Abstract
We examined United States Renal Data System registry records for Medicare-insured kidney transplant recipients in 2000–2011 to study the clinical and cost impacts of urinary tract infections (UTI), pneumonia, and sepsis in the first year post-transplant among a contemporary, national cohort. Infections were identified by billing diagnostic codes. Among 60,702 recipients, 45% experienced at least one study infection in the first year post-transplant, including UTI in 32%, pneumonia in 13%, and sepsis in 12%. Older recipient age, female sex, diabetic kidney failure, non-standard criteria organs, sirolimus-based immunosuppression and steroids at discharge were associated with increased risk of first-year infections. By time-varying, multivariate Cox regression, all study infections predicted increased first-year mortality, ranging from 41% (aHR 1.41, 95%CI 1.25–1.56) for UTI alone, 6-to-12-fold risk for pneumonia or sepsis alone, to 34-fold risk (aHR 34.38, 95%CI 30.35–38.95) for those with all three infections. Infections also significantly increased first-year costs, from $17,691 (standard error (SE) $591) marginal cost increase for UTI alone, to approximately $40,000–$50,000 (SE $1054–1238) for pneumonia or sepsis alone, to $134,773 (SE $1876) for those with UTI, pneumonia and sepsis. Clinical and economic impacts persisted in years 2–3 post-transplant. Early infections reflect important targets for management protocols to improve post-transplant outcomes and reduce costs of care.
Keywords: Economics, Infections, Kidney transplantation, Medicare, Registries
INTRODUCTION
Advances in the clinical management of kidney transplant recipients have yielded substantial improvements in short-term allograft survival in recent decades, mediated in part by reduction in the incidence of acute rejection (1). While 50% to 60% of renal allograft recipients in the 1980s experienced at least one acute rejection episode, the current incidence of acute rejection in the first post-transplant year is less than 15% (1, 2). Unfortunately, lower rates of acute rejection have not been accompanied by a substantial increase in long-term graft survival (3). The lack of improvement in long-term survival graft has been attributed in part to complications driven by potent immunosuppression, as well as by increasing comorbidity burden among recipients at the time of transplantation (4).
Although prophylactic antimicrobial medications are commonly prescribed in the first 6 to 12 months after kidney transplantation (5, 6), infections are a common complication in the early post-transplant period (7, 8). Infections clearly contribute to post-transplant morbidity, mortality and costs, but estimates of these impacts in contemporary, national samples are lacking. Earlier analyses of United States Renal Data System (USRDS) registry data for transplant recipients in 1994 to 1997 found that a diagnosis of septicemia was associated with an average six-year reduction in subsequent patient survival (9). Based on data from the same period, Abbott et al identified a 64% increase in subsequent mortality after pneumonia hospitalization among transplanted patients (10). While some single center studies and a meta-analysis report no impact of urinary tract infections (UTI) on patient and allograft survival (11–14), UTI diagnosis was associated with a 33% increase in death among a national sample in 1996 to 2000, and outpatient UTI was associated with increased risk of graft loss in that cohort (15).
In addition to survival implications, infections also increase the intensity and cost of post-transplant care. UTI, respiratory tract infections, and sepsis rank among the ten most common causes of re-hospitalization in the first and second years after kidney transplantation (16, 17). In a prior study of recipients in 1995 to 2001, Medicare costs in the first year post-transplant rose $29,787 in those who developed sepsis and $18,107 in those with pneumonia, and an additional $10,964 in patients who had evidence of both infections (18). Moreover, the cost impact of sepsis and pneumonia persisted beyond the first year post-transplantation (18, 19). The cost implications of UTI have not been previously addressed.
Importantly, these studies were performed in a prior era when induction, tacrolimus, mycophenolate mofetil (MMF) and sirolimus were not widely used and characteristics of transplant recipients differed somewhat from recipients in current practice (16). Given the shift to more potent immunosuppressive therapies, increased average age and medical complexity of recipients along with changes in practice patterns, we examined the clinical and economic implications of important first-year infections in a recent, national sample of kidney transplant recipients. Using USRDS data for Medicare-insured United States (U.S.) kidney transplant recipients that integrates national transplant registry with Medicare billing claims, we sought to assess the clinical correlates of UTI/pyelonephritis, pneumonia and sepsis in the first-year after transplant, three leading infectious complications captured in Medicare claims data, and to quantify associated impacts on post-transplant patient survival, graft survival, and Medicare expenditures.
METHODS
Data Source, Study Samples and Approvals
Study data were drawn from records of the USRDS, which integrate Organ Procurement and Transplantation Network (OPTN) records with Medicare billing claims. The primary study sample comprised recipients of first, single-organ kidney transplants in the U.S. from 2000 to 2011 with Medicare as the primary payer at time of transplantation (20). The similarities and differences of patients in the USRDS with and without Medicare as their primary payer have been described previously (21). The study was approved by the Saint Louis University and Johns Hopkins University Institutional Review Boards, and by the USRDS.
Infection Event Definitions
Diagnoses of key infections in the first year after transplant were identified by billing claims with corresponding ICD9-CM diagnosis codes for UTI/pyelonephritis, pneumonia, and sepsis (Appendix 1). Claims from a hospitalization include diagnoses associated with all physician encounters and procedures recorded during the course of the admission. We required one inpatient claim or two outpatient claims on separate dates to define serious complications, as performed in previous studies of claims data to identify these conditions in the kidney transplant population (18, 21–23). Patients were categorized as having a single infection type alone or combinations over the first year post-transplant.
Outcome and Covariate Definitions
The primary clinical outcomes of interest were time to death and time to all-cause graft loss. Mortality was defined as death from any cause. Graft failure was defined as the earliest reported date of return to maintenance dialysis or “preemptive” re-transplantation. Patients were censored from survival analyses at the date of their last expected follow-up or end of study data (December 2013).
The primary economic measure was actual payments for all healthcare services made by Medicare. Payments were evaluated during the first year, and then in the second through third year after transplantation. The cost analysis was limited to three years, as Medicare transplant benefits expire at three years except in the cases of people age ≥65 years or in those with certain disabilities. Patient costs were included in analysis of an interval if: 1) the recorded Medicare eligibility extended continuously from the beginning to the end of the period, or if 2) Medicare eligibility ended in an interval because of death or graft loss. Monetary figures were adjusted to the prices in the year 2011 Medical Care Component of the Consumer Price Index (24).
Baseline recipient demographic and clinical characteristics, donor traits and transplant factors were included as reported by transplant centers to the OPTN registry (Table 1). Immunosuppression information included induction regimen and maintenance agents prescribed at transplant discharge, but doses, drug levels and use of immunosuppression after discharge were not available.
Table 1.
Associations of baseline recipient, donor and transplant factors with study first-year infection categories.
| Full sample (N=60,702) |
Any Infection (n=27,139) |
UTI alone (n=14,817) |
Pneumonia alone (n=3,451) |
Sepsis alone (n=2,498) |
UTI & Pneumoniaa (n=1,456) |
UTI & Sepsisa (n=1,884) |
Pneumonia & Sepsis (n=1,977) |
UTI, Pneumonia & Sepsis (n=1056) |
|
|---|---|---|---|---|---|---|---|---|---|
| % | aOR | aOR | aOR | aOR | aOR | aOR | aOR | aOR | |
| Recipient Characteristics | |||||||||
| Age (yrs) | |||||||||
| <18 | 4.0% | 1.07 | 0.90 | 1.38* | 1.38* | 1.79* | 0.87 | 1.26 | 1.24 |
| 18–30 | 11.1% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 31–44 | 24.6% | 0.95 | 0.92* | 0.95 | 1.01 | 0.99 | 0.87 | 1.23 | 1.04 |
| 45–59 | 36.3% | 1.10* | 0.97 | 1.16* | 1.28* | 1.56† | 1.03 | 1.69‡ | 1.99‡ |
| >=60 | 24.1% | 1.61‡ | 1.28‡ | 1.77‡ | 1.66‡ | 2.83‡ | 1.60‡ | 2.92‡ | 4.17‡ |
| Female sex | 39.5% | 2.05‡ | 2.58‡ | 1.09* | 1.36‡ | 2.24‡ | 2.36‡ | 1.19* | 2.00‡ |
| Race | |||||||||
| White | 57.6% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Black | 30.2% | 0.96* | 0.99 | 0.90* | 0.94 | 0.79† | 0.93 | 0.88* | 0.85* |
| Other | 12.2% | 0.89† | 0.93* | 0.86* | 0.89 | 0.80* | 0.86 | 0.83* | 0.82 |
| BMI (kg/m2) | |||||||||
| >18.5 | 4.8% | 1.12* | 1.11* | 1.24* | 1.13 | 0.96 | 1.05 | 1.12 | 1.17 |
| 18.5 to 25 | 35.3% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 25 to <30 | 32.6% | 0.95* | 0.99 | 0.83‡ | 0.94 | 0.91 | 1.06 | 0.90 | 0.90 |
| >=30 | 27.4% | 1.03 | 1.06* | 0.88* | 1.12* | 1.04 | 1.24† | 0.89 | 1.12 |
| Cause of ESRD | |||||||||
| Diabetes | 23.9% | 1.17‡ | 1.10* | 1.14* | 1.38‡ | 1.16 | 1.52‡ | 1.13 | 1.34* |
| Glomerulonephritis | 18.4% | 0.81‡ | 0.83‡ | 0.84* | 0.80† | 0.79* | 0.78* | 0.68‡ | 0.69* |
| Hypertension | 22.5% | 0.91‡ | 0.96 | 0.91 | 0.92 | 0.84* | 0.82* | 0.73‡ | 0.83 |
| Polycystic kidney disease | 5.9% | 0.79‡ | 0.91* | 0.68‡ | 0.78* | 0.52‡ | 0.85 | 0.57‡ | 0.48‡ |
| Other | 29.2% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Pretransplant dialysis | |||||||||
| Preemptive | 6.6% | 0.83‡ | 0.86† | 0.74* | 0.80* | 0.98 | 0.67* | 0.81* | 0.70 |
| >0–24 | 21.6% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 25–36 | 13.5% | 1.10* | 1.08* | 1.10 | 1.04 | 1.29* | 1.14 | 1.12 | 1.32* |
| >=37 | 58.3% | 1.20‡ | 1.14‡ | 1.18* | 1.22† | 1.22* | 1.34‡ | 1.28† | 1.81‡ |
| Comorbidities | |||||||||
| ASCVD | 13.1% | 1.18‡ | 1.09* | 1.19† | 1.20* | 1.20* | 1.18* | 1.55‡ | 1.27* |
| COPD | 1.1% | 1.31* | 1.00 | 2.22‡ | 0.96 | 1.64* | 0.89 | 1.97‡ | 1.73* |
| Smoking | 0.3% | 1.03 | 0.96 | 0.82 | 0.99 | 1.43 | 1.72 | 1.27 | 1.39 |
| Peak PRA | |||||||||
| < 10 | 70.9% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 10–79 | 20.0% | 1.07* | 1.02 | 1.13* | 1.22† | 1.06 | 1.16* | 1.13 | 1.12 |
| >=80 | 9.1% | 1.17‡ | 1.01 | 1.29† | 1.46‡ | 1.18 | 1.21* | 1.78‡ | 1.52† |
| HLA mismatches | |||||||||
| Zero A, B, and DR | 10.6% | 0.92* | 0.99 | 0.88* | 0.89 | 0.95 | 0.83* | 0.72† | 0.87 |
| Zero DR | 16.2% | 0.95* | 0.98 | 0.93 | 0.87* | 1.04 | 1.04 | 0.79† | 0.84 |
| Donor Type | |||||||||
| Living | 26.5% | 0.82‡ | 0.88‡ | 0.75‡ | 0.76‡ | 0.74† | 0.80* | 0.59‡ | 0.76* |
| SCD | 50.4% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| ECD | 17.6% | 1.17‡ | 1.08* | 1.24† | 1.23* | 1.33† | 1.27* | 1.09 | 1.43† |
| DCD | 5.5% | 1.06 | 0.95 | 1.20* | 1.22* | 1.49† | 1.10 | 1.22* | 1.24 |
| Donor Characteristics | |||||||||
| Age, mean (SD), yr | 38.1(15.6) | 1.00‡ | 1.00 | 1.00* | 1.01‡ | 1.01* | 1.01* | 1.01‡ | 1.01† |
| Female | 45.4% | 1.02 | 1.01 | 0.99 | 1.06 | 1.01 | 1.13* | 0.97 | 0.99 |
| Race | |||||||||
| White | 74.4% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Black | 14.3% | 1.10† | 1.03 | 1.13* | 1.29‡ | 1.15 | 1.10 | 1.36‡ | 1.37† |
| Other | 11.3% | 1.08* | 1.02 | 1.08 | 1.12 | 1.27* | 1.32† | 1.17 | 0.96 |
| Cytomegalovirus sero-pairing | |||||||||
| Donor −/Recipient − | 13.1% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Donor −/Recipient + | 21.9% | 1.00 | 0.98 | 1.05 | 0.97 | 1.07 | 1.02 | 1.05 | 0.90 |
| Donor + /Recipient + | 40.8% | 1.01 | 1.00 | 1.01 | 0.98 | 1.04 | 1.10 | 1.12 | 0.85 |
| Donor + / Recipient − | 16.5% | 1.08* | 0.98 | 1.16* | 1.14 | 1.25* | 1.20* | 1.30* | 1.28* |
| Induction | |||||||||
| No Induction | 32.9% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Thymoglobulin | 38.2% | 0.96* | 1.01 | 0.92* | 0.87* | 1.00 | 0.87* | 0.92 | 0.79* |
| IL2R-Ab | 28.5% | 0.90‡ | 0.92* | 0.91* | 0.91 | 0.90 | 0.93 | 0.84 | 0.73† |
| OKT3 | 0.4% | 0.96 | 0.78 | 0.88 | 0.94 | 0.73 | 1.59 | 1.29* | 1.10 |
| Steroids at discharge | 77.7% | 1.18‡ | 1.13‡ | 1.16* | 1.15* | 1.23* | 1.48‡ | 1.37‡ | 1.30* |
| Maintenance ISx at discharge | |||||||||
| Tacrolimus and MMF | 61.9% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Sirolimus and CNI | 5.0% | 1.19‡ | 1.06 | 1.43‡ | 1.42† | 1.23 | 1.33* | 1.54‡ | 1.62† |
| Sirolimus without CNI | 5.4% | 1.29‡ | 1.01 | 1.65‡ | 1.55‡ | 1.42* | 1.36* | 2.35‡ | 1.77‡ |
| Other | 27.7% | 1.06* | 0.95* | 1.23‡ | 1.25‡ | 1.00 | 1.13* | 1.31‡ | 1.22* |
| Year of Transplant | |||||||||
| 2000–2005 | 59.1% | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 2006–2011 | 40.9% | 1.13‡ | 1.16‡ | 0.98 | 0.99 | 1.11 | 1.28‡ | 1.22† | 1.36‡ |
P-values:
P 0.02–0.04;
P 0.0001–0.01;
P < 0.0001
Composites including UTI consider UTI up to/concomitant with other events
ASCVD, atherosclerotic cardiovascular (coronary, cerebral or peripheral vascular) disease; CNI, calcineurin inhibitor; COPD, chronic obstructive pulmonary disease; IL2R-Ab, inter-leukin-2 receptor antibodies; PRA, panel reactive antibody ; UTI, urinary tract infection
Statistical Analyses
Data management and analysis were performed with SAS for Windows software, version 9.3 (SAS Institute Inc., Cary, NC). Distributions of baseline traits in the full study sample were summarized as proportions. We performed multivariate logistic regression to identify independent correlates (adjusted odds ratios, aOR) of first-year infection categories.
Associations of first-year infection events with subsequent mortality and all-cause graft loss risks (adjusted hazards ratio, aHR) were estimated with time-varying, multivariate Cox regression including adjustment for recipient, donor, and transplant clinical factors captured in the OPTN registry (as listed in Table 1). Time-varying models allow estimation of the relative risks of an outcome associated with post-transplant events (infections in this case), as previously illustrated in the transplant literature (25–27). In the case of infection categories including multiple types, risk was estimated following the last diagnosis date in the group. Based on a priori and empirical evidence of lower clinical impact of UTI/pyelonephritis, this infection was considered as part of a combination category if it preceded or was concomitant with sepsis or pneumonia. The risk of subsequent death and graft loss associated with first-year infections was partitioned as within or after the first transplant anniversary.
The marginal cost impacts of first-year infection categories on costs in year 1 and in years 2–3 after transplant were computed by ordinary least squares (OLS) regression equations as: E(Y) =β1X1 + β2X2 +… βkXk, where E(Y) = Medicare payments within a period of interest, Xk = the value of a given predictor variable, and βk = the marginal costs associated with a 1-unit change in a given variable after adjustment for other observed factors in the model. Thus, for binary variables such as infections, the βk parameters quantify the marginal costs associated with the infection categories, adjusted for the recipient, donor and transplant factors. Cost period models were also adjusted for the impact of death and graft failure within the period of interest, as previously described (19, 28). Predicted costs at year 1 and years 2–3 post-transplant based on first-year infection status were computed from the resulting multivariable regression equations, with values of covariates set to the sample averages.
RESULTS
Frequency and Clinical Correlates of Infections in the First-Year Post-Transplant
Among 60,702 eligible transplant recipients, 39.5% were women; 57.6% were Caucasian, 30.2% African American, and 12.2% other races (Table 1). Transplants were donated from standard criteria deceased donors in 50.4%, other deceased donors in 23.1%, and living donors in 26.5%. Induction immunosuppression was administered in 67.1% of transplants across the study period; 78% of recipients received steroids at discharge, and tacrolimus with MMF was the most common maintenance immunosuppression regimen (administered to 61.9% of recipients). In the first year after transplantation, 44.7% (n=27,139) developed any study infection including the following patterns over the year: UTI alone, 24.4%; pneumonia alone, 5.7%; sepsis alone, 4.1%; UTI and pneumonia, 2.4 %; UTI and sepsis, 3.1%; pneumonia and sepsis, 3.3%; and UTI, pneumonia and sepsis, 1.7%. Overall, 32.0%, 13.0% and 12.0% of recipients were affected by UTI, pneumonia, and sepsis, respectively. Distributions of subcategories of infections identified in the first year post-transplant are provided in Appendix 2.
Compared to younger adults, recipients aged 45–59 years had an increased likelihood of developing any study infection (Table 1). Recipients age 60 years and older had a 61% higher adjusted likelihood of all the first-year infection categories compared to recipients aged 18–30 years. Women had twice the odds of developing any infection compared with men, driven by more than twice the odds of infection categories that included UTI alone or in combination. Obese (BMI >30 kg/m2) transplant recipients had an increased likelihood of developing a UTI (OR=1.06), sepsis with UTI (OR 1.24) and sepsis alone (OR 1.12), but lower likelihood of pneumonia (OR 0.88). Recipient chronic obstructive pulmonary disease was associated with a 31 % increased risk of any first-year infection (aOR 1.31) including twice the likelihood of pneumonia alone or with sepsis, while the presence of atherosclerotic cardiovascular disease was associated with 18% increased likelihood of any infection. Smoking was reported infrequently, perhaps due to the common requirement for smoking cessation as a criterion for transplant candidacy, and we did not detect significant associations of smoking with infection risk. Patients with diabetic renal failure had increased likelihood of developing any study infection, driven by increased likelihood of all categories including sepsis. Recipients of preemptive transplants had 17% lower adjusted odds of developing a first year infection (aOR 0.83) compared with patients who were on dialysis for up to 2 years before transplant, while patients who received more than 3 years of dialysis had the highest odds of developing infectious complications.
Compared to recipients of SCD allografts, recipients of ECD and DCD kidneys had increased odds of all infectious complications except for UTI, while recipients of live donor transplants had lower adjusted odds of any infectious complication (aOR 0.82). Use of female donors was associated with modestly higher odds of UTI with sepsis compared to male donors (aOR 1.13). Compared to patients with low-risk Cytomegalovirus sero-pairing (Donor(D)−/Recipient (R)−), those with high-risk (D+/R−) sero-pairing had 8% higher risk for any infectious complication, and 16%–30% increased risk of infections including pneumonia, or sepsis with another infection. Sirolimus-based maintenance therapy was associated with a 19%–29% higher odds of any study infection compared to tacrolimus and MMF-based regimens, with appearance of somewhat higher risk when sirolimus was used without compared to with a calcineurin inhibitor (CNI) especially for pneumonia with sepsis (135% risk increase for sirolimus without CNI, and 54% risk increase for sirolimus with CNI, compared to reference of tacrolimus and MMF). Patients who received steroids at discharge had increased likelihood of any study infection and nearly all categories, with highest risk for combinations that included sepsis. After adjustment for maintenance immunosuppression and other recipient and transplant factors, thymoglobulin and inter-leukin-2 receptor antibody (IL2R-Ab) induction immunosuppression agents were associated with approximately 4–10% decreased likelihood of first-year infections compared with no induction. OKT3 was used in less 1% of this cohort, but was associated with significantly increased with of pneumonia with sepsis. “Era effects” were also noted, with increased odds of any study infection (predominantly driven by UTI) for patients transplanted in 2006–2011 compared to those transplanted in 2000–2005.
Associations for First-Year Infections with Death and Allograft Loss
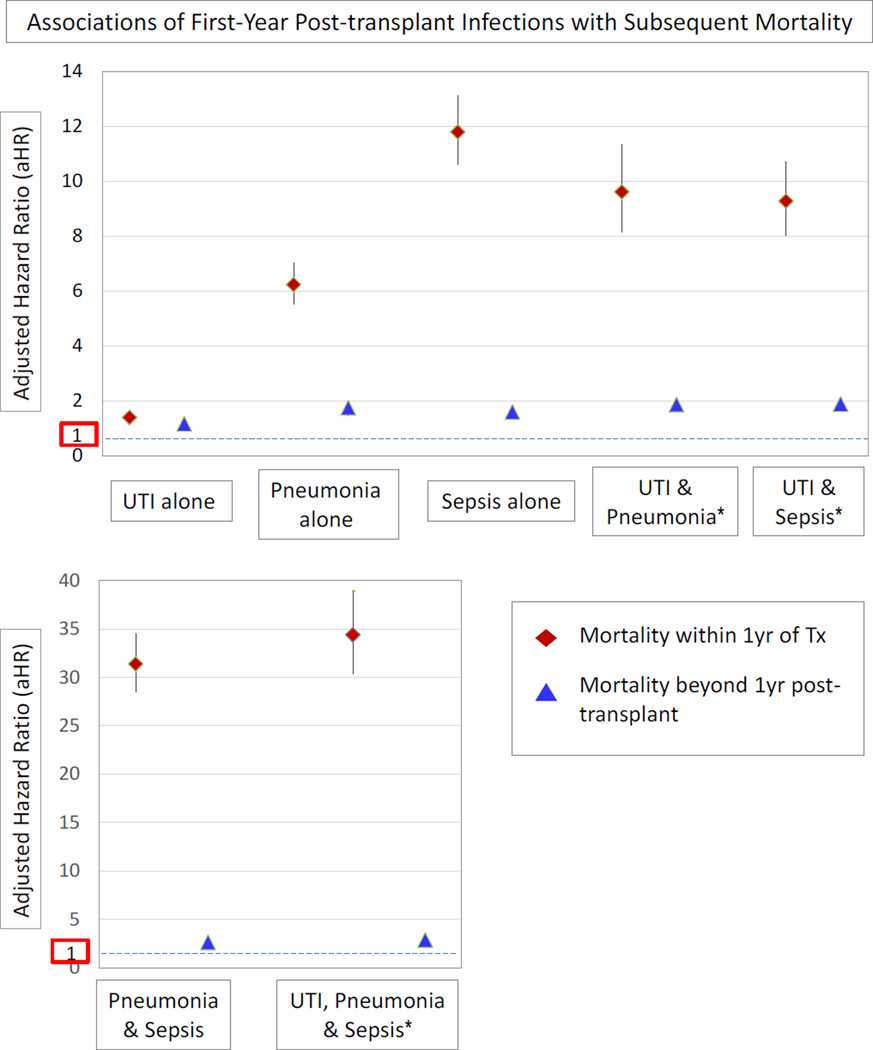
Median post-transplant follow-up of the cohort was 4.5 years. Patient survival at 5 years post-transplant was 73.3% and 83.1% among deceased donor and living donor allograft recipients, respectively. All the first-year study infections were associated with increased risk of death within the first year post-transplant. Relative risks of death compared to no infection ranged from 41% risk increase with UTI alone (aHR 1.41, 95% CI 1.25–1.56), six-fold risk with pneumonia alone (aHR 6.23, 95% CI 5.54–7.02), to nearly 12-fold risk with sepsis alone (aHR 11.79, 95% CI 10.61–13.12) (Figure 1, Appendix 3). Adjusted mortality risk was highest in those who developed more than one study infection in the first year, with nearly 9-times the risk of death in those with UTI and pneumonia (aHR 9.61, 95% CI 8.15–11.33) or UTI and sepsis (aHR 9.27, 95%CI 8.03–10.71) compared to recipients without a study infection. Patients who developed pneumonia and sepsis in the first year had 31-times the adjusted mortality of those without study infections (aHR 31.37, 95%CI 28.51–34.50), while risk was increased 34-fold in those with UTI, pneumonia and sepsis (aHR 34.38, 95% CI 30.35–38.95). Significant mortality risks associated with first-year infections persisted at a lower level beyond the first transplant anniversary, from a modest 16% later risk for those with UTI alone, to almost 3-times the risk of later death in those with combined UTI, pneumonia and sepsis (aHR 2.85, 95% CI 2.54–3.20). After adjustment for the impact of infections, preemptive transplantation, use of thymoglobulin or IL2R-Ab induction therapy and of steroids were associated with lower mortality risk, while advancing age, underweight BMI, ESRD due to diabetes, high levels of sensitization (PRA ≥80%), receipt of an expanded criteria allograft, and sirolimus-based immunosuppression (with and without CNIs) were associated with increased mortality (Appendix 3). The first-year infection categories were associated with similar patterns of increased risk of all-cause graft loss during the first year of transplantation, and multiple infections had the greatest impacts (Appendix 4).
Figure 1.
Adjusted associations of first-year infections with risk of death after transplantation. Adjusted for all recipient, donor and transplant factors in Table 1. Please see Appendix 2 for complete survival regression results.
First-Year Infections and Healthcare Expenditures
After adjustment for baseline recipient, donor and transplant factors, as well as for death or graft failure events in the period, all of the study infection categories had significant impacts on first-year costs, ranging from a $17,691 marginal cost increase for UTI alone, $39,593 for pneumonia alone and $53,965 for sepsis alone (Table 2). Marginal cost associations were higher for those who experienced more than one infection in the first year. First-year infections were also associated with significant downstream cost effects in years 2–3 after transplant, ranging from $8,372 for UTI alone to $36,000–$38,000 for pneumonia with sepsis, and for combined UTI, pneumonia and sepsis. Associations of other baseline recipient, donor and transplant factors with post-transplant costs in the first year and in years 2–3 post-transplant are provided in Appendix 5.
Table 2.
Adjusted associations of first-year infections with marginal costs in the first year, and in years 2–3 after transplantation.*
| 1 yr costs (including Tx) | 2–3 yr Costs | |
|---|---|---|
| Parameter Estimate, $ per period |
Parameter Estimate, $ per period |
|
| Infection Events | ||
| UTI alone | 17,691‡ | 8,372‡ |
| Pneumonia alone | 39,593‡ | 15,247‡ |
| Sepsis alone | 53,965‡ | 20,676‡ |
| UTI + Pneumonia | 60,615‡ | 25,171‡ |
| UTI + Sepsis | 65,195‡ | 28,191‡ |
| Pneumonia + Sepsis | 123,242‡ | 38,053‡ |
| UTI + Pneumonia + Sepsis | 134,773‡ | 36,055‡ |
Adjusted for all recipient, donor and transplant factors in Table 1. Please see Appendix 4 for complete cost regression results.
P-values compared to no infection:
P 0.0001–0.001;
P < 0.0001
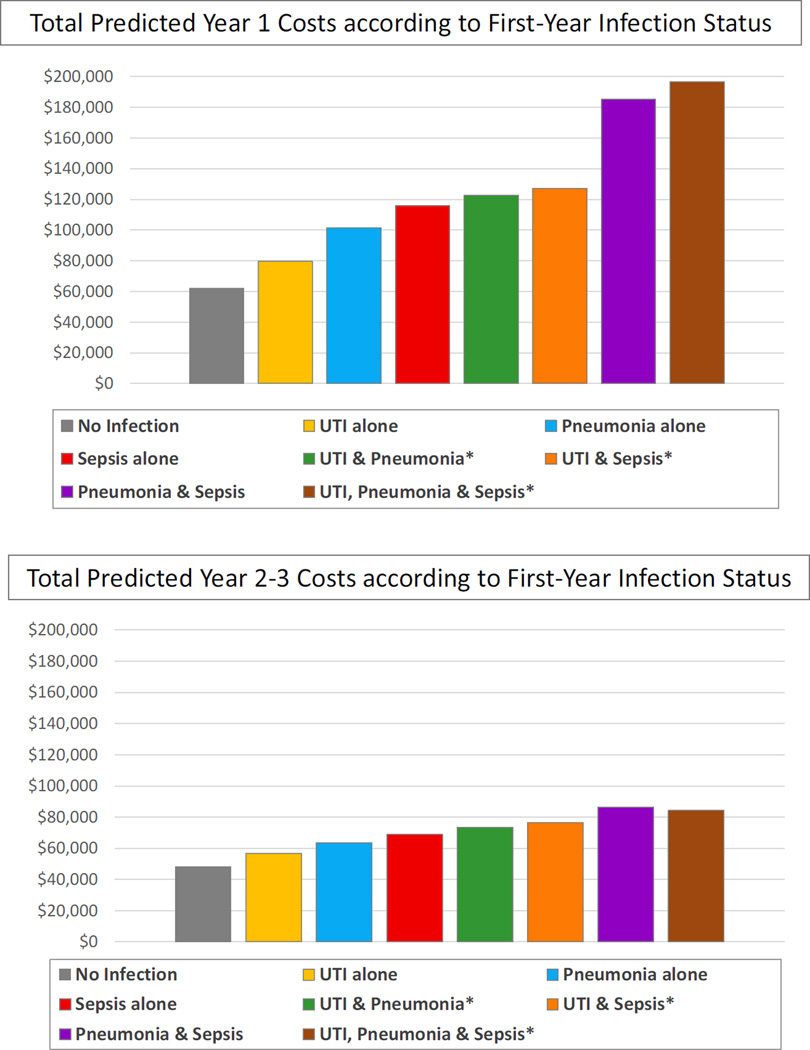
Total predicted costs in the first year post transplant rose from $61,909 in those with no infections to the following levels according to infection categories: UTI alone, $79,600; pneumonia alone, $101,502; sepsis alone, $115,874; UTI and pneumonia, $122,525; UTI and sepsis, $127,104 (Figure 2). Total predicted first-year costs increased to $185,151 for those who experienced pneumonia and sepsis, and climbed to $196,682 for those who developed UTI, pneumonia and sepsis in year one. Total expenditures in years 2–3 post-transplant were also higher after first-year infections, although differences were smaller than observed for first-year costs, ranging from $48,235 in those with no infections, to $56,607 in those with first-year UTI alone, to $84,000–$86,000 in those with combined first-year pneumonia and sepsis, or UTI, pneumonia and sepsis (Figure 2).
Figure 2.
Total predicted period costs according to first-year infection status for an average transplant recipient.
DISCUSSION
While long-term graft survival after kidney transplant has continued to improve in recent decades (29), the improvement has not been commensurate with reductions in the risk of acute rejection. Given the dramatic reduction in early immunological graft failure, efforts to reduce non-immune complications of transplantation including infections have become an important goal in optimizing post-transplant care. Previous registry-based studies have demonstrated adverse clinical and economic impacts of some post-transplant infections (15, 18). However, the patients examined in those cohorts were transplanted 15 to 20 years ago. In the current era, transplant recipients are older on average and have higher comorbidity burdens; moreover, clinical practice has evolved to include more common use of induction and potent maintenance immunosuppressive therapies (30).
We examined USRDS registry data for Medicare-insured kidney transplant recipients in 2000 to 2011 to quantify the clinical and economic impacts of first-year UTI, pneumonia and sepsis in a contemporary national sample, and observed several key findings. Consistent with prior single center reports (7, 8, 31), nearly 45% of the national cohort experienced at least one of these infection events in the first year after transplantation, including UTI in 32%, pneumonia in 13%, and sepsis in 12%. Older recipients, women, those with diabetic renal failure, recipients of non-standard donor organs, and those who received sirolimus-based immunosuppression or steroids at discharge were more likely to develop first-year infections. All study infections were associated with increased risk of subsequent death in the first year, ranging from 41% risk increase with UTI alone, 6- to 12-fold risk with pneumonia or sepsis alone, to 34-fold risk after all three infections. Finally, all of the study infections significantly increased first-year post-transplant costs, ranging from a $17,691 marginal cost increase for UTI alone, $39,593 for pneumonia alone and $53,965 for sepsis alone, to $134,773 for those with UTI, pneumonia and sepsis in the first year. Clinical and economic impacts persisted beyond the first anniversary of the transplant.
Identification of UTI as a particularly common early post-transplant infection is consistent with prior studies (7, 8, 32). Interestingly, we observed increased likelihood of UTI events in more recent years. This finding may reflect the rising incorporation of prophylactic stenting over selective ureteral stenting in surgical protocols. A recent Cochrane review concluded that while use of prophylactic ureteral stenting has reduced the incidence of major urologic complications after kidney transplantation, the practice has been associated with increased risk of UTI (33). Some single center studies have questioned whether UTI significantly affects long-term outcomes (11–13), but using a large national cohort, we were able to demonstrate that first-year UTI adversely impacts subsequent patient and graft survival. Bacteriuria and UTI in kidney transplant recipients have been associated with elevated local and systematic cytokine levels (34, 35), which may in part mediate detrimental consequences for graft and patient survival. While the relative impacts of UTI on death and graft loss are lower than the consequences of pneumonia or sepsis at an individual case level, the high frequency of UTI produces large population-level consequences, making UTI an important target for prevention especially in those at high risk (32).
Not surprisingly, pneumonia and sepsis were associated with large increases in patient mortality and all-cause graft loss, and the risk was extremely high among patients who experienced combined events in the first year. In addition to patient factors including older age and diabetic ESRD, risks of these infections were correlated with longer pre-transplant dialysis duration, use of non-standard organs, maintenance steroids and early use of sirolimus-based maintenance therapy. Sirolimus-based immunosuppression has been associated with an increased risk of infectious complications in a prior single-center retrospective study and in a randomized controlled trial (7, 36), while other randomized trials (not powered for assessment of complications) have reported numerically higher although statistically similar infection rates in patients receiving sirolimus compared with other maintenance regimens (37–39). Interestingly, we also observed associations of induction therapy with lower infection risk. Induction therapy can allow for reduction in cumulative post-transplant immunosuppression (eg, lower anti-metabolite dosing) which may explain the lower likelihood of early post-transplant infections. Targeting prophylactic and management strategies to groups at highest risk of post-transplant infections, including older recipients, women and diabetic patients, may help reduce the incidence of first-year infections, and associated clinical and economic consequences. Strategies warranting evaluation include early ureteral stent removal and management, identification and management of bladder dysfunction, diabetes control, and extended use of prophylactic antimicrobial therapies for patients at increased risk of post-transplant infections (5, 40, 41).
Post-transplant complications are associated with significant and substantial increases in Medicare expenditures for the treatment of both infectious and immunologic complications. Our study provided updated estimates of the cost impacts of pneumonia and sepsis generated by Kutinova et al for patients transplanted in the late 1990s (18), and adds new estimates of the cost implications of UTI. The economic implications are similar for mild infectious conditions (UTI alone) and the cost of acute rejection not requiring antibody therapy in the first year post-transplant ($14,122 per case) (19). Rejection requiring intravenous cell depleting antibody treatment incurs higher costs ($22,407 per case), but is less expensive than treatment of pneumonia or sepsis (19). Finally, estimated costs of humoral rejection requiring intensive therapies including high-dose intravenous immunoglobulin and rituximab exceed $50,000–$100,000 per case (42), similar to costs of sepsis or multiple infections. Although the costs per case appear similar for rejection and infections of graded severity, the total economic burden appears substantially higher for infections as the incidence of UTI (32%), pneumonia (13%) and sepsis (12%) far exceed incidence of acute rejection without antibody treatment (6.9%) or with antibody treatment (2.5%) (19), and humoral rejection (0.7%–1.9% of compatible transplants) (43). Moreover, the economic impact of post-transplant infection complications is expected to increase with the greater use of immunosuppression to prevent rejection in highly sensitized patients who are prioritized in the new allocation system (4, 44). The economic impacts of early infectious complications are particularly relevant for contemporary transplant programs given the lack of reimbursement under global insurance contracts.
Finally, we found that the clinical and economic consequences of early UTI, pneumonia and sepsis persist beyond the first year post-transplant. Such “downstream effects” of early complications have been previously been demonstrated for infections and events such as acute rejection (18, 19). Hence strategies that reduce the burden of early infectious complications have the potential to improve patient survival, allograft survival and costs of care beyond the first transplant anniversary.
Limitations of the current study include use of billing claims as surrogate measures for diagnoses. Laboratory values (such as blood counts) and diagnostic test results (such as cultures and chest x-rays) were not available to adjudicate the clinical outcomes in our study. While coding errors are possible, the use of claims data provides the sole option for long-term, nationally representative data collection given that infection events are not tracked in the national registry. Medicare billing structure does not allow resolution of additional procedures relevant to infection risk from the transplant surgery procedure (e.g., placement of stents, drains and catheters). We also lacked information on some potential risk factors such as prior urologic surgery, use of maintenance immunosuppression over time, drug levels, and use of co-medications. In addition, kidney transplant recipients who have Medicare as their primary insurer may differ systematically from those who use other reimbursement systems. However, Medicare claims are particularly relevant to research among kidney transplant recipients because, unlike the eligibility requirements of age >65 or disability in the general population, renal allograft recipients are offered disease-specific Medicare entitlement and Medicare is the largest single insurer in this population. As a result, Medicare billing claims have been used to study a variety of complications after kidney transplantation (18, 21, 45, 46). We recently applied the coding algorithms used in the current study to investigate UTI, pneumonia and sepsis after ABO-incompatible live donor transplantation (23). Regarding our costs regression approach, alternatives to our OLS models, such as regressions estimating the determinants of the natural log of Medicare payments, may be more efficient but also may produce biased estimates and are difficult to interpret. Because we have access to cost data for very large samples, we employ the unbiased estimator. Our past work has demonstrated nearly identical results with OLS cost regression and regressions on the natural log of Medicare payments (47), and OLS has become our standard in analyses of the economic impact of complications in transplantation (19, 28).
In conclusion, UTI, pneumonia and sepsis in the first-year post-transplant are associated with increased risks of death, allograft loss and Medicare spending in contemporary transplant practice. The consequences appear to be greatest for patients who experience multiple types of infections. Overall, UTI remains the most common first-year infection, and the likelihood of post-transplant UTI appears to have increased over the last few years. Patients at particularly increased risk of first-year infections include older recipients, women, those with diabetic renal failure, recipients of non-standard donor organs, and those managed with sirolimus-based immunosuppression. Development of management strategies to minimize post-transplant infectious complications without an increase in the risk of immunological graft failure is an important priority. Given the large population-level impact of UTI, evaluation of the efficacy of antimicrobial prophylaxis and risk factor management including ureteral stent protocols are especially warranted.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government. This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) RC1-DK86450.
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
ABBREVIATIONS
- aHR
adjusted hazards ratio
- aOR
adjusted odds ratio
- CMV
Cytomegalovirus
- DCD
Donation after cardiac death
- ESRD
End-stage renal disease
- ECD
Expanded criteria donor kidney
- MMF
Mycophenolate mofetil
- OLS
Ordinary least squares
- OPTN
Organ Procurement and Transplantation Network
- SCD
Standard criteria donor
- USRDS
United States Renal Data System
- UTI
Urinary tract infection
Footnotes
Author Roles and Support:
Participated in study design, interpretation, and writing of the paper
Participated in study design, interpretation, and writing of the paper. Support provided to the author’s institution by the NIH/NIDDK
Participated in study design, acquisition of data and regulatory approvals, data analysis, and writing of the paper. Support provided to the author’s institution by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Participated in data analysis and manuscript preparation. Support provided to the author’s institution by the NIH/NIDDK
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
CONFLICTS OF INTEREST: The authors have no conflicts of interest related to this work.
An abstract describing portions of this work was presented at the 2015 American Transplant Congress in Philadelphia, PA, May 3, 2015.
REFERENCES
- 1.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 2.Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L'Italien G, Schnitzler MA. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94(4):369–376. doi: 10.1097/TP.0b013e318259407f. Epub 2012/07/28. [DOI] [PubMed] [Google Scholar]
- 3.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(6):1226–1235. doi: 10.1111/j.1600-6143.2011.03539.x. Epub 2011/05/14. [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. Epub 2015/01/30. [DOI] [PubMed] [Google Scholar]
- 5.Horwedel TA, Bowman LJ, Saab G, Brennan DC. Benefits of sulfamethoxazole-trimethoprim prophylaxis on rates of sepsis after kidney transplant. Transplant infectious disease : an official journal of the Transplantation Society. 2014;16(2):261–269. doi: 10.1111/tid.12196. Epub 2014/03/14. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Renal Data System (USRDS) Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Altas of ESRD, Transplantation, Figure 7.30; [Accessed September 1, 2015]. 2010 Annual Data Report. Available at: http://www.usrds.org/2010/pdf/V2_07.pdf. [Google Scholar]
- 7.Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clinical transplantation. 2006;20(4):401–409. doi: 10.1111/j.1399-0012.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 8.Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand M. Infectious complications after kidney transplantation: a single-center experience. Transplant Infectious Disease. 2007;9(4):302–309. doi: 10.1111/j.1399-3062.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 9.Abbott KC, Oliver JD, 3rd, Hypolite I, Lepler LL, Kirk AD, Ko CW, et al. Hospitalizations for bacterial septicemia after renal transplantation in the united states. American journal of nephrology. 2001;21(2):120–127. doi: 10.1159/000046234. Epub 2001/05/19. [DOI] [PubMed] [Google Scholar]
- 10.Tveit DJ, Hypolite IO, Poropatich RK, Hshieh P, Cruess D, Hawkes CA, et al. Hospitalizations for bacterial pneumonia after renal transplantation in the United States. Journal of nephrology. 2002;15(3):255–262. [PubMed] [Google Scholar]
- 11.Ariza-Heredia EJ, Beam EN, Lesnick TG, Cosio FG, Kremers WK, Razonable RR. Impact of urinary tract infection on allograft function after kidney transplantation. Clinical transplantation. 2014;28(6):683–690. doi: 10.1111/ctr.12366. [DOI] [PubMed] [Google Scholar]
- 12.Fiorante S, Fernandez-Ruiz M, Lopez-Medrano F, Lizasoain M, Lalueza A, Morales JM, et al. Acute graft pyelonephritis in renal transplant recipients: incidence, risk factors and long-term outcome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(3):1065–1073. doi: 10.1093/ndt/gfq531. [DOI] [PubMed] [Google Scholar]
- 13.Papasotiriou M, Savvidaki E, Kalliakmani P, Papachristou E, Marangos M, Fokaefs E, et al. Predisposing factors to the development of urinary tract infections in renal transplant recipients and the impact on the long-term graft function. Renal failure. 2011;33(4):405–410. doi: 10.3109/0886022X.2011.568137. [DOI] [PubMed] [Google Scholar]
- 14.Green H, Rahamimov R, Gafter U, Leibovitci L, Paul M. Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: a systematic review and meta-analysis. Transplant infectious disease : an official journal of the Transplantation Society. 2011;13(5):441–447. doi: 10.1111/j.1399-3062.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 15.Abbott KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44(2):353–362. doi: 10.1053/j.ajkd.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Lentine KL, Hurst FP, Jindal RM, Villines TC, Kunz JS, Yuan CM, et al. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis. 2010;55(1):152–67. doi: 10.1053/j.ajkd.2009.06.032. Epub 2009/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnitzler MA, Skeans MA, Axelrod DA, Lentine KL, Tuttle-Newhall JE, Snyder JJ, et al. OPTN/SRTR 2013 Annual Data Report: economics. Am J Transplant. 2015;15(Suppl 2):1–24. doi: 10.1111/ajt.13201. Epub 2015/01/30. [DOI] [PubMed] [Google Scholar]
- 18.Kutinova A, Woodward RS, Ricci JF, Brennan DC. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant. 2006;6(1):129–139. doi: 10.1111/j.1600-6143.2005.01156.x. Epub 2006/01/26. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghian A, Schnitzler MA, Axelrod DA, Kalsekar A, L'Italien G, Lentine KL. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241–249. doi: 10.1097/TP.0b013e318255f839. Epub 2012/08/10. [DOI] [PubMed] [Google Scholar]
- 20.Whiting JF, Woodward RS, Zavala EY, Cohen DS, Martin JE, Singer GG, et al. Economic cost of expanded criteria donors in cadaveric renal transplantation: analysis of Medicare payments.[see comment] Transplantation. 2000;70(5):755–760. doi: 10.1097/00007890-200009150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178–185. doi: 10.1034/j.1600-6143.2003.00010.x. Epub 2003/02/27. [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(6):905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 23.Lentine KL, Axelrod D, Klein C, Simpkins C, Xiao H, Schnitzler MA, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation. 2014;98(1):54–65. doi: 10.1097/TP.0000000000000029. Epub 2014/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bureau of Labor Statistics. [Accessed May 15, 2015];Consumer Price Index Databases. Available at: http://www.bls.gov/cpi/data.htm.
- 25.Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC. Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney international. 2003;63(2):647–653. doi: 10.1046/j.1523-1755.2003.00761.x. [DOI] [PubMed] [Google Scholar]
- 26.Lentine KL, Xiao H, Brennan DC, Schnitzler MA, Villines TC, Abbott KC, et al. The impact of kidney transplantation on heart failure risk varies with candidate body mass index. Am Heart J. 2009;158(6):972–982. doi: 10.1016/j.ahj.2009.10.009. Epub 2009/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvalaggio PR, Dzebisashvili N, Pinsky B, Schnitzler MA, Burroughs TE, Graff R, et al. Incremental value of the pancreas allograft to the survival of simultaneous pancreas-kidney transplant recipients. Diabetes Care. 2009;32(4):600–602. doi: 10.2337/dc08-1718. Epub 2009/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnitzler MA, Johnston K, Axelrod D, Gheorghian A, Lentine KL. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91(12):1347–1356. doi: 10.1097/TP.0b013e31821ab993. Epub 2011/06/11. [DOI] [PubMed] [Google Scholar]
- 29.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-Term Renal Allograft Survival in the United States: A Critical Reappraisal. American Journal of Transplantation. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 30.United States Renal Data System. [Accessed May 15, 2015];Chapter 6:Transplantation. 2014 Available at: http://www.usrds.org/2014/download/V2_Ch_06_Transplantation_14.pdf. [Google Scholar]
- 31.Kosmadakis G, Daikos GL, Pavlopoulou ID, Gobou A, Kostakis A, Tzanatou-Exarchou H, et al. Infectious complications in the first year post renal transplantation. Transplantation proceedings. 2013;45(4):1579–1583. doi: 10.1016/j.transproceed.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients--an analysis of USRDS data. Am J Transplant. 2007;7(3):653–661. doi: 10.1111/j.1600-6143.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. The Cochrane database of systematic reviews. 2013;6:CD004925. doi: 10.1002/14651858.CD004925.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi M, Daniel V, Naujokat C, Wiesel M, Hergesell O, Opelz G. Strong inflammatory cytokine response in male and strong anti-inflammatory response in female kidney transplant recipients with urinary tract infection. Transpl Int. 2005;18(2):177–185. doi: 10.1111/j.1432-2277.2005.00007.x. Epub 2005/02/05. [DOI] [PubMed] [Google Scholar]
- 35.Ciszek M, Paczek L, Bartlomiejczyk I, Mucha K. Urine cytokines profile in renal transplant patients with asymptomatic bacteriuria. Transplantation. 2006;81(12):1653–1657. doi: 10.1097/01.tp.0000226072.20185.f8. Epub 2006/06/24. [DOI] [PubMed] [Google Scholar]
- 36.Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67(7):1036–1042. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 37.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 38.Flechner SM, Glyda M, Cockfield S, Grinyo J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant. 2011;11(8):1633–1644. doi: 10.1111/j.1600-6143.2011.03573.x. Epub 2011/06/15. [DOI] [PubMed] [Google Scholar]
- 39.Guerra G, Ciancio G, Gaynor JJ, Zarak A, Brown R, Hanson L, et al. Randomized trial of immunosuppressive regimens in renal transplantation. Journal of the American Society of Nephrology : JASN. 2011;22(9):1758–1768. doi: 10.1681/ASN.2011010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dharnidharka VR, Araya CE, Wadsworth CS, McKinney MC, Howard RJ. Assessing the value of ureteral stent placement in pediatric kidney transplant recipients. Transplantation. 2008;85(7):986–991. doi: 10.1097/TP.0b013e318169bf11. Epub 2008/04/15. [DOI] [PubMed] [Google Scholar]
- 41.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. Epub 2009/10/23. [DOI] [PubMed] [Google Scholar]
- 42.Tanriover B, Wright SE, Foster SV, Roush KS, Castillo-Lugo JA, Fa K, et al. High-dose intravenous immunoglobulin and rituximab treatment for antibody-mediated rejection after kidney transplantation: a cost analysis. Transplantation proceedings. 2008;40(10):3393–3396. doi: 10.1016/j.transproceed.2008.08.131. Epub 2008/12/23. [DOI] [PubMed] [Google Scholar]
- 43.Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015;15(2):489–498. doi: 10.1111/ajt.12982. Epub 2015/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. Journal of the American Society of Nephrology : JASN. 2014;25(8):1842–1848. doi: 10.1681/ASN.2013070784. Epub 2014/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lentine KL, Rocca Rey LA, Kolli S, Bacchi G, Schnitzler MA, Abbott KC, et al. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol. 2008;3(4):1090–1101. doi: 10.2215/CJN.03080707. Epub 2008/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology : JASN. 2005;16(2):496–506. doi: 10.1681/ASN.2004070580. Epub 2004/12/24. [DOI] [PubMed] [Google Scholar]
- 47.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3(5):590–598. doi: 10.1034/j.1600-6143.2003.00082.x. Epub 2003/05/20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.