology. 2015;16:299–309. doi: 10.1038/nrm3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell stem cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Wang ZH. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- Harris RE, Ashe HL. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12:519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nature reviews Molecular cell biology. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M, Buszczak M, Yamashita YM. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature. 2015;523:329–332. doi: 10.1038/nature14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47:236–241. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation; research in biological diversity. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature medicine. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans . Developmental biology. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- Krieger T, Simons BD. Dynamic stem cell heterogeneity. Development. 2015;142:1396–1406. doi: 10.1242/dev.101063. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell stem cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. The Journal of experimental medicine. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhou J, Chowdhury F, Cheng J, Wang N, Wang F. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229–240. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila Stem Cell Niches: A Decade of Discovery Suggests a Unified View of Stem Cell Regulation. Developmental cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. The Journal of experimental medicine. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;32:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66:25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell stem cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Morgner J, Ghatak S, Jakobi T, Dieterich C, Aumailley M, Wickstrom SA. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nature communications. 2015;6:8198. doi: 10.1038/ncomms9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. The Journal of investigative dermatology. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, Chang E, Resnik SR, Page C, Kim D, et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell stem cell. 2015;17:139–151. doi: 10.1016/j.stem.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell stem cell. 2010;6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell stem cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Ouspenskaia T, Matos I, Mertz AF, Fiore VF, Fuchs E. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow R, Prabhu M, Gridley T, Rajagopal J. Parent stem cells can serve as niches for their daughter cells. Nature. 2015;523:597–601. doi: 10.1038/nature14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Frontiers in bioscience : a journal and virtual library. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila . PLoS biology. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Roman AK, Jayewickreme CD, Murtaugh LC, Shivdasani RA. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo . Stem cell reports. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Seminars in cell & developmental biology. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Current biology : CB. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell. 2015;161:569–580. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- Song XQ, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, Schug A, Raz E, Scholpp S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nature communications. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA, Zon LI. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160:241–252. doi: 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell stem cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Molecular and cellular biology. 2012a;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature cell biology. 2012b;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. The EMBO journal. 2015;34:1164–1179. doi: 10.15252/embj.201490386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nature reviews Molecular cell biology. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila . Nature. 2008;455 doi: 10.1038/nature07214. 72-U49. [DOI] [PubMed] [Google Scholar]
- Webster MT, Manor U, Lippincott-Schwartz J, Fan CM. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell stem cell. 2015 doi: 10.1016/j.stem.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Cheng CQ, Shen CJ, Gao L, Olarerin-George AO, Won KJ, Hogenesch JB, Chen CS. Adhesion regulates MAP kinase/ternary complex factor exchange to control a proliferative transcriptional switch. Current biology : CB. 2012;22:2017–2026. doi: 10.1016/j.cub.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature medicine. 2014;20:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- Zielins ER, Atashroo DA, Maan ZN, Duscher D, Walmsley GG, Hu M, Senarath-Yapa K, McArdle A, Tevlin R, Wearda T, et al. Wound healing: an update. Regen Med. 2014;9:817–830. doi: 10.2217/rme.14.54. [DOI] [PubMed] [Google Scholar]
- Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]