Abstract
The source of hyperpolarized (HP) [13C]bicarbonate in the liver during metabolism of HP [1‐13C]pyruvate is uncertain and likely changes with physiology. Multiple processes including decarboxylation through pyruvate dehydrogenase or pyruvate carboxylase followed by subsequent decarboxylation via phosphoenolpyruvate carboxykinase (gluconeogenesis) could play a role. Here we tested which metabolic fate of pyruvate contributed to the appearance of HP [13C]bicarbonate during metabolism of HP [1‐13C]pyruvate by the liver in rats after 21 h of fasting compared to rats with free access to food. The 13C NMR of HP [13C]bicarbonate was observed in the liver of fed rats, but not in fasted rats where pyruvate carboxylation and gluconeogenesis was active. To further explore the relative fluxes through pyruvate carboxylase versus pyruvate dehydrogenase in the liver under typical conditions of hyperpolarization studies, separate parallel experiments were performed with rats given non‐hyperpolarized [2,3‐13C]pyruvate. 13C NMR analysis of glutamate isolated from the liver of rats revealed that flux from injected pyruvate through pyruvate dehydrogenase was dominant under fed conditions whereas flux through pyruvate carboxylase dominated under fasted conditions. The NMR signal of HP [13C]bicarbonate does not parallel pyruvate carboxylase activity followed by subsequent decarboxylation reaction leading to glucose production. In the liver of healthy well‐fed rats, the appearance of HP [13C]bicarbonate exclusively reflects decarboxylation of HP [1‐13C]pyruvate via pyruvate dehydrogenase. © 2016 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Keywords: hyperpolarization, bicarbonate, pyruvate carboxylase, pyruvate dehydrogenase, NMR, glutamate, gluconeogenesis, phosphoenolpyruvate carboxykinase
Abbreviations used
- DHAP
dihydroxyacetone phosphate
- GA3P
D‐glyceraldehyde 3‐phosphate
- Gln
glutamine
- Glu
glutamate
- gww
gram wet weight
- HP
hyperpolarized
- IDH
isocitrate dehydrogenase
- α‐KGDH
α‐ketoglutarate dehydrogenase
- MAG
monoacetone glucose
- OAA
oxaloacetate
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- PEP
phosphoenolpyruvate
- PEPCK
phosphoenolpyruvate carboxykinase
- Suc‐CoA
succinyl‐coenzyme A
- TCA
tricarboxylic acid.
Introduction
Multiple alternative pathways are available for metabolism of pyruvate in the liver including carboxylation to oxaloacetate via pyruvate carboxylase (PC), decarboxylation to acetyl‐CoA via pyruvate dehydrogenase (PDH), transamination to alanine via alanine transaminase or reduction to lactate via lactate dehydrogenase. Carbons from pyruvate may enter the tricarboxylic acid (TCA) cycle via either PC or PDH, but the biochemical significance of each pathway is quite different. Pyruvate carboxylation produces oxaloacetate to replenish intermediates of the TCA cycle whereas pyruvate decarboxylation yields acetyl‐CoA for oxidation in the TCA cycle. The relative importance of these pathways has been extensively studied in hepatocytes or rodent models using 14C‐enriched pyruvate 1, 2 and 13C‐enriched pyruvate or lactate 3, 4. Some reports assume that PDH flux is zero 5 whereas others assume that pyruvate metabolism involves both the carboxylation and decarboxylation pathways 2, 4. These differences likely reflect the uncertainties in simultaneously measuring fluxes through both PDH and PC by radiotracer methods as well as substantial influence of nutritional and experimental conditions on the rates of pyruvate metabolism via PDH or PC.
The use of hyperpolarized (HP) [1‐13C]pyruvate in studies of hepatic metabolism using dynamic nuclear polarization has rekindled interest in the fate of pyruvate in the liver. The technique enhanced the sensitivity of MRS dramatically 6 and enabled real‐time detection of hepatic metabolism in vivo 7, 8, 9, but the source of the HP [13C]bicarbonate (H13CO3 −) signal from the liver during metabolism of HP [1‐13C]pyruvate is uncertain. The appearance of HP [13C]bicarbonate could arise from pyruvate metabolism in multiple alternative pathways 7, 10, 11. These include [1‐13C]pyruvate decarboxylation through PDH, carboxylation of [1‐13C]pyruvate followed by reorientation in the fumarate pool and subsequent decarboxylation of oxaloacetate carbon 4 (C4) by phosphoenolpyruvate carboxykinase (PEPCK), or carboxylation through PC to generate [1‐13C]oxaloacetate followed by flux through citrate synthase to generate [6‐13C]citrate and subsequent decarboxylation at the level of isocitrate dehydrogenase (Fig. 1). In a previous study, we found that isolated, perfused mouse livers generate HP [13C]bicarbonate from HP [1‐13C]pyruvate. Knockout of PEPCK eliminated production of HP [13C]bicarbonate and consequently we proposed that the appearance of HP [13C]bicarbonate from the liver might be an index of gluconeogenesis 10. In contrast, other investigators assumed that HP [13C]bicarbonate arises only via flux of HP [1‐13C]pyruvate through PDH 7. The relevance of these findings to liver metabolism in vivo remains to be investigated.
Figure 1.
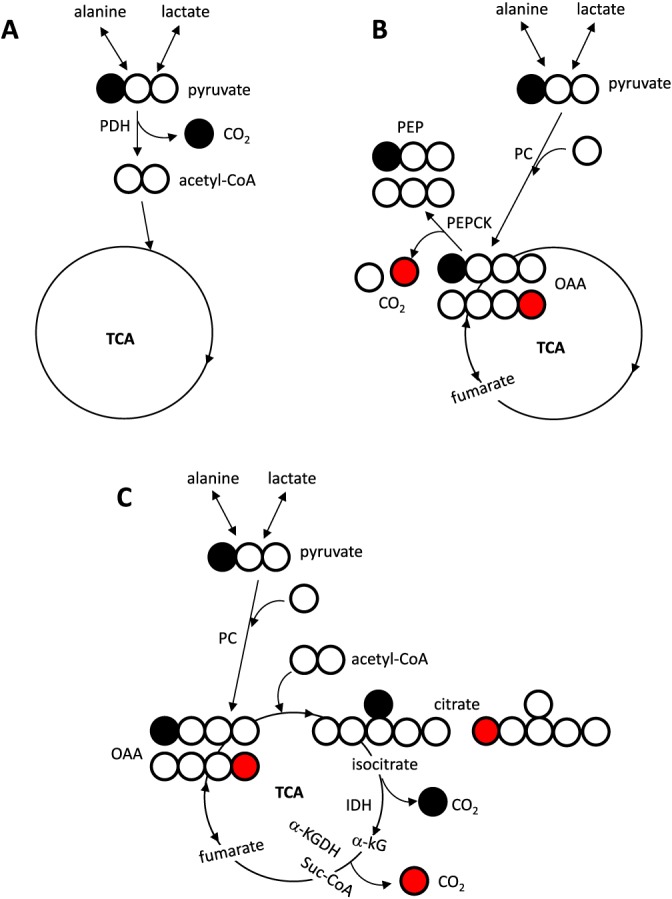
Potential pathways for production of [13C]bicarbonate from [1‐13C]pyruvate in the liver. Decarboxylation of [1‐13C]pyruvate through PDH yields 13CO2 and unlabeled acetyl‐CoA (A). After carboxylation through PC, [1‐13C]pyruvate yields [1‐13C]oxaloacetate that is in rapid exchange with a symmetric molecule, fumarate, producing [4‐13C]oxaloacetate and [1‐13C]oxaloacetate in equal proportions. When [4‐13C]oxaloacetate is converted to PEP through PEPCK, the 13C from the oxaloacetate is lost (B). When [1‐13C]oxaloacetate becomes [6‐13C]citrate after condensation with unlabeled acetyl‐CoA, the 13C from the citrate is lost at the level of IDH through the forward turn of the TCA cycle. The 13C from [1‐13C]citrate after condensation of [4‐13C]oxaloacetate and unlabeled acetyl‐CoA is lost at the level of α‐KGDH (C). Open circle =12C; filled circle =13C; red circle =13C after oxaloacetate exchange with fumarate.
The present study was designed to determine the sources of HP [13C]bicarbonate in rat liver in vivo after intravenous injection of HP [1‐13C]pyruvate, focusing on the relative importance of pyruvate metabolism through PDH and PC depending on nutritional states. Metabolism of non‐hyperpolarized [2,3‐13C]pyruvate was further examined in separate 'bench‘ experiments to determine the relative fluxes through PDH and PC by 13C isotopomer analysis of glutamate isolated from the liver of rats. These experiments demonstrated that the NMR signal of HP [13C]bicarbonate was observed only in fed animals with active PDH flux, but not detectable in fasted animals with active PC flux followed by gluconeogenesis. Thus, PDH is the source of the HP [13C]bicarbonate signal from the liver in vivo exposed to HP [1‐13C]pyruvate, but the appearance of HP [13C]bicarbonate is not indicative of pyruvate carboxylation followed by the subsequent decarboxylation process occurring through PEPCK or in the TCA cycle.
Materials and Methods
Dynamic nuclear polarization
Hyperpolarization of [1‐13C]pyruvic acid (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) was performed using a home built 4.6 T pre‐polarizer. Neat [1‐13C]pyruvic acid was doped with trityl OX063 radical (15 mM) and ProHance® (1 mM) and was polarized at 128.915 GHz for 1.5 h at 1.15 K. Microwave irradiation was turned off and the sample was rapidly dissolved with 4 mL of superheated 100 mM phosphate buffer (pH 7.4) and 3 mL of the solution was transferred into a beaker near the fringe field (~ 5 G) of a GE 3 T 750 W. Polarization was measured as 10–12% at the time of injection. The final concentration of the HP pyruvate was 80 mM.
Animal studies
The study was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Two groups of Sprague–Dawley rats (300–350 g), fed (ad libitum) and fasted (21 h) with free access to water, were studied. General anesthesia was induced with Isoflurane® and maintained throughout the experiment. HP [1‐13C]pyruvate (2.5 ml, 80 mM) was injected via a tail vein over a period of approximately 30 s. 13C NMR data were collected within 1 s after the start of the injection using parameters described below. Upon completion of data acquisition, blood was harvested from the inferior vena cava and the liver was excised and freeze clamped using liquid nitrogen. The total time from the start of injection to freeze clamping the liver was approximately 8 min.
Parallel bench experiments were performed mimicking the anesthesia, timing of injection, concentration of pyruvate, timing of tissue clamping and other factors used in the observations of HP metabolites. However, [2,3‐13C]pyruvate (Cambridge Isotope) was used in place of [1‐13C]pyruvate and rats were not studied in the magnet. Rats were injected with 2.5 mL of 80 mM [2,3‐13C]pyruvate through a tail vein under general anesthesia. Blood and liver were harvested after 8 min.
Sample processing for NMR analysis
Blood was deproteinized by adding perchloric acid to a final concentration of 7% and centrifuged. The supernatant was neutralized with KOH, centrifuged and lyophilized. The dried residue was applied to an ion‐exchange column containing 15 ml of cation‐exchange resin (Dowex 50Wx8–200; Sigma, St. Louis, MO, USA) and 15 ml of anion‐exchange resin (Amberlite IRA‐67; Sigma) with water as eluent to purify glucose, and it was lyophilized. To convert glucose into monoacetone glucose (MAG), dried glucose was suspended in 3.0 ml of acetone containing 120 μl of concentrated sulfuric acid. The mixture was stirred for 4 h at room temperature to yield diacetone glucose. After adding 3 ml of water, the pH was adjusted to 2.0 by adding Na2CO3 (1.5 M). The mixture was stirred for 24 h at room temperature to convert diacetone glucose into MAG. The pH was then further increased to 8.0 with Na2CO3. Acetone was evaporated under a vacuum, and the sample was lyophilized. MAG was extracted into 5 mL of hot ethyl acetate (5×), and ethyl acetate was removed by vacuum evaporation. The resulting MAG was further purified by passage through a 3‐ml DSC‐18 cartridge, using 5% acetonitrile as eluent. The effluent was lyophilized and stored dry before NMR analysis.
Frozen liver tissue (7–8 g) was pulverized and extracted with cold perchloric acid (10%), neutralized with KOH and lyophilized. Glutamate was isolated by ion‐exchange chromatography as described previously 4.
Data acquisition
13C NMR data were collected with a General Electric Dual‐Tuned (1H/13C) birdcage volume rat coil using a 15 mm single, axial slice on the rat liver, 12 cm field of view, 32124710‐Hz center frequency, 10° fid pulse, 1‐s repetition time, 5 kHz bandwidth and 120 acquisitions for a total time of 2 min on a GE MR750W 3 T clinic scanner. These data were apodized and zero‐filled prior to Fourier transformation and the relative peak areas were measured by integration using the iNMR for Windows version 2.35 (Nucleomatica, Molfetta, Italy). Spectra were scaled to the maximum pyruvate signal, but were not referenced to an external standard.
All high‐resolution NMR spectra were collected using a 14.1 T spectrometer (Varian INOVA, Agilent, Santa Clara, CA, USA) equipped with a 3‐mm broadband probe with the observe coil tuned to 1H (600 MHz) or 13C (150 MHz). MAG was resuspended in 160 μL of deuterated acetonitrile and 10 μL of water. After shimming, 13C NMR spectra were collected using a 52° observe pulse, 60992 data points collected over a sweep‐width of 20330 Hz and a 1.5‐s acquisition time with 1.5‐s interpulse delay at 25°C. Proton decoupling was performed using a standard WALTZ‐16 pulse sequence. Typically 25000 scans were averaged, requiring 21 h. Liver tissue extracts or isolated glutamate samples were dissolved in 2H2O (180 μl) for 1 H and 13C NMR acquisition. 1H NMR spectra of liver extracts were obtained using a 90° observe pulse, a 2‐s acquisition time and 1‐s delay between pulses. 1000–3500 scans were summed requiring ~1–3 h. 13C NMR spectra of liver extracts or glutamate were obtained using a 60° pulse, a 36765‐Hz sweep width, 110294 data points and a 1.5‐s acquisition time with 1.5‐s interpulse delay at 25°C. Spectra were averaged over 25000 scans. All NMR spectra were analyzed using ACD/Labs NMR spectral analysis program (Advanced Chemistry Development, Inc., Toronto, Canada).
Calculation of excess enrichments in glucose
Excess 13C enrichment in glucose was measured using the internal references of two methyl resonances of MAG as described previously 12, 13. As the two methyl groups were added in the process of conversion of glucose to MAG, these two resonances reflect natural abundant levels of 13C and their intensities are, therefore, independent of metabolism. As an example, the enrichment of [3‐13C]glucose in plasma was calculated using the ratio of the area of singlet of C3 resonance (SC3) at 75.3 ppm over the areas of the two methyl resonance singlets (2SMethyl) at 26.1 and 26.7 ppm. Thus, any increase in the ratio of SC3/2SMethyl over natural abundance MAG must reflect excess 13C enrichment in [3‐13C]glucose. Similarly, excess enrichment from [3,4‐13C]glucose was measured using the ratio of doublet reflecting J34 over 2SMethyl. In the present study, the 13C enrichment in glucose is the sum of individual 13C enrichment from each glucose isotopomer with excess 13C above natural abundance.
Metabolite assays
The levels of alanine and lactate from liver tissues were measured using 1H NMR analysis of perchloric acid extracts. The extracts and standard samples were dissolved in 2H2O containing 4,4‐Dimethyl‐4‐silapentane‐1‐sulfonic acid as a NMR reference. Sixty‐four scans were summed for each sample requiring 5 min. The levels of alanine and lactate were calculated using standard curves. The level of plasma glucose was estimated using the glucose oxidase method (YSI 2300 Glucose Analyzer; GMI, Inc., Ramsey, MN, USA).
Statistical analysis
Data are expressed as the mean ± standard error. Comparisons between two groups were made using a Student's one‐tailed t‐test, where P < 0.05 was considered significant.
Results
After intravenous injection of HP [1‐13C]pyruvate, serial 13C NMR spectra were acquired every second for 2 min; examples of summed spectra are illustrated in Figure 2. Large signals from HP [1‐13C]pyruvate, pyruvate hydrate, [1‐13C]lactate and [1‐13C]alanine were observed. The signal of HP [13C]bicarbonate was detected in fed animals (n = 6), but not in fasted animals (n = 6).
Figure 2.
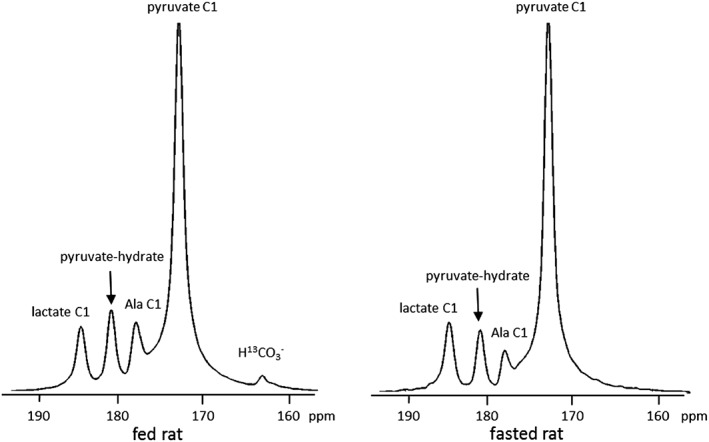
13C NMR spectra of rat liver in vivo after intravenous injection of HP [1‐13C]pyruvate. HP [1‐13C]pyruvate (80 mM, 2.5 ml) was injected into rats through a tail vein, and the spectra are the sum of 120 scans acquired over 2 min in a 3 T clinical scanner. Dual‐tuned (1H/13C) birdcage volume rat coil was used with a 15 mm single, axial slice on the rat liver, 12 cm field of view, 10° fid pulse, 1‐s repetition time and 5 kHz bandwidth. Livers of both fed and fasted rats show HP signals from [1‐13C]lactate, [1‐13C]alanine, [1‐13C]pyruvate and the hydrate of [1‐13C]pyruvate, but only livers of fed animals show the signal from HP [13C]bicarbonate (H13CO3 −).
Pyruvate entry to the TCA cycle through PC versus PDH varies depending on nutritional states
In parallel bench experiments designed to evaluate the relative fluxes through PC versus PDH and to determine the source(s) of the HP [13C]bicarbonate signal, rats received non‐hyperpolarized [2,3‐13C]pyruvate instead of HP [1‐13C]pyruvate. The protocol, animal preparation and timing used for these experiments were otherwise identical to those used in the HP experiment in the magnet. The metabolic scheme shown in Figure 3A summarizes the multiplets one would expect to see if [2,3‐13C]pyruvate was metabolized to glutamate via PC versus PDH. If [2,3‐13C]pyruvate passes through PDH, [1,2‐13C]acetyl‐CoA will be generated. Condensation of [1,2‐13C]acetyl‐CoA and oxaloacetate will produce [4,5‐13C]citrate and consequently [4,5‐13C]α‐ketoglutarate. As glutamate exchanges rapidly with α‐ketoglutarate, doublets in the C4 and C5 resonances of glutamate (i.e. [4,5‐13C]glutamate) as a result of J45 would be evident. Metabolism of [2,3‐13C]pyruvate via pyruvate carboxylation cannot transfer label into glutamate C4 and C5 positions. Instead, carboxylation of [2,3‐13C]pyruvate generates [2,3‐13C]oxaloacetate. Backwards scrambling into the symmetric fumarate pool would not change this labeling pattern. Forward flux of [2,3‐13C]oxaloacetate through citrate synthase and subsequent steps in the TCA cycle would eventually generate doublets in the C2 and C3 resonances of glutamate (i.e. [2,3‐13C]glutamate) both as a result of J23. In theory, the appearance of 13C in C2 and C3 of glutamate could also be because of enrichment in acetyl‐CoA via PDH activity plus extensive turnover in the TCA cycle. However, if [1,2‐13C]acetyl‐CoA is a relatively small fraction of the acetyl‐CoA pool, as it is in this experiment (i.e. < 3%), then a doublet in glutamate C2 as a result of J23 (D23) produced through PDH will be negligible. In this experiment, the doublet because of J23 (Fig. 3B) is dominant and can only arise via pyruvate carboxylation.
Figure 3.
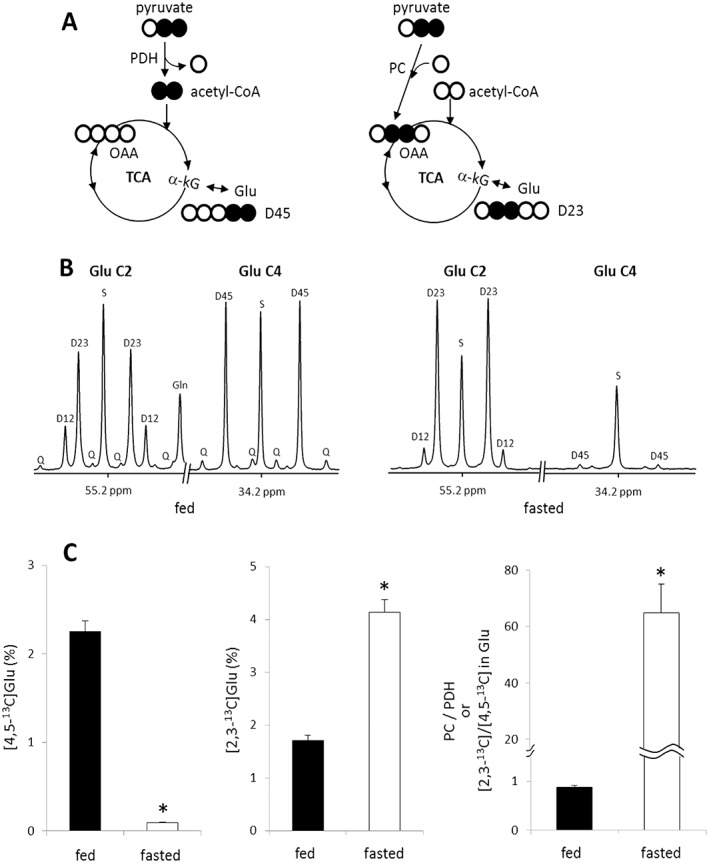
Detection of pyruvate entry to the TCA cycle through PC or PDH Based on NMR Analysis of Hepatic Glutamate in Rats Given [2,3‐13C]pyruvate. The entry of [2,3‐13C]pyruvate to the TCA cycle through PDH yields [4,5‐13C]glutamate while the entry through PC produces [2,3‐13C]glutamate after the condensation of [2,3‐13C]oxaloacetate and acetyl‐CoA followed by the forward turn through the TCA cycle (A). In 13C NMR spectra of C2 and C4 regions of glutamate isolated from liver, the signal from [4,5‐13C]glutamate is very strong in fed animals, but negligible in fasted rats. The signal from [2,3‐13C]glutamate is strong in fed animals and very strong in fasted animals (B). The fraction of D45 ([4,5‐13C]glutamate) was measured by assuming the singlet at C4 ([4‐13C]glutamate) as natural abundance 13C, and it was 2.25% in fed animals and only 0.09% in fasted animals. Likewise, the fraction of D23 ([2,3‐13C]glutamate) was 1.70% in fed animals and 4.13% in fasted animals. The ratio of [2,3‐13C]/[4,5‐13C] in glutamate, reflecting the ratio of anaplerosis/PDH, was dramatically higher in fasted rats compared with fed rats (D). Other small resonances in the spectra are from glutamate isotopomers produced through extensive turnover of the TCA cycle. D12, doublet from coupling of C1 with C2; D23, doublet from coupling of C2 with C3; D34, doublet from coupling of C3 with C4; Q, doublet of doublets, or quartet, arising from coupling of C2 with both C1 and C3 or from coupling of C4 with both C3 and C5; D45, doublet from coupling of C4 with C5; S, singlet; *, P < 0.001. n = 6–7 in each group.
The impact of nutritional states on the relative activity of PC and PDH is illustrated in Figure 3B and C. The 13C NMR spectra of glutamate isolated from the liver of fed animals showed a dominant doublet owing to J45 (i.e. D45) in the C4 resonance, demonstrating [1,2‐13C]acetyl‐CoA production through PDH. If one assumes the singlet represents natural abundance 13C, then 2.25 ± 0.12% (n = 7) of the acetyl‐CoA pool was derived from the bolus of [2,3‐13C]pyruvate. The 13C NMR spectra of glutamate isolated from the liver of fasted animals were quite different. In this case, very little D45 was observed in glutamate C4, indicating that flux through PDH was negligible. The C4 spectrum was dominated by the singlet as a result of natural abundance 13C, and the calculated fraction of [4,5‐13C]glutamate was only 0.09 ± 0.01% in these animals (n = 6). In contrast, the C2 region of glutamate was dominated by D23 in fasted animals. As there is essentially no excess 13C enrichment in C4 of glutamate, the signal from [2,3‐13C2]glutamate in the C2 must be derived exclusively via PC activity. Thus PDH flux was negligible in the fasted state, but pyruvate carboxylation was the overwhelming source of entry of pyruvate into the TCA cycle. The calculated fraction of [2,3‐13C]glutamate was 4.13% in the liver of fasted animals whereas it was 1.70% in that of fed animals. Under these experimental conditions, the distribution of 13C in the TCA cycle has not achieved a steady state so a quantitative analysis of PC versus PDH flux is not possible. Nevertheless, the ratio of [2,3‐13C]glutamate/[4,5‐13C]glutamate provides an index of anaplerosis versus oxidation of pyruvate (i.e. relative flux of PC versus PDH) in liver. This ratio was dramatically different depending on nutritional states; 0.88 ± 0.04 in fed animals versus 64.80 ± 10.09 in fasted animals (Fig. 3C).
PC and PEPCK activities do not correlate with HP [13C]bicarbonate signal from in vivo liver
Instead of remaining in the TCA cycle intermediates, 13C‐labeled oxaloacetate formed via pyruvate carboxylation may exit the cycle through PEPCK generating PEP and subsequently glucose (Fig. 4A). As flux through PEPCK is a potential source of HP [13C]bicarbonate in the present study, gluconeogenesis was examined by the analysis of 13C‐labeled glucose from the plasma or liver tissues of rats given HP [1‐13C]pyruvate in the magnet or rats given non‐hyperpolarized [2,3‐13C]pyruvate in the separate bench experiment. The results from both studies were the same: negligible 13C enrichment was observed in glucose from fed animals, but highly 13C‐enriched glucose was observed from fasted animals, exactly as anticipated based on differences in nutritional states. As examples, the 13C NMR spectra shown in Figure 4 reflect plasma glucose isolated from rats after exposure to HP [1‐13C]pyruvate. The entry of [1‐13C]pyruvate into the pool of 4‐carbon TCA cycle intermediates would result in symmetrization of label and loss of 13C during conversion of [4‐13C]oxaloacetate to PEP. The fraction remaining as [1‐13C]oxaloacetate would quickly equilibrate into the pool of 3‐carbon intermediates, glyceraldehyde 3‐phosphate (GA3P) and dihydroxyacetone phosphate (DHAP), along the path to glucose. This would be detected as excess 13C enrichments in C3 and C4 of glucose. As any [4‐13C]oxaloacetate would have been lost as 13CO2 at the level of PEPCK (shown as the red circle in Figure 4A), the excess 13C enrichment in glucose represents only a fraction of the original [1‐13C]pyruvate given as a bolus. Quantitatively, excess 13C enrichment in fasting animals was 3.21 ± 0.30% but only 0.21 ± 0.06% in fed animals receiving HP [1‐13C]pyruvate. Interestingly, production of [3,4‐13C]glucose was evident by the appearance of D34 in both C3 and C4 resonances only in plasma glucose from fasting animals. This isotopomer could only have been produced by condensation of two 13C‐enriched trioses, [3‐13C]DHAP and [1‐13C]GA3P. The levels of plasma glucose were 6.3 ± 0.3 mmol/L in fasted animals and 15.5 ± 0.8 mmol/L in fed animals (Fig. 4C). In the separate bench experiment using non‐hyperpolarized [2,3‐13C]pyruvate, [1,2‐13C]glucose and [5,6‐13C]glucose were produced through carboxylation of [2,3‐13C]pyruvate followed by gluconeogenesis (spectra not shown). The excess 13C enrichment in plasma glucose from this experiment was 11.34 ± 0.94% in fasted rats (n = 6) whereas it was only 0.72 ± 0.14% in fed rats (n = 7).
Figure 4.
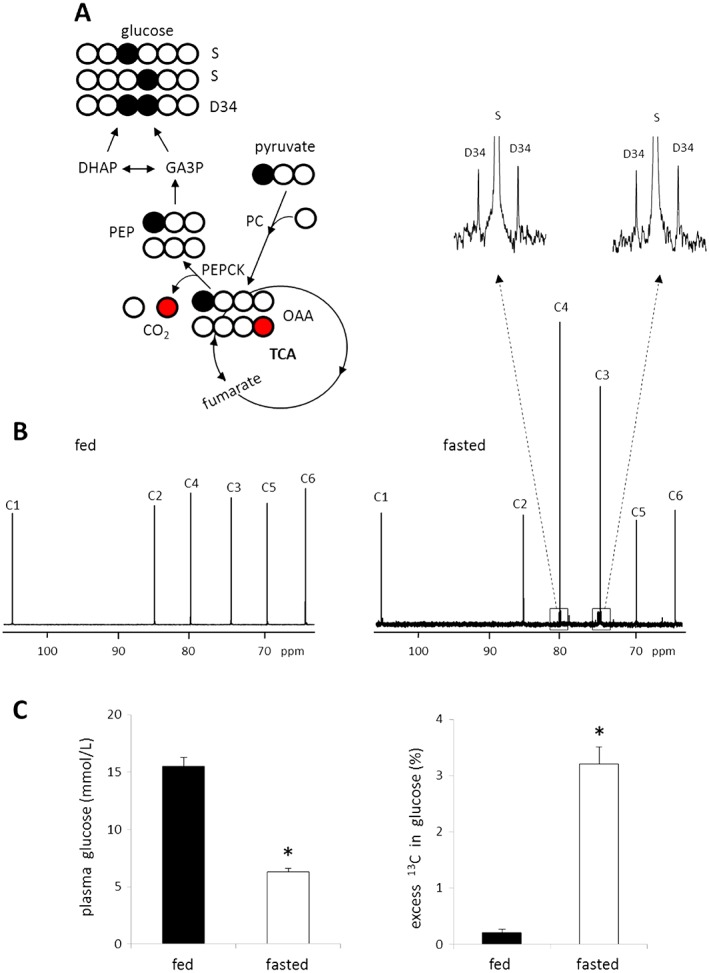
NMR evidence for gluconeogenesis from [1‐13C]pyruvate after a single bolus injection of HP pyruvate. [1‐13C]pyruvate carboxylation through PC produces [1‐13C]oxaloacetate, which exchanges with fumarate (a symmetric molecule) to yield [4‐13C]oxaloacetate. [1‐13C]oxaloacetate may exit the TCA cycle via PEPCK becoming [1‐13C]PEP after losing 12C while [4‐13C]oxaloacetate becoming unlabeled PEP after losing 13C (producing 13CO2 and then [13C]bicarbonate). [1‐13C]PEP can enrich either C3 or C4 of glucose through gluconeogenesis or both C3 and C4 if two labeled triose units condense (A). In the 13C NMR spectra of MAG derived from plasma glucose, fed rats show negligible excess 13C while fasted rats show obvious excess 13C in C3 and C4 positions (B). The level of plasma glucose from fasted animals is lower than that from fed animals, but excess 13C enrichment in glucose is much higher in fasted animals (C). D34, doublet from coupling of C3 with C4; S, singlet; Open circle =12C; filled circle =13C; red circle =13C after oxaloacetate exchange with fumarate; *P < 0.001. n = 6–8 in each group.
A small portion of lactate and alanine originated from exogenous pyruvate
As noted, after injection of HP [1‐13C]pyruvate, large signals from HP [1‐13C]lactate and [1‐13C]alanine were detected in vivo. To estimate the portions of lactate and alanine derived from the injected pyruvate, the pool size and 13C enrichment of lactate C3 position (1.32 ppm) or alanine C3 (1.47 ppm) were measured using 1H NMR spectra of liver extracts from the rats given [2,3‐13C]pyruvate (Fig. 5A). The pool sizes varied with nutritional states; lactate was 6.58 ± 0.28 μmole/gww from fed animals (n = 7) and 2.63 ± 0.15 μmole/gww from fasted animals (n = 6) whereas alanine was 3.90 ± 0.19 μmole/gww from fed animals and 1.52 ± 0.09 μmole/gww from fasted animals (Fig. 5B). The fraction of 13C‐labeled lactate was 1.93 ± 0.15% in fed animals and 5.88 ± 0.57% in fasted animals as measured from the signal of proton attached to 13C (H‐13C) over total proton signals attached to C3 of lactate (H‐13C + H‐12C). Similarly, the fraction of alanine derived from 13C‐labeled pyruvate was 2.73 ± 0.42% in fed animals and 5.36 ± 0.31% in fasted animals (Fig. 5C). Although HP [1‐13C]lactate and HP [1‐13C]alanine signals were readily detected in the 13C NMR spectrum (Fig. 2), the actual enrichment in the total lactate and alanine pool of the liver was small.
Figure 5.
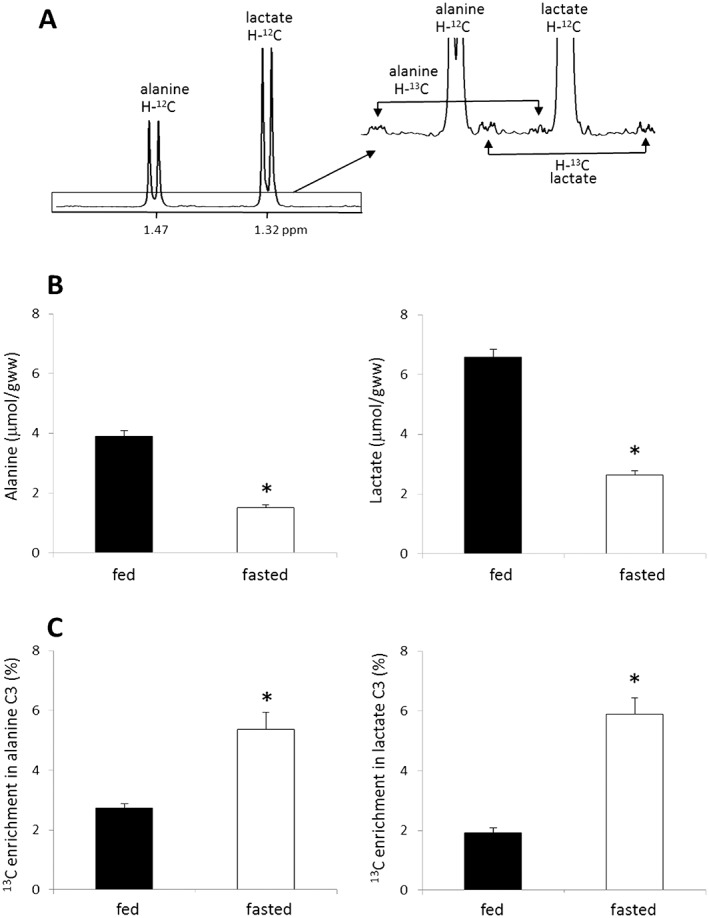
1H NMR analysis of Lactate and Alanine from the Liver of Rats Receiving [2,3‐13C]pyruvate. Rats received [2,3‐13C]pyruvate through a tail vein and liver was harvested after 8 min. 13C enrichments in lactate and alanine were measured by 1H NMR analysis of liver tissue extracts. 1H NMR spectrum from a fed rat (A) shows resonances of lactate C3 (at 1.32 ppm) and alanine C3 (at 1.47 ppm) with dominant peaks from proton attached to 12C (H‐12C) and small satellite peaks from proton attached to 13C (H‐13C). Fed animals have higher levels of alanine and lactate in the liver compared with fasted animals (B) whereas they have lower 13C enrichments in both lactate and alanine than fasted animals (C). *P < 0.001. n = 5–7 in each group.
Discussion
As anticipated, metabolism of HP [1‐13C]pyruvate to HP lactate and HP alanine was easily detected by 13C NMR spectroscopy in rat liver in vivo with a temporal resolution of a few seconds 8, 9. Unlike prior reports, HP [13C]bicarbonate was also detected but the magnitude of the signal was heavily dependent on the nutritional state of the animals. The signal of HP [13C]bicarbonate was evident in livers of fed rats but it was undetectable in livers of fasting animals. Subsequent experiments demonstrated that [1‐13C]pyruvate was indeed converted to glucose in fasted animals even although HP [13C]bicarbonate was not detected. This interesting finding then begs the question, why is HP [13C]bicarbonate not observed in livers undergoing active gluconeogenesis?
The HP [13C]bicarbonate signal from HP [1‐13C]pyruvate reflects PDH activity
The appearance of HP [13C]bicarbonate in the liver after injection of [1‐13C]pyruvate could reflect flux through multiple pathways including direct production via PDH, PC followed by gluconeogenesis via PEPCK, or PC followed by forward flux in the TCA cycle. Metabolism of non‐hyperpolarized [2,3‐13C]pyruvate was used to sort through these metabolic possibilities. As evidenced by the dramatic difference in [4,5‐13C]glutamate enrichment, PDH was active in livers of fed animals but largely inactive in livers of fasting animals. This is consistent with the presence of a HP [13C]bicarbonate signal in livers of fed animals and the absence of the signal in fasting rats. However, the absence in fasting animals is inconsistent with the observations that (i) much of the 13C originating in [1‐13C]pyruvate or [2,3‐13C]pyruvate appears in plasma or liver glucose of fasting animals demonstrating active pyruvate carboxylation followed by gluconeogenesis via PEPCK and (ii) fasted rats have higher enrichment by [2,3‐13C]glutamate in liver glutamate pools than fed rats demonstrating again active pyruvate carboxylation followed by forward flux through the TCA cycle. Given that pyruvate flux through PDH was low in fasted animals, we exclude the possibility of [2,3‐13C]glutamate production through PDH followed by extensive turnover of the TCA cycle. This indicates much of the HP [1‐13C]pyruvate presented to livers of fasting animals must undergo pyruvate carboxylation and subsequent gluconeogenesis or the forward flux through the cycle without production of HP [13C]bicarbonate.
[2,3‐13C]pyruvate used in bench experiments has some advantages for estimation of the relative importance of PC and PDH in the liver where typically extensive 13C scrambling is expected. Carboxylation of [2,3‐13C]pyruvate produces [2,3‐13C]oxaloacetate. The 13C‐13C labeling in oxaloacetate C2 and C3 remains the same carbon positions even after back‐scrambling with fumarate or after pyruvate cycling (oxaloacetate➔PEP➔pyruvate➔oxaloacetate). However, the quantitation of [2,3‐13C]glutamate in the present study is a low detection limit of pyruvate carboxylation especially in fasted animals, as a significant portion of [2,3‐13C]oxaloacetate exits the TCA cycle through PEPCK. This was confirmed by the markedly increased fractions of [1,2‐13C]‐ and [5,6‐13C]glucose in plasma and liver tissues of fasted rats receiving [2,3‐13C]pyruvate. Despite the dramatic increase in PC activity followed by gluconeogenesis, the lack of HP [13C]bicarbonate indicates that the signal was not associated with PEPCK activity in livers of fasting animals. It should be noted that the HP [13C]bicarbonate signal reflects the concentration of bicarbonate in the tissue, fractional enrichment with 13C, and polarization, so the absence of a signal in a particular physiological states could reflect multiple factors. Nevertheless, these experiments demonstrated that HP [13C]bicarbonate from HP [1‐13C]pyruvate under these conditions reflected flux through PDH only even in livers undergoing active gluconeogenesis.
Both magnetization loss and negligible PDH flux may contribute to the observed reduction in HP [13C]bicarbonate in fasted animals
PC activity in the liver was higher in fasted animals than fed animals. As PC itself requires bicarbonate, the absence of HP [13C]bicarbonate in the fasted liver could be simply as a result of a low bicarbonate concentration in the association with PC activity. However, PC‐related [13C]bicarbonate consumption in these animals seems not to be important as the signal from [1,2,3‐13C]glutamate in glutamate C2 region (quartet or Q) was trivial from the liver of fasted animals receiving [2,3‐13C]pyruvate (Fig. 3B). If [13C]bicarbonate is incorporated for the carboxylation of [2,3‐13C]pyruvate to oxaloacetate via PC, [2,3,4‐13C]oxaloacetate would be produced and consequently [1,2,3‐13C]glutamate.
However, the absence of a HP [13C]bicarbonate signal in livers of fasting animals presumably reflects negligible PDH activity and loss of hyperpolarization owing to T1. While PDH was clearly active in livers of fed animals, fasted animals showed 25‐fold less labeling of [4,5‐13C]glutamate. This substantially reduces the possibility of detecting HP [13C]bicarbonate derived from PDH after a fast. In contrast, although pyruvate carboxylation followed by gluconeogenesis and the forward flux in the TCA cycle were both shown to be highly active in livers of fasting animals, a HP [13C]bicarbonate signal was not observed in this metabolic state. Thus, after carboxylation of HP [1‐13C]pyruvate, 13C must be released at the levels of PEPCK, isocitrate dehydrogenase and α‐ketoglutarate dehydrogenase, but the absence of a HP [13C]bicarbonate signal suggests magnetization loss owing to T1 relaxation in the multiple enzyme‐catalyzed reactions. While HP [13C]bicarbonate is produced from [1‐13C]pyruvate in the first step of the PDH complex, [13C]bicarbonate release at the levels of PEPCK, isocitrate dehydrogenase and α‐ketoglutarate dehydrogenase are multiple steps away from [1‐13C]pyruvate. For example, [13C]bicarbonate release at the level of PEPCK after [1‐13C]pyruvate administration requires six enzyme‐catalyzed steps: pyruvate C1➔ oxaloacetate C1 ➔ malate C1➔ fumarate C1 or C4 (symmetric) ➔ malate C4➔ oxaloacetate C4 ➔ PEP plus [13C]bicarbonate. Similarly, [13C]bicarbonate release at the level of isocitrate dehydrogenase and α‐ketoglutarate dehydrogenase occurs 5 and 10 enzyme catalyzed downstream of [1‐13C]pyruvate, respectively. Unlike fasted animals, fed animals showed a HP [13C]bicarbonate signal despite negligible gluconeogenesis and lower forward flux through the TCA cycle after pyruvate carboxylation. Thus, the HP [13C]bicarbonate signal observed in livers of fed animals must be largely attributed to PDH activity.
Comparison of results from in vivo liver versus isolated liver
As noted, we proposed that PEPCK activity was responsible for the HP [13C]bicarbonate signal based on the studies of isolated mouse livers 10 whereas others simply assumed that PDH was responsible for the signal in the mouse liver in vivo 7. In the present study, we demonstrated that the HP [13C]bicarbonate signal reflects decarboxylation through PDH in the rat liver in vivo. Experimental conditions in studies of isolated livers do not duplicate conditions in vivo and may play a role in our ability to detect HP [13C]bicarbonate originating from the PEPCK pathway. The acquisition conditions were different, as large flip angle pulses were used for the perfused liver. The isolated livers were also studied without albumin in the perfusion medium. As the T 1 relaxation time of HP [1‐13C]pyruvate is reduced in the presence of albumin 14, 15, the polarization state of [1‐13C]pyruvate was presumably higher in isolated livers, likely increasing the chances of detecting more HP products. In addition, octanoate was used in the previous HP [13C]bicarbonate study using isolated livers from mice including PEPCK‐knockout mice 10. Octanoate is known to decrease the intracellular NAD+/NADH ratio 16, which would serve to further lower the PDH activity and stimulate PEPCK, again altering the detection of HP [13C]bicarbonate. As the TCA cycle activity in the liver of PEPCK‐knockout mice is impaired 17, reduced PDH activity in the liver of these mice could also play a role in the decrease of the HP [13C]bicarbonate signal observed in these animals. Thus, it is not reasonable to assume that conditions in vivo duplicate the isolated liver. As perfused liver is often supplied with a non‐physiological mixture of substrates and liver is metabolically flexible 18, the relative importance of PC and PDH in perfused liver could be different from in vivo liver.
The fractions of HP lactate C1 and alanine C1 from HP [1‐13C]pyruvate are small
The signals from HP lactate 13C and alanine 13C were strong in both fed and fasted animals given HP [1‐13C]pyruvate. The pool size of lactate and alanine was higher in a fed condition compared with a fasted condition. In contrast, as demonstrated in the parallel bench study with [2,3‐13C]pyruvate, 13C enrichment in liver lactate and alanine was lower in fed animals compared with fasted animals. Thus, the intensities of HP alanine and lactate signals in the fed versus fasted liver spectra appear to be similar (Fig. 2). Under these conditions, it is possible that time was insufficient to allow exchange of 13C into all lactate and alanine pools in the liver. Despite the low enrichments, in vivo 13C NMR spectroscopy showed strong HP lactate 13C and alanine 13C. These experiments demonstrate the remarkable sensitivity of detecting 13C by hyperpolarization methods in vivo.
Summary
The metabolic fate of pyruvate in the liver may play key roles in high‐impact disorders including type 2 diabetes and fatty liver disease. The present study demonstrated that the real‐time detection of HP [13C]bicarbonate signal from the livers of healthy animals given HP [1‐13C]pyruvate reflects the activity of PDH rather than PEPCK or TCA cycle activity after pyruvate carboxylation. Hyperpolarization technology offers a new approach to understanding real‐time liver biochemistry in vivo, but presently it has limitations in detecting the majority of metabolites of interest due to the loss of magnetization through multiple step reactions. However, the combination of hyperpolarization technology and non‐hyperpolarized 13C isotopomer experiments provides important quantitative insights into intermediary metabolism in the in vivo liver.
Acknowledgements
We appreciate expert advice by William Mander from Oxford Instruments Molecular Biotools. We thank Nicholas Carpenter, Xiaodong Wen and Thomas Hever for their excellent technical support. This study was supported by NIH grants DK099289 to E.S.J., RR 002584, DK058398 and EB 015908 to C.R.M., and HL34557 to A.D.S.
Jin, E. S. , Moreno, K. X. , Wang, J.‐X. , Fidelino, L. , Merritt, M. E. , Sherry, A. D. , and Malloy, C. R. (2016) Metabolism of hyperpolarized [1‐13C]pyruvate through alternate pathways in rat liver. NMR Biomed., 29: 466–474. doi: 10.1002/nbm.3479.
References
- 1. Katz J. Determination of gluconeogenesis in vivo with 14C‐labeled substrates. Am. J. Physiol. 1985; 248: R391–R399. [DOI] [PubMed] [Google Scholar]
- 2. Magnusson I, Schumann WC, Bartsch GE, Chandramouli V, Kumaran K, Wahren J, Landau BR. Noninvasive tracing of Krebs cycle metabolism in liver. J. Biol. Chem. 1991; 266: 6975–6984. [PubMed] [Google Scholar]
- 3. Agius L, Alberti KG. Regulation of flux through pyruvate dehydrogenase and pyruvate carboxylase in rat hepatocytes. Effects of fatty acids and glucagon. Eur. J. Biochem. 1985; 152: 699–707. [DOI] [PubMed] [Google Scholar]
- 4. Katz J, Wals P, Lee WN. Isotopomer studies of gluconeogenesis and the Krebs cycle with 13C‐labeled lactate. J. Biol. Chem. 1993; 268: 25509–25521. [PubMed] [Google Scholar]
- 5. Fernandez CA, Des Rosiers C. Modeling of liver citric acid cycle and gluconeogenesis based on 13C mass isotopomer distribution analysis of intermediates. J. Biol. Chem. 1995; 270: 10037–10042. [DOI] [PubMed] [Google Scholar]
- 6. Ardenkjaer‐Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal‐to‐noise ratio of >10,000 times in liquid‐state NMR. Proc. Natl. Acad. Sci. U. S. A. 2003; 100: 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee P, Leong W, Tan T, Lim M, Han W, Radda GK. In vivo hyperpolarized carbon‐13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin‐resistant mouse model. Hepatology 2013; 57: 515–524. [DOI] [PubMed] [Google Scholar]
- 8. Spielman DM, Mayer D, Yen YF, Tropp J, Hurd RE, Pfefferbaum A. In vivo measurement of ethanol metabolism in the rat liver using magnetic resonance spectroscopy of hyperpolarized [1‐13C]pyruvate. Magn. Reson. Med. 2009; 62: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu S, Chen AP, Zierhut ML, Bok R, Yen YF, Schroeder MA, Hurd RE, Nelson SJ, Kurhanewicz J, Vigneron DB. In vivo carbon‐13 dynamic MRS and MRSI of normal and fasted rat liver with hyperpolarized 13C‐pyruvate. Mol. Imaging Biol. 2009; 11: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merritt ME, Harrison C, Sherry AD, Malloy CR, Burgess SC. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc. Natl. Acad. Sci. U. S. A. 2011; 108: 19084–19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moreno KX, Moore CL, Burgess SC, Sherry AD, Malloy CR, Merritt ME. Production of hyperpolarized 13CO2 from [1‐13C]pyruvate in perfused liver does reflect total anaplerosis but is not a reliable biomarker of glucose production. Metabolomics In Press. 2015; 11(5): 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal. Biochem. 2004; 327: 149–155. [DOI] [PubMed] [Google Scholar]
- 13. Jin ES, Sherry AD, Malloy CR. Evidence for reverse flux through pyruvate kinase in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009; 296: E748–E757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreno KX, Sabelhaus SM, Merritt ME, Sherry AD, Malloy CR. Competition of pyruvate with physiological substrates for oxidation by the heart: implications for studies with hyperpolarized [1‐13C]pyruvate. Am. J. Physiol. Heart Circ. Physiol. 2010; 298: H1556–H1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marco‐Rius I, Tayler MC, Kettunen MI, Larkin TJ, Timm KN, Serrao EM, Rodrigues TB, Pileio G, Ardenkjaer‐Larsen JH, Levitt MH, Brindle KM. Hyperpolarized singlet lifetimes of pyruvate in human blood and in the mouse. NMR Biomed. 2013; 26: 1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGarry JD, Foster DW. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J. Biol. Chem. 1971; 246: 1149–1159. [PubMed] [Google Scholar]
- 17. Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 2004; 279: 48941–48949. [DOI] [PubMed] [Google Scholar]
- 18. Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 1992; 72(2): 419–448. [DOI] [PubMed] [Google Scholar]


