Abstract
Developmental ethanol exposure can lead to long-lasting cognitive impairment, hyperactivity, and emotional dysregulation among other problems. In healthy adults, sleep plays an important role in each of these behavioral manifestations. Here we explored circadian rhythms (activity, temperature) and slow-wave sleep in adult mice that had received a single day of ethanol exposure on postnatal day 7 and saline littermate controls. We tested for correlations between slow-wave activity and both contextual fear conditioning and hyperactivity. Developmental ethanol resulted in adult hyperactivity within the home cage compared to controls but did not significantly modify circadian cycles in activity or temperature. It also resulted in reduced and fragmented slow-wave sleep, including reduced slow-wave bout duration and increased slow-wave/fast-wave transitions over 24 hour periods. In the same animals, developmental ethanol exposure also resulted in impaired contextual fear conditioning memory. The impairment in memory was significantly correlated with slow-wave sleep fragmentation. Furthermore, ethanol treated animals did not display a post-training modification in slow-wave sleep which occurred in controls. In contrast to the memory impairment, sleep fragmentation was not correlated with the developmental ethanol-induced hyperactivity. Together these results suggest that disruption of slow-wave sleep and its plasticity are a secondary contributor to a subset of developmental ethanol exposure's long-lasting consequences.
Keywords: Fetal alcohol disorder, sleep fragmentation, slow-wave sleep, insomnia, circadian rhythm
1. INTRODUCTION
Fetal alcohol spectrum disorder (FASD) is a primary cause of intellectual disability (Abel and Sokol, 1986, May and Gossage, 2001, Fox and Druschel, 2003), with neurobehavioral hallmarks such as deficits in learning, memory and mood. Developmental ethanol exposure disrupts proliferation, differentiation, migration, and survival of neurons (Bonthius and West, 1990, West et al., 1990, Ikonomidou et al., 2000, Klintsova et al., 2007, Gil-Mohapel et al., 2010), and FASD is associated with cognitive, behavioral, memory and sensory impairments, as well as heightened susceptibility to seizures (West et al., 1990, Berman and Hannigan, 2000, Riley and McGee, 2005, Morasch and Hunt, 2009, Bell et al., 2010, Carr et al., 2010, Mattson et al., 2010). The specific set of symptoms are dependent on the age, duration and intensity of the ethanol exposure (Riley and McGee, 2005, Sadrian et al., 2014). Beyond the initial wave of ethanol induced damage such as cell death, there is a cascade of cellular, synaptic and network consequences induced by early ethanol exposure – some as a direct result of the ethanol insult, and some as a secondary response to cellular changes induced by that initial insult. One of the many functions that are disrupted by early ethanol exposure is sleep-wake structure (Criado et al., 2008, Pesonen et al., 2009, Jan et al., 2010, Wengel et al., 2011, Volgin and Kubin, 2012).
Slow-wave sleep (SWS) is characterized by up- and down-states in cell excitability in thalamocortical regions (Buzsaki, 2006), and by sharp-wave/ripple activity in both the hippocampal formation (Buzsaki, 1986) and olfactory (piriform) cortex (Murakami et al., 2005, Wilson, 2010, Manabe et al., 2011). These coordinated, brief (100s ms) periods of high-excitability appear to provide windows of opportunity for replay of recent experiences, and binding and/or transfer of learned information across distributed brain regions (Stickgold et al., 2001, Buzsaki, 2006, Stickgold and Walker, 2007, Barnes and Wilson, 2014). This activity may also be important for homeostatic regulation of synaptic strength (Liu et al., 2010, Bushey et al., 2011).
In individuals with FASD (Troese et al., 2008, Pesonen et al., 2009, Wengel et al., 2011, Chen et al., 2012) and in animal models of early ethanol exposure (Criado et al., 2008, Volgin and Kubin, 2012) sleep becomes more fragmented. Sleep fragmentation refers to shortened sleep bouts and frequent transitions between sleep and wake states. In both healthy and pathological populations, sleep deprivation and fragmentation are associated with impaired cognitive function, attention and emotional regulation (Durmer and Dinges, 2005, Abel et al., 2013, Basner et al., 2013). Sleep onset and transitions between sleep states are controlled by a variety of sub-cortical nuclei, including regions of thalamus, hypothalamus and brainstem (Jones, 2005, Abel et al., 2013), and GABAergic neurons in these regions play a crucial role in sleep regulation (Brown and McKenna, 2015). Commonly used hypnotics target GABAergic receptors (Manfridi et al., 2001, Walsh et al., 2007, Brickley and Mody, 2012), and insomnia and sleep fragmentation have been associated with impaired GABAergic neuron function in these regions (Lundahl et al., 2007, Kalume et al., 2015). Developmental ethanol exposure results in dysregulation of GABAergic neurons, including the parvalbumin expressing subset, throughout many brain regions (Coleman et al., 2012, Sadrian et al., 2014, Skorput et al., 2015, Smiley et al., 2015), potentially further raising the possibility of sleep dysfunction.
Here, in an extension of previous work that focused on analysis of developmental ethanol effects on relatively brief periods of sleep/waking (Stone et al., 1996, Criado et al., 2008, Volgin and Kubin, 2012), we explored slow-wave sleep over a multi-day period in adult mice exposed to a single exposure of ethanol on postnatal day 7 (P7). In the same mice, we examined developmental ethanol induced behavioral hyperactivity in the home cage and hippocampal-dependent contextual fear memory impairment, to assess whether these behavioral outcomes correlated with sleep disruption. The results demonstrate both reduced time in slow-wave sleep, and severe sleep fragmentation following developmental ethanol, as well as a significant correlation between sleep disruption and memory impairment. In contrast, developmental ethanol-induced hyperactivity was not correlated with sleep structure. The results suggest that slow-wave sleep disruption may be an important secondary contributor to the long-lasting neurobehavioral consequences of developmental ethanol exposure.
2. Experimental Procedures
2.1 Subjects
C57BL/6By mice, bred at the Nathan Kline Institute animal facility, were maintained on ad lib food and water at all times. Lights were on from 9am to 9pm for most animals, though for a subset lights were on from 8am to 8pm. Circadian measurements (see below) are expressed relative to the light cycle. All procedures were approved by the Nathan Kline Institute IACUC and were in accordance with NIH guidelines for the proper treatment of animals. On P7 pups were injected subcutaneously with saline or ethanol (EtOH) as described (Olney et al., 2002b, Saito et al., 2007). Each mouse in a litter was assigned to the saline or EtOH group at an equivalent proportion of the total number of mice with a distributed gender ratio. EtOH treatment (2.5 g/kg) was delivered twice in the same day at a 2-hr interval as originally described for C57BL/6 mice (Olney et al., 2002b). Pups were returned to their home cage immediately following injections. Our previous studies showed that this P7 EtOH treatment induced a peak blood alcohol level (BAL) of 0.5 g/dL when truncal blood was collected at 0.5, 1, 3, and 6 hr following the second EtOH injection and analyzed with an Alcohol Reagent Set (Pointe Scientific, Canton, MI, USA) (Saito et al., 2007). Under the same P7 EtOH treatment conditions, it has been reported that initial BAL peaks are attained approximately 1 h after each injection, with BAL falling below half of this level 8 h after first EtOH exposure (Wozniak et al., 2004, Young and Olney, 2006). Pups were weaned at P28 into group cages of littermates. Same-sex mice were housed together in cages in numbers between two and four per cage. Body weights measured using a set of C57BL/6By mice before (at P7) and after (at P14 and at 3-month old) saline/EtOH injections were as follows: P7 mice, 3.5±0.12g/3.44±0.17g (mean±SEM of males/ mean±SEM of females) for saline-assigned groups and 3.64±0.12g/3.5±0.13g for EtOH-assigned groups; P14 mice, 6.73±0.14g/6.21±0.29g for saline-treated groups and 6.07±0.14g/5.47±0.17g for EtOH-treated groups; 3-month-old mice, 26.3±1.0g/20.2±0.4g for saline-treated groups and 25.5±0.6g/20.0±0.3g for EtOH-treated groups. ANOVA indicated no significant main effects for gender or assigned groups and no significant interaction between these variables at P7. At P14, there were significant main effects for genders [F(1,40)=6.3 (p=0.016)] and for treatment groups [F(1,40)=10.0 (p=0.003)] without significant interaction between gender and treatment. Three-month-old mice only showed a significant main effect of gender [F(1,42)=78.5 (p=0.001)] without a significant main effect of treatment or a significant interaction between the variables. Thus, the differences in body weights observed in P14 mice between saline and EtOH groups seem to be diminished by 3 months of age when behavioral and electrophysiological studies were undertaken in the present study. Gender differences in the effects of P7 EtOH were not observed when studied previously (Wilson et al., 2011, Sadrian et al., 2012, Sadrian et al., 2014), nor were any significant differences observed between genders here, thus for most analyses both genders were combined.
2.2 Telemetry recordings and slow-wave analyses
Animals (postnatal age 85-100) were anesthetized with isoflurane and surgically implanted with a single stainless steel (125μ diameter) electrode in the frontal cortex. The electrode and reference were connected to a single-channel telemetry device implanted under the skin of the back. This telemetry transmitter (DSI, model ETA-F10) also transmitted body temperature and movement, which were extracted separately for analysis in a subset of animals. These transmitters did not allow EMG measures, and thus REM sleep was not monitored. Following surgery, animals were allowed to recover alone in their home cage for 4-7 days before 24hr recordings were begun. Basal sleep/wake and circadian activity were recorded continuously for 2-3 days in the home cage. All recordings were made from animals housed individually in a pairwise design, with a developmentally ethanol exposed mouse and their littermate control recorded simultaneously. Data from no more than one pair/litter are included here.
Local field potentials (LFP's) were acquired and digitized at 1000Hz and analyzed using Spike2 software (CED, Inc). Slow-wave activity was identified by analysis of delta frequency (0.1-5Hz) oscillations. LFP's were low-pass filtered and r.m.s. delta power extracted. Epochs (14s) of high delta power were identified as being at least 1 standard deviation above the mean power over a given 24hr period. Artifacts were removed before mean and standard deviation calculations. Analyses of slow-wave bouts included: mean percent time in slow-wave over a given period, mean slow-wave bout duration, mean number of slow-wave/fast-wave bout transitions, and mean spectral power (Fast-Fourier Transform [FFT] using 2Hz bins) during slow-wave bouts.
Sleep spindles, 8-15Hz oscillations most commonly associated with slow-wave sleep (Eschenko et al., 2006, Dang-Vu et al., 2010, Halassa et al., 2011) were also examined. Spindle events were identified during slow-wave bouts as previously described (Eschenko et al., 2006). Briefly, LFP's were band-pass filtered at 12-15Hz and root mean square (r.m.s.) was calculated with a time window of 0.1s across the complete 24hr recording. Spindle events were identified as r.m.s. amplitudes > 2 S.D. above the mean r.m.s., and these thresholded events were counted for comparison between groups. Counts were obtained during five randomly selected slow-wave bouts from each of four time periods – early morning, late morning, early evening and late evening and averaged within each animal.
In those animals where body temperature and activity data were acquired, data were blocked into 3hr bins, and mean time-dependent changes in activity and temperature were calculated over 2-3 consecutive days. Comparisons were made both over time and between groups.
2.3 Contextual fear conditioning
Following basal sleep recording, a subset of animals underwent contextual fear conditioning. Conditioning occurred at the beginning of the light cycle. Animals were placed in a conditioning chamber (9 × 22 × 20cm; W × L × H) with a shock grid floor, and a peppermint scent. Animals were allowed to explore the chamber for 5 min before receiving four 0.5mA, 1s foot shocks, with an average inter-shock interval of 2 min. Following the end of the training, animals were returned to their home cage, and LFP recordings resumed. Based on previous work (Barnes et al., 2011, Barnes and Wilson, 2014), time spent in slow-wave sleep during the 4hr post-training period was also quantified and compared across groups. The following day, at the start of the light cycle, animals were returned to the peppermint scented conditioning chamber for a 5min test of contextual freezing. Testing was videotaped for blind analysis. Time spent freezing was quantified for comparison across groups.
3. RESULTS
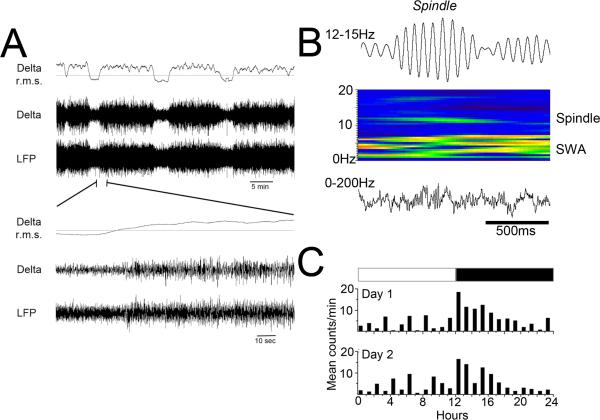
A total of 28 animals were used in the sleep analyses here. Not all animals were tested in all manipulations (see n's identified for each assay). In most cases, data were analyzed using tests of repeated measures between same sex littermate pairs, with one littermate exposed to saline on P7 and the other to EtOH on P7 (n=14 pairs from 14 litters). Recordings and tests were performed in animals at least 85 days of age. As shown in Figure 1, measures in all animals included LFP recordings from frontal cortex, which were low pass filtered to extract delta wave (0.1-5Hz) activity as an indicant of slow-wave activity (Fig. 1A). In addition, LFP's were bandpass filtered (12-15Hz) to extract sleep spindle activity from randomly selected slow-wave bouts (Fig. 1B). In a subset of animals, activity and body temperature were also extracted from the telemetry implant (Fig. 1C), to allow analysis of circadian rhythms in these indices.
Figure 1.
Representative examples of sleep related activity recordings used here. A) Example of local field potential (LFP) recording of slow-wave/fast-wave transitions. LFP trace is filtered 0-200Hz and Delta trace is filtered 0.1-5Hz. Delta root mean square (r.m.s.) plot shows the clear transitions between states. Periods of high delta power are considered slow-wave activity. B) Cortical spindles were extracted from LFP recordings (filtered 12-15Hz) and spindle events identified and counted. C) The telemetry transmitters provided activity measures based on animal movement. The circadian oscillation of this activity measure was quantified for comparison across treatment groups.
3.1 Adults exposed to developmental EtOH are hyperactive but display normal circadian rhythm
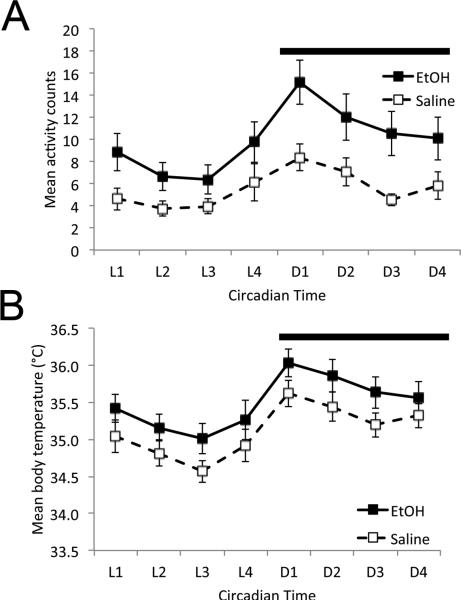
In 10 pairs of animals, general behavioral activity and body temperature were extracted from the telemetry signal (Fig. 2). Both EtOH and saline treated animals displayed circadian rhythms in behavioral activity and body temperature. Repeated measures ANOVA (time × developmental treatment) revealed a main effect of time for both activity (F(7,126) = 10.45, p < 0.001) and temperature (F(7,126) = 34.68, p < 0.001). In addition, EtOH-treated animals were significantly hyper-active in their home cage (Fig. 2A) compared to saline controls across the cycle (main effect of treatment, F(1,126) = 6.88, p < 0.02; time × treatment interaction (F(7,126) = 1.19, N.S.). While saline treated mice spent 14.7±1.3% of the 24hr cycle active in their home cage based on the telemetry activity monitor, EtOH treated mice spent 25.9±1.6% of the time active (paired t-test, t(9), = 6.04, p<0.01). There was no significant main effect of developmental treatment on body temperature (Fig. 2B; F(1,126) = 1,126) = 2.13, N.S.), nor time × treatment interaction (F(7,126) = 0.33, N.S.). Thus, exposure to ethanol on P7 resulted in hyperactivity in adult mice, though circadian rhythmicity was maintained.
Figure 2.
Developmental EtOH-treated adult mice displayed home cage hyperactivity, but circadian rhythmicity of both activity (A) and body temperature (B) were not significantly different from saline controls.
3.2 Adult sleep structure is impaired in adult mice exposed to EtOH during development
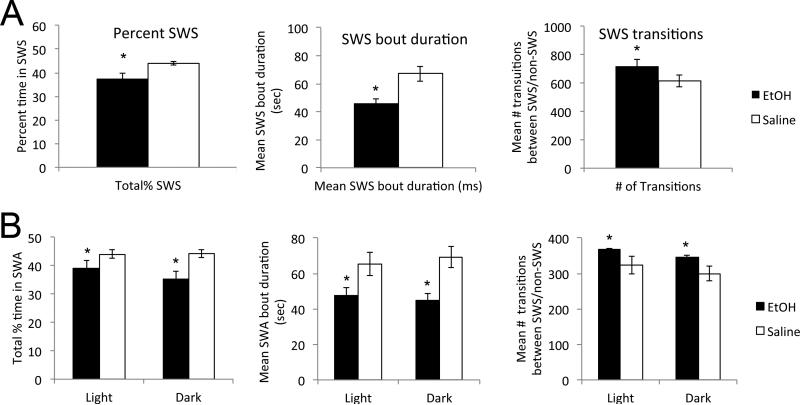
In 14 pairs of animals, slow-wave activity was quantified in terms of percent time in slow-wave state over 24hrs, mean slow-wave bout duration, and mean number of transitions to slow-wave (Fig. 3). Animals that had received developmental EtOH exhibited reduced time in slow-wave state (paired t-test, t(13) = 2.714, p < 0.02), reduced slow-wave bout duration (t(13) = 3.36, p < 0.01) and increased slow-wave state transitions (t(13) = 2.28, p < 0.05). Given the difference in percent time spent in slow-wave state between groups, we also compared number of slow-wave state transitions/hour of slow-wave activity (Fig. 3A). Again, EtOH-treated animals had an enhanced number of slow-wave state transitions (t(13) = 3.46, p < 0.01). In accord with the modified sleep bouts, EtOH treated animals also had significantly prolonged bouts of activity compared to saline controls (saline mean = 28.1 ± 2.6 sec; EtOH mean = 36.9 ± 2.6 sec, paired-t-test, t(8) = 3.59, p < 0.01). There was no detectable effect of sex on developmental ethanol-induced sleep fragmentation (e.g., percent time in slow-wave sleep, sex × postnatal treatment ANOVA, main effect of treatment F(1,24) = 6.82, p < 0.02; main effect of sex F(1,24) = 2.00, N.S.; sex × treatment interaction F(1,24) = 0.29, N.S. There were similar results for slow-wave sleep bout duration and transitions/hour. Data not shown). Together, these results suggest severely fragmented slow-wave sleep in adults exposed to ethanol during development.
Figure 3.
Sleep fragmentation in developmental EtOH-treated adult mice. A) Developmental EtOH-treated adult mice displayed a significant reduction in percent time in slow-wave sleep recorded over 24hr, decreased slow-wave sleep bout duration and enhanced slow-wave sleep transitions/hr than saline controls. B) This sleep fragmentation was apparent during both the light and dark phases of the 24hr day. Asterisks signify significant difference from saline controls.
This fragmentation was apparent during both the light and dark phases of the 24hr cycle (Fig. 3B). All three measures of sleep fragmentation, decreased percent time in slow-wave activity, decreased slow-wave bout duration and enhanced slow-wave transitions were significantly different between EtOH and saline treated animals during both the light and dark phases (treatment × light phase repeated measures ANOVA, main effect of group, percent time, F(1,26) = 8.99, p < 0.01; bout duration, F(1,26) = 16.48, p < 0.01; slow-wave transitions, F(1,26) = 6.96, p < 0.02; no significant main effect of phase or treatment × phase interaction).
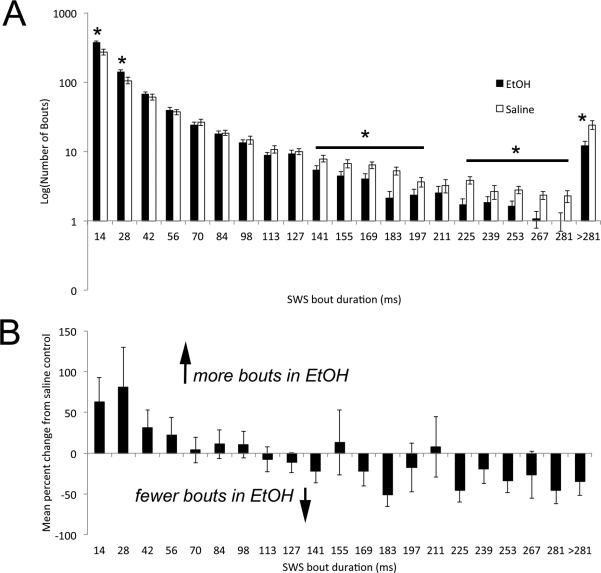
The distribution of sleep bout durations over 24hrs (Fig. 4) was significantly different between adults exposed on P7 to saline and EtOH (bout duration × developmental treatment, repeated measures ANOVA, main effect of treatment, F(1,273) = 5.01, p < 0.05). Developmental EtOH exposure resulted in enhanced numbers of short duration slow-wave bouts and decreased numbers of long duration slow-wave bouts compared to saline treated animals (bout durations × treatment interaction, F(20,273) = 5.36, p < 0.01).
Figure 4.
A) Frequency histogram of slow-wave sleep bout durations in developmental EtOH-exposed and saline controls adult mice. EtOH exposed mice show significantly fewer long duration bouts and more short duration bouts than saline controls. B) EtOH-Saline differences in bout durations. Asterisks signify significant difference from saline controls.
In addition to disruption of sleep structure, we also examined the effects of developmental EtOH exposure on delta frequency power during slow-wave bouts compared to adults exposed to saline at P7. FFT analyses were performed on all slow-wave bouts over the course of a 24hr period for each animal. Delta power was was reduced in adults exposed to developmental EtOH compared to saline controls, though this reduction did not reach significance (saline mean 24hr delta power = 0.43 ± 0.05 μV2, EtOH = 0.37 ± 0.05 μV2, paired t-test, t(9) = 0.74, N.S.).
Thus, P7 EtOH resulted in reduce and fragmented SWS. As a final measure of sleep structure, we examined the frequency of sleep spindles in five randomly chosen SWS bouts during four different periods across the 24hr cycle. Sleep spindles have been demonstrated to be important for some forms of sleep-dependent memory consolidation (Eschenko et al., 2006, Molle et al., 2009, Diekelmann and Born, 2010). Spindles were identified as described in the Method and shown in Fig. 1. No difference was observed in sleep spindle density (number of spindles per minute of NREM sleep) during SWS between groups (n=8 pairs, mean ± S.D.; EtOH; mean = 22.02 ± 0.7351; Saline, mean = 21.56 ± 0.5824; paired t(7) = 1.12, p = 0.30, N.S.).
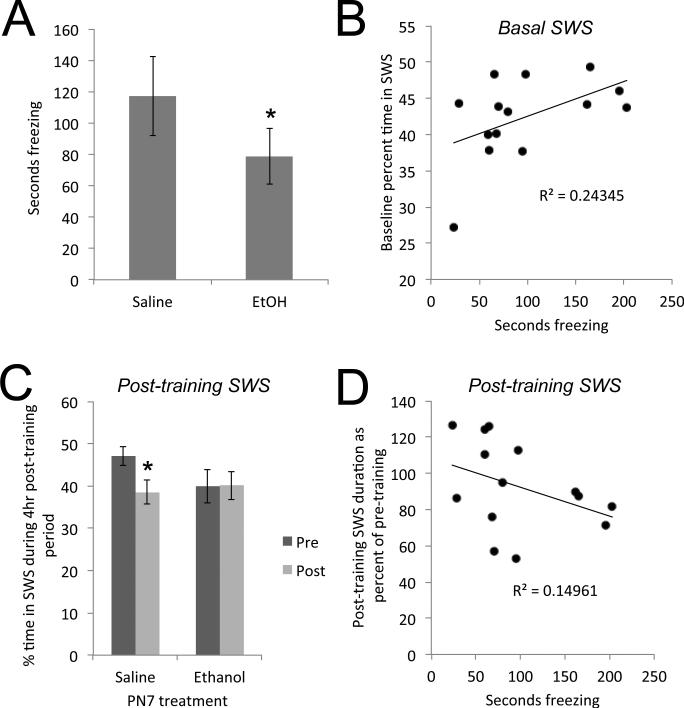
3.3 Impaired memory induced by developmental EtOH is correlated with impaired SWS
As previously reported (Berman and Hannigan, 2000, Wozniak et al., 2004, Gil-Mohapel et al., 2010, Subbanna et al., 2013, Sadrian et al., 2014), developmental ethanol resulted in impaired contextual fear memory in adult mice (Fig. 5A). Developmental ethanol exposed animals displayed impaired learned contextual fear memory compared to saline controls (7 pairs, paired t(6) = 2.61, p < 0.05). We next examined whether the developmental EtOH exposure-induced sleep fragmentation predicted this memory impairment. Percent time in slow-wave sleep over the days prior to fear conditioning was significantly correlated with learned fear (time spent freezing; r = 0.49, p < 0.05). Thus, the more time spent in slow-wave sleep prior to conditioning, the better the memory expression (Fig. 5B). The correlation between memory and slow-wave bout duration was not significant (r = 0.15, N.S.).
Figure 5.
A) Adult mice exposed to ethanol during early development showed impaired long-term (24hr) hippocampal-dependent context fear memory. B) This memory impairment was significantly correlated with percent time in slow-wave sleep recorded during the 24 hour period preceding training. C) Saline control mice show a significant decrease in time spent in slow-wave sleep during the 4hr post contextual fear conditioning. Developmental ethanol exposed mice show no plasticity in post-training slow-wave sleep. D) The ability to adjust time spent in post-conditioning slow-wave sleep is significantly correlated with contextual fear memory 24hr later. Asterisks signify significant difference from saline controls.
Post-training sleep also differed between EtOH and saline treated animals. Sleep related activity can be modulated by learning events (Eschenko et al., 2006, Stickgold and Walker, 2007, Molle et al., 2009, Barnes et al., 2011, Abel et al., 2013). For example, in rats odor-cued fear conditioning is associated with enhanced post-training slow-wave activity recorded in piriform cortex during the 4hr post-conditioning period, and this change in SWS is correlated with memory strength the following day (Barnes et al., 2011, Barnes and Wilson, 2014). In mice, fear conditioning is associated with decreased post-training sleep, especially REM sleep though to a lesser extent SWS (Sanford et al., 2003a, Sanford et al., 2003c, Wellman et al., 2013). Here, the amount of time spent in SWS during the 4hr post-training was significantly reduced compared to the same 4hr period on pre-conditioning days in adult mice exposed to saline at P7 (Fig. 5C). In contrast, mice developmentally exposed to EtOH did not modify their sleep patterns after contextual fear conditioning (repeated measures ANOVA, group × time interaction, F(1,14) = 4.41, p = 0.05). Post-hoc Fisher tests revealed a significant (p < 0.05) decreased time in SWS during the 4hr post-fear conditioning in saline treated mice, while the mice exposed to developmental EtOH did not. The ability to modify post-conditioning SWS was related to subsequent memory (Fig. 5D). The correlation between time spent freezing (memory strength) and the amount of post-training change in SWS trended towards significance (r = −0.387, p = 0.08). Animals that reduced time in SWS during the 4hrs post-conditioning showed enhanced contextual fear memory the following day.
Finally, in contrast to the observed relationship between SWS and contextual memory, there was no significant correlation between SWS and homecage hyperactivity (n = 10 pairs, r = −0.00).
4. DISCUSSION
The present results demonstrate that binge EtOH exposure during early development results in long-lasting slow-wave sleep fragmentation in adult mice. Within animals, the severity of this sleep fragmentation is significantly correlated with contextual fear memory impairment. Contextual fear conditioning is generally considered strongly influenced by hippocampal function (Gewirtz et al., 2000, Maren et al., 2013, Hunt and Barnet, 2016). While the developmental EtOH exposed mice were also behaviorally hyperactive in their homecage, 24hr hyperactivity levels did not correlate with sleep disturbance. Given the important role of sleep in memory consolidation and synaptic plasticity (Durmer and Dinges, 2005, Stickgold and Walker, 2007, Diekelmann and Born, 2010, Abel et al., 2013), the results suggest that developmental EtOH exposure not only induces immediate neural circuit disruption and cell death (Abel and Sokol, 1986, Bonthius and West, 1990, Ikonomidou et al., 2000, Olney et al., 2002a, Olney et al., 2002b, Saito et al., 2007, Gil-Mohapel et al., 2010, Saito et al., 2010, Sadrian et al., 2012, Smiley et al., 2015), but also induces the sustained and repeated insult of sleep deprivation, which in itself can lead to cognitive and emotional impairments (Durmer and Dinges, 2005, Killgore, 2010, LeGates et al., 2014).
Slow-wave sleep fragmentation in EtOH treated mice was characterized by reduced time in slow-wave sleep, reduced slow-wave bout duration, enhanced active bout duration, and increased numbers of slow-wave/fast wave transitions. Sleep fragmentation was expressed during both the active (dark) and inactive (light) portions of the day. This sleep fragmentation occurred without any concomitant change in circadian rhythm as assessed with body temperature and movement. It should be noted that more prolonged developmental exposure to EtOH can impair circadian rhythms as measured by running wheel activity (Allen et al., 2005). In the present study, activity levels were enhanced in EtOH treated animals, but normal rhythm was maintained.
Disruption of sleep following developmental EtOH exposure has been reported in both human infants (Troese et al., 2008, Chen et al., 2012) and rodents (Stone et al., 1996, Criado et al., 2008, Volgin and Kubin, 2012). The previous rodent work has focused on monitoring sleep over short periods (3-5hrs), and/or focused on infant animals (Hilakivi, 1986). Here we demonstrate that in adult mice slow-wave sleep across the circadian cycle is disrupted, and that this sleep disruption is significantly correlated with impaired memory. Although not reaching statistical significance, the power of delta oscillations during SWS was reduced by developmental ethanol. Stronger delta oscillations during SWS are associated with enhanced memory (Ngo et al., 2013). Thus, not only did the developmental EtOH exposure reduce the amount and stability of SWS, but reduced delta power suggests reduced efficacy even during successful SWS bouts. The present techniques did not allow analysis of REM sleep, although previous work has suggested sleep disruption includes REM state (Stone et al., 1996, Troese et al., 2008).
In addition to fragmentation of basal sleep, adults exposed to EtOH during development also had impaired sleep plasticity following conditioning. Various forms of conditioning can result in modified sleep states in the post-conditioning period in both humans and rodents (Sanford et al., 2003b, Eschenko et al., 2006, Stickgold and Walker, 2007, Molle et al., 2009, Diekelmann and Born, 2010, Barnes et al., 2011). The post-conditioning changes in sleep, or sleep oscillations can be local to the involved circuits (Huber et al., 2004, Pugin et al., 2015) Replay of recently acquired information can occur during post-conditioning sleep, strengthening those memories (Skaggs and McNaughton, 1996, Stickgold and Walker, 2007, Popa et al., 2010, Abel et al., 2013, Barnes and Wilson, 2014). In addition, post-conditioning reset of synaptic strength can occur during sleep, as a homeostatic mechanism for maintaining synapses and circuits within their most dynamic range (Huber et al., 2004, Liu et al., 2010). Loss of the ability to adjust sleep during the post-conditioning period in developmental EtOH exposed adults may be another significant contributor to impaired memory in these animals.
How does developmental ethanol exposure produce long-lasting impairment in SWS? There are a variety of potential mechanisms that could contribute to this sleep dysfunction. For example, previous work has demonstrated that neonatal ethanol exposure in mice severely reduces GABAergic neurons in the cortical regions of adult brains (Sadrian et al., 2014, Smiley et al., 2015), contributing to hyper-excitability of limbic circuits (Wilson et al., 2011, Sadrian et al., 2012), and seizure development (Bonthius et al., 2001, Bell et al., 2010). This induced change in excitation/inhibition balance could modify both plasticity and function of forebrain circuits underlying cognition and emotion (Hensch, 2005, Sadrian et al., 2013). However, GABAergic neurons are also important for sleep-related oscillations and switching between sleep states (Hermanstyne et al., 2010, Halassa et al., 2011, Abel et al., 2013, Qiu et al., 2014, Brown and McKenna, 2015, Xu et al., 2015), and impaired function of GABAergic neurons impairs sleep (Kalume et al., 2015). If developmental EtOH exposure impairs GABAergic neuron function and/or reduces GABAergic cell number in subcortical areas known to regulate sleep, this may be an important link between early ethanol and sleep, as well as a potential therapeutic target.
In summary, it is known that developmental ethanol exposure can induce widespread cell death, lasting decreases in adult neurogenesis, and neural circuit dysfunction. These events can contribute to the cognitive, emotional and behavioral sequelae of developmental EtOH. However in addition, the present results suggest that sleep deprivation, sleep inefficiency, and impaired sleep plasticity may be a continuing, lifelong insult following early EtOH exposure. This link is most pronounced with memory impairment, though does not appear to contribute to behavioral home cage hyperactivity. Work is ongoing to further explore the relationship between GABAergic neurons and sleep in the consequences of developmental EtOH exposure. The work suggests treatment of insomnia and improved sleep hygiene may be important treatments for the long-term cognitive impact of developmental EtOH exposure.
HIGHLIGHTS.
Developmental ethanol exposure induces slow-wave sleep fragmentation in adults
Early ethanol induced sleep fragmentation correlates with cognitive impairment
Early ethanol also impairs sleep plasticity following conditioning
Life-long sleep disruption may be a critical factor in cognitive disability following early ethanol
ACKNOWLEDGEMENTS
This work was supported by a grant from NIAAA (R01- AA023181) to M.S. and D.A.W. The authors thank Taylor Mustapich for assistance with data analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;2:1222. doi: 10.1016/s0140-6736(86)92234-8. [DOI] [PubMed] [Google Scholar]
- Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Current biology : CB. 2013;23:R774–788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GC, West JR, Chen WJ, Earnest DJ. Neonatal alcohol exposure permanently disrupts the circadian properties and photic entrainment of the activity rhythm in adult rats. Alcohol Clin Exp Res. 2005;29:1845–1852. doi: 10.1097/01.alc.0000183014.12359.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Chapuis J, Chaudhury D, Wilson DA. Odor fear conditioning modifies piriform cortex local field potentials both during conditioning and during post-conditioning sleep. PLoS One. 2011;6:e18130. doi: 10.1371/journal.pone.0018130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Wilson DA. Slow-wave sleep-imposed replay modulates both strength and precision of memory. J Neurosci. 2014;34:5134–5142. doi: 10.1523/JNEUROSCI.5274-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Rao H, Goel N, Dinges DF. Sleep deprivation and neurobehavioral dynamics. Current opinion in neurobiology. 2013;23:854–863. doi: 10.1016/j.conb.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SH, Stade B, Reynolds JN, Rasmussen C, Andrew G, Hwang PA, Carlen PL. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34:1084–1089. doi: 10.1111/j.1530-0277.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Pantazis NJ, Karacay B, Bonthius NE, Taggard Da, Lothman EW. Alcohol exposure during the brain growth spurt promotes hippocampal seizures, rapid kindling, and spreading depression. Alcohol Clin Exp Res. 2001;25:734–745. [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT. Turning a Negative into a Positive: Ascending GABAergic Control of Cortical Activation and Arousal. Frontiers in neurology. 2015;6:135. doi: 10.3389/fneur.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res. 2010;34:1022–1032. doi: 10.1111/j.1530-0277.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- Chen ML, Olson HC, Picciano JF, Starr JR, Owens J. Sleep problems in children with fetal alcohol spectrum disorders. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012;8:421–429. doi: 10.5664/jcsm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr., Oguz I, Lee J, Styner M, Crews FT. Postnatal day 7 ethanol treatment causes persistent reductions in adult mouse brain volume and cortical neurons with sex specific effects on neurogenesis. Alcohol. 2012;46:603–612. doi: 10.1016/j.alcohol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Current biology : CB. 2010;20:R626–627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DJ, Druschel CM. Estimating prevalence of fetal alcohol syndrome (FAS): effectiveness of a passive birth defects registry system. Birth Defects Res A Clin Mol Teratol. 2003;67:604–608. doi: 10.1002/bdra.10108. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Is the hippocampus necessary for contextual fear conditioning? Behav Brain Res. 2000;110:83–95. doi: 10.1016/s0166-4328(99)00187-4. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hermanstyne TO, Kihira Y, Misono K, Deitchler A, Yanagawa Y, Misonou H. Immunolocalization of the voltage-gated potassium channel Kv2.2 in GABAergic neurons in the basal forebrain of rats and mice. J Comp Neurol. 2010;518:4298–4310. doi: 10.1002/cne.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi L. Effects of prenatal alcohol exposure on neonatal sleep-wake behaviour and adult alcohol consumption in rats. Acta pharmacologica et toxicologica. 1986;59:36–42. doi: 10.1111/j.1600-0773.1986.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Barnet RC. Adolescent and adult rats differ in the amnesic effects of acute ethanol in two hippocampus-dependent tasks: Trace and contextual fear conditioning. Behav Brain Res. 2016;298:78–87. doi: 10.1016/j.bbr.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jan JE, Asante KO, Conry JL, Fast DK, Bax MC, Ipsiroglu OS, Bredberg E, Loock CA, Wasdell MB. Sleep Health Issues for Children with FASD: Clinical Considerations. Int J Pediatr. 2010 doi: 10.1155/2010/639048. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends in pharmacological sciences. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kalume F, Oakley JC, Westenbroek RE, Gile J, de la Iglesia HO, Scheuer T, Catterall WA. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol Dis. 2015;77:141–154. doi: 10.1016/j.nbd.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Progress in brain research. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl J, Staner L, Staner C, Loft H, Deacon S. Short-term treatment with gaboxadol improves sleep maintenance and enhances slow wave sleep in adult patients with primary insomnia. Psychopharmacology (Berl) 2007;195:139–146. doi: 10.1007/s00213-007-0866-0. [DOI] [PubMed] [Google Scholar]
- Manabe H, Kusumoto-Yoshida I, Ota M, Mori K. Olfactory cortex generates synchronized top-down inputs to the olfactory bulb during slow-wave sleep. J Neurosci. 2011;31:8123–8133. doi: 10.1523/JNEUROSCI.6578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfridi A, Brambilla D, Mancia M. Sleep is differently modulated by basal forebrain GABA(A) and GABA(B) receptors. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R170–175. doi: 10.1152/ajpregu.2001.281.1.R170. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34:1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- Morasch KC, Hunt PS. Persistent deficits in heart rate response habituation following neonatal binge ethanol exposure. Alcohol Clin Exp Res. 2009;33:1596–1604. doi: 10.1111/j.1530-0277.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D'Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002a;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002b;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Matthews K, Heinonen K, Paavonen JE, Lahti J, Komsi N, Lemola S, Jarvenpaa AL, Kajantie E, Strandberg T. Prenatal origins of poor sleep in children. Sleep. 2009;32:1086–1092. doi: 10.1093/sleep/32.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin F, Metz AJ, Wolf M, Achermann P, Jenni OG, Huber R. Local increase of sleep slow wave activity after three weeks of working memory training in children and adolescents. Sleep. 2015;38:607–614. doi: 10.5665/sleep.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Yao QL, Vetrivelan R, Chen MC, Lu J. Nigrostriatal Dopamine Acting on Globus Pallidus Regulates Sleep. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Sadrian B, Lopez-Guzman M, Wilson DA, Saito M. Distinct neurobehavioral dysfunction based on the timing of developmental binge-like alcohol exposure. Neuroscience. 2014;280:204–219. doi: 10.1016/j.neuroscience.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience. 2012;206:122–135. doi: 10.1016/j.neuroscience.2011.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrian B, Wilson DA, Saito M. Long-lasting neural circuit dysfunction following developmental ethanol exposure. Brain Sciences. 2013;3:704–727. doi: 10.3390/brainsci3020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Hegde M, Ohsie J, Paik SM, Vadasz C, Saito M. Involvement of ceramide in ethanol-induced apoptotic neurodegeneration in the neonatal mouse brain. J Neurochem. 2010;115:168–177. doi: 10.1111/j.1471-4159.2010.06913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Mao RF, Wang R, Vadasz C, Saito M. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol Clin Exp Res. 2007;31:665–674. doi: 10.1111/j.1530-0277.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003a;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003b;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003c;26:527–540. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skorput AG, Gupta VP, Yeh PW, Yeh HH. Persistent interneuronopathy in the prefrontal cortex of young adult offspring exposed to ethanol In Utero. Journal of Neuroscience. 2015;35:10977–10988. doi: 10.1523/JNEUROSCI.1462-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN, Gerum S, Wilson DA, Vadasz C. Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol. 2015 doi: 10.1016/j.alcohol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Altman HJ, Hall J, Arankowsky-Sandoval G, Parekh P, Gold PE. Prenatal exposure to alcohol in adult rats: relationships between sleep and memory deficits, and effects of glucose administration on memory. Brain Res. 1996;742:98–106. doi: 10.1016/s0006-8993(96)00976-6. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci. 2013;33:6350–6366. doi: 10.1523/JNEUROSCI.3786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troese M, Fukumizu M, Sallinen BJ, Gilles AA, Wellman JD, Paul JA, Brown ER, Hayes MJ. Sleep fragmentation and evidence for sleep debt in alcohol-exposed infants. Early human development. 2008;84:577–585. doi: 10.1016/j.earlhumdev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Kubin L. Reduced sleep and impaired sleep initiation in adult male rats exposed to alcohol during early postnatal period. Behav Brain Res. 2012;234:38–42. doi: 10.1016/j.bbr.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic GABAA agonist, gaboxadol, improves traditional hypnotic efficacy measures and enhances slow wave activity in a model of transient insomnia. Sleep. 2007;30:593–602. doi: 10.1093/sleep/30.5.593. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013;36:471–480. doi: 10.5665/sleep.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengel T, Hanlon-Dearman AC, Fjeldsted B. Sleep and sensory characteristics in young children with fetal alcohol spectrum disorder. J Dev Behav Pediatr. 2011;32:384–392. doi: 10.1097/DBP.0b013e3182199694. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14:813–818. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 2010;30:1760–1765. doi: 10.1523/JNEUROSCI.5636-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Peterson J, Basavaraj BS, Saito M. Local and regional network function in behaviorally relevant cortical circuits of adult mice following postnatal alcohol exposure. Alcohol Clin Exp Res. 2011;35:1974–1984. doi: 10.1111/j.1530-0277.2011.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, Dan Y. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiology of disease. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]