Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) contributes to premature death along with obesity, diabetes, and cardiovascular disease. We examined whether hepatic steatosis on ultrasound and liver enzyme activities were associated with increased liver disease mortality in the U.S. National Health and Nutrition Examination Survey (NHANES), 1988-1994, with up to 23 years of linked-mortality data.
Methods
Survey-linked National Death Index records were analyzed among 14,527 adult participants who were negative for viral hepatitis B and C and iron overload. Hepatic steatosis on ultrasound was categorized as normal, mild, moderate, or severe. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma glutamyltransferase (GGT) elevation was defined as the highest sex-specific decile.
Results
Cumulative mortality was 36.2% from all causes, including 16.3% from cardiovascular disease, 10.8% from cancer, 5.4% from diabetes, and 1.1% from liver disease. Severe hepatic steatosis was associated with increased liver disease mortality in both age-adjusted (hazard ratio [HR], 3.92; 95% confidence interval [CI], 1.49-10.27, p for trend, 0.011) and multivariate-adjusted analyses (HR, 2.68; 95% CI, 1.02-7.03; p for trend, 0.072). Hepatic steatosis was not independently associated with mortality from all-causes, cardiovascular disease, cancer, or diabetes. Higher liver disease mortality was found with elevated ALT (HR, 4.08; 95% CI,1.99-8.33), AST (HR, 4.33; 95% CI, 2.18-8.59), and GGT (HR, 7.91; 95% CI, 3.06-20.46). GGT elevation was associated with increased overall mortality (HR, 1.45; 95% CI, 1.21-1.74). Liver enzymes were, otherwise, unrelated to overall or cause-specific mortality.
Conclusion
In the U.S. population, severe hepatic steatosis on ultrasound and liver enzyme elevation were associated with increased liver disease mortality, but were not independently associated with mortality from all causes (except for GGT), cardiovascular disease, cancer, or diabetes.
Keywords: hepatic steatosis, non-alcoholic fatty liver disease, alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase
Fatty liver disease has become the most common liver disease in the United States and other Western countries and non-alcoholic steatohepatitis has emerged as the second leading etiology of liver disease among adults listed for liver transplantation in the U.S.(1-3) The prevalence of fatty liver disease in the U.S. population has ranged from 21% to 34% when measured by ultrasound or proton nuclear magnetic resonance spectroscopy(1, 4) and fatty liver prevalence has increased over the past two decades.(5, 6) Fatty liver disease encompasses a spectrum of liver injury ranging from steatosis to severe steatohepatitis that can progress to fibrosis, cirrhosis, liver failure, or hepatocellular carcinoma in a subgroup of patients.(7) However, the natural history remains incompletely understood. NAFLD is considered to contribute to premature death along with obesity, diabetes, and cardiovascular disease.(8, 9) However, studies of the general U.S. population have not found higher mortality from all-causes, cardiovascular disease, or diabetes among viral hepatitis negative persons with fatty liver on abdominal ultrasound or with abnormal serum ALT activity.(10-13) Higher liver disease mortality was found among persons with ALT elevation.(12, 14) We questioned whether recent survey-linked National Death Index records would reveal a stronger relationship of fatty liver disease with mortality outcomes. Mortality ascertainment is now complete for the third U.S. NHANES through 2011, providing up to 23 years of follow-up. We examined whether hepatic steatosis on ultrasound and abnormal serum activities of ALT, AST, and GGT, among persons without viral hepatitis or iron overload, were associated with all-cause and disease-specific mortality in this national, population-based, prospective survey.
METHODS
The NHANES III was conducted in the United States from 1988 through 1994 by the National Center for Health Statistics of the Centers for Disease Control and Prevention.(15) The survey consisted of cross-sectional interview, examination, and laboratory data collected from a complex multistage, stratified, clustered probability sample representative of the civilian, noninstitutionalized population with oversampling of non-Hispanic blacks, Mexican Americans, and persons aged 60 years or older. The survey was approved by the institutional review board of the Centers for Disease Control and Prevention, and all participants provided written informed consent to participate.
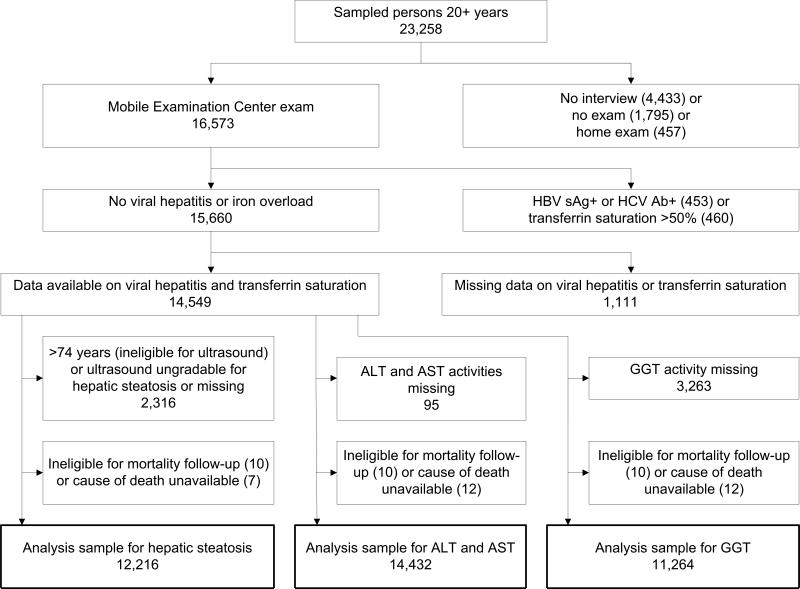
Of 23,258 sampled persons aged 20 years and older in NHANES III, 16,573 (71%) attended a study visit at a mobile examination center. Participants were excluded if they were positive for serum hepatitis B surface antigen or hepatitis C antibody (n=453), had a serum transferrin saturation >50% (n=460), were missing viral hepatitis serology or transferrin saturation (n=1,111), were ineligible for mortality follow-up (n=10), or did not have a cause of death available (n=12) (Figure 1). For analyses of hepatic steatosis, we additionally excluded persons who were ineligible for abdominal ultrasonography due to age older than 74 years or whose ultrasound was missing or ungradable for hepatic steatosis (n=2,316), resulting in an analysis sample of 12,216. For analyses of aminotransferases, we excluded persons who were missing data on ALT or AST activities (n=95) resulting in an analysis sample of 14,432. An additional 3,263 participants were surveyed before GGT was added to the protocol and, therefore, were excluded from analyses of GGT, resulting in an analysis sample of 11,264.
Figure 1.
Derivation of NHANES III sample for analysis of noninvasive fatty liver markers and mortality.
NHANES III, third National Health and Nutrition Examination Survey; HBV sAg+, hepatic B virus surface antigen positive; HCV Ab+, hepatitis C virus antibody positive; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase.
A serum sample was collected and shipped weekly at −20°C to a testing laboratory. ALT, AST, and GGT activities were assayed by using a Hitachi 737 Analyzer (Boehringer-Mannheim Diagnostics, Indianapolis, IN) at the White Sands Research Center, Alamogordo, NM.(16) Because ALT and AST activities are higher in men compared with women, and their relationships with mortality were previously found to be U-shaped,(14) ALT and AST were categorized as sex-specific deciles 1-3, 4-9, and 10 with deciles 4-9 serving as the comparison group. ALT cut-points were 13 IU/L and 34 IU/L, respectively, for men and 9 IU/L and 22 IU/L, respectively, for women and AST cut-points were 18 IU/L and 30 IU/L, respectively, for men and 15 IU/L and 26 IU/L, respectively, for women. The relationship of GGT activity with mortality was previously found to be linear and abnormal GGT activity was defined as the 10th decile using cut-points of 58 IU/L for men and 40 IU/L for women.(14)
Hepatic steatosis was ascertained among participants aged 20-74 by abdominal ultrasound. Ultrasounds were originally performed in 1988 to 1994 to evaluate gallbladder disease. Archived video tapes of ultrasounds were reviewed in 2009-2010 to assess the presence of fat within the hepatic parenchyma using standard criteria.(10, 17) Evaluation of hepatic steatosis was performed using five criteria: parenchymal brightness, liver to kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition. Readers under the supervision of a radiologist who was an expert in ultrasonography evaluated each scan. Hepatic steatosis was categorized as normal, mild, moderate or severe.
Data were collected at baseline on factors believed to be related to liver injury and included as covariates in multivariate analyses:(12, 18-25) age (years), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), education (years; <12, 12, >12), alcohol intake (never, former, >0-<1 drink/day, 1-2 drinks/day, >2 drinks/day), cigarette smoking (never, former, >0-<1 pack per day, ≥1 pack per day), caffeine intake from beverages (mg/day; deciles), physical activity (metabolic equivalents/month; deciles), diagnosed diabetes, body mass index [BMI; weight (kg) / height (m2)]; waist-to-hip circumference ratio, and systolic and diastolic blood pressure (mmHg). Serum was tested for hepatitis B virus surface antigen, hepatitis C virus antibody, transferrin saturation (%), platelet count (1000 cells/uL), and concentrations of hemoglobin A1C (%), total and high-density lipoprotein (HDL) cholesterol (mg/dL), C-reactive protein (mg/dL; 0-0.3, >0.3), estimated glomerular filtration rate (ml/min/1.73m2; ≥60, <60), alkaline phosphatase (IU/L), total bilirubin (mg/dL), and albumin (g/dL) as previously described.(16) Diabetes was defined as a diagnosis or hemoglobin A1C ≥6.5%.
Participants were passively followed for mortality through December 31, 2011, by linking NHANES III participants with National Death Index records through a probabilistic match, a well-established matching method.(26) In a validation study of a prior NHANES cohort, the accuracy of the method was high, with 96.1% of decedents and 99.4% of living participants being classified correctly.(27) Mortality outcomes were based on death certificate underlying or other cause of death coded according to the International Classification of Diseases, Ninth Revision (ICD-9), for deaths occurring between 1988 and 1998 and the International Classification of Diseases, Tenth Revision (ICD-10), for deaths occurring between 1999 and 2011. Outcomes for this analysis consisted of all-cause mortality and cause-specific mortality from cardiovascular disease (ICD-9 codes 410-414, 428, 429.2, 433-435, 437.0-437.1, 440, and 444; ICD-10 codes G45, I20-I25, I50, I63, I65-I66, I67.2, I67.8, I69.3, I70, and I74), neoplasms (ICD-9 codes 140-239; ICD-10 codes C00-D48), diabetes (ICD-9 code 250; ICD-10 codes E10-E14), and liver disease (ICD-9 codes 570-573; ICD-10 codes K70-K76). Deaths with liver cancer coded as underlying or other cause of death (n=11) were included with neoplasms. Restricted NHANES III mortality data were used for this analysis through the National Center for Health Statistics Research Data Center.(28)
Statistical analysis
Baseline characteristics of participants by hepatic steatosis status, or by liver enzyme activity deciles, were examined by comparing means (standard deviations) of continuous factors using a t test and percentages of categorical factors using a chi-square (χ2) test. Cumulative mortality during follow-up by hepatic steatosis status or by liver enzyme activity deciles was calculated using Kaplan-Meier analysis. HR estimates (relative risk) and 95% CIs for mortality outcomes were calculated using Cox proportional hazard regression analysis (SUDAAN PROC SURVIVAL) to control for effects of potential risk factors while taking into consideration varying lengths of follow-up. Factors included in multivariate analyses consisted of age, sex, race-ethnicity, education, alcohol intake, cigarette smoking, caffeine intake from beverages, physical activity, BMI, waist-to-hip circumference ratio, diabetes, total and HDL cholesterol, systolic and diastolic blood pressure, C-reactive protein, and estimated glomerular filtration rate. Continuous factors whose distributions were skewed to the right were expressed as deciles (10th percentiles) before being added to regression models. For analyses of hepatic steatosis, HRs were computed for each steatosis category relative to normal liver by categorizing steatosis status as indicator variables. The trend in HRs across steatosis categories was tested by treating steatosis status as an ordinal variable of 4 levels. For analyses of ALT and AST activities, HRs were computed for deciles 1-3 and decile 10 relative to deciles 4-9 by categorizing these aminotransferases as indicator variables. Time at risk was from the date of the NHANES III examination to the date of death or to December 31, 2011. For analyses of cause-specific mortality, participants who died from other causes were censored at the date of death. All factors met the proportional hazard assumption of a relatively constant risk ratio through examination of -log (-log) plots of survival versus time by categories.(29) Multivariate analyses excluded persons with missing values for any factor included in the model. P-values were two-sided, and a P-value of < 0.05 was considered to indicate statistical significance. All analyses utilized sample weights that accounted for unequal selection probabilities and nonresponse. All variance calculations accounted for the design effects of the survey using Taylor series linearization.(30)
RESULTS
Baseline characteristics
Among 12,216 participants 20-74 years of age without viral hepatitis or iron overload, the prevalence (standard error) of mild, moderate, and severe hepatic steatosis on ultrasound was 13.7% (0.6%), 13.7% (0.6%), and 6.8% (0.6%), respectively. Consistent with previous reports,(5, 10) persons with hepatic steatosis were older, more likely to be male, Mexican American, former or heavier drinkers, former smokers, and diabetic, and less likely to be non-Hispanic black or moderate drinkers, and had higher BMI, waist-to-hip circumference ratio, blood pressure, total cholesterol, C-reactive protein, and liver enzyme activities, and lower education levels, physical activity, and HDL cholesterol (Table 1). For most of these known NAFLD risk factors, increasing hepatic steatosis on ultrasound was associated with worsening health outcomes.
Table 1.
Characteristics* of NHANES III examination participants by category of hepatic steatosis on ultrasound, United States, 1988-1994
| Hepatic steatosis |
||||
|---|---|---|---|---|
| Normal (n=7,739) | Mild (n=1,662) | Moderate (n=1,909) | Severe (n=923) | |
| ALT (IU/L) | 15.4 (10.0) | 17.2 (12.1)1 | 22.9 (17.5)1,2 | 28.1 (20.6)1,2,3 |
| AST (IU/L) | 19.8 (8.0) | 20.5 (10.8)1 | 23.5 (12.6)1,2 | 27.4 (19.2)1,2,3 |
| GGT (IU/L) | 24.6 (27.5) | 26.8 (28.0) | 40.3 (51.7)1,2 | 45.4 (51.4)1,2,3 |
| Alkaline phosphatase (IU/L) | 79.0 (27.7) | 81.1 (26.2)1 | 87.4 (30.1)1,2 | 89.2 (30.0)1,2 |
| Albumin (g/dL) | 4.21 (0.37) | 4.17 (0.34)1 | 4.20 (0.35)2 | 4.17 (0.35) |
| Total bilirubin (mg/dL) | 0.60 (0.3) | 0.60 (0.3) | 0.60 (0.3) | 0.64 (0.3) |
| Platelet count (1000 cells/uL) | 270.5 (66.0) | 272.4 (68.4) | 276.7 (72.0)1 | 266.2 (69.2)3 |
| Age (years) | 41.1 (14.8) | 42.6 (14.8)1 | 46.6 (14.8)1,2 | 48.6 (14.1)1,2 |
| Women | 54.0 | 52.7 | 47.41,2 | 41.81,2,3 |
| Race-ethnicity | ||||
| Non-Hispanic white | 76.8 | 75.5 | 75.9 | 75.4 |
| Non-Hispanic black | 11.0 | 9.9 | 8.21 | 8.9 |
| Mexican American | 4.8 | 5.5 | 7.71,2 | 7.31 |
| Education (years) | 12.7 (3.0) | 12.4 (3.1)1 | 11.9 (3.3)1,2 | 11.9 (3.4)1,2 |
| Alcohol intake | ||||
| Never | 11.6 | 14.0 | 13.5 | 12.9 |
| Former | 30.6 | 30.9 | 35.91 | 35.4 |
| >0-<1 drink/day | 42.8 | 40.4 | 35.61 | 32.91,2 |
| 1-2 drinks/day | 9.5 | 8.5 | 7.11 | 8.5 |
| >2 drinks/day | 5.4 | 6.3 | 8.01 | 10.31 |
| Cigarette smoking | ||||
| Never | 46.5 | 48.0 | 42.21,2 | 43.4 |
| Former | 23.1 | 25.2 | 34.71,2 | 33.81,2 |
| >0-<1 pack/day | 13.5 | 12.7 | 8.91,2 | 9.41 |
| ≥1 pack/day | 16.9 | 14.01 | 14.21 | 13.4 |
| Caffeine intake (mg/day) | 236.8 (297.2) | 213.2 (224.3)1 | 223.1 (252.2) | 225.1 (260.1) |
| Physical activity (METs/month) | 116.8 (131.9) | 117.8 (144.7) | 94.9 (119.9)1,2 | 93.6 (113.6)1,2 |
| BMI (kg/m2) | 25.5 (4.6) | 27.0 (6.0)1 | 30.3 (7.1)1,2 | 30.8 (5.9)1,2 |
| Waist-to-hip ratio | 89.1 (8.2) | 90.4 (9.2)1 | 95.3 (10.0)1,2 | 97.6 (7.8)1,2,3 |
| Diabetes | 3.3 | 7.01 | 13.91,2 | 19.11,2,3 |
| Total cholesterol (mg/dL) | 201.4 (41.1) | 201.9 (41.9) | 213.6 (46.3)1,2 | 214.4 (43.8)1,2 |
| HDL cholesterol (mg/dL) | 52.2 (14.9) | 49.8 (15.3)1 | 45.8 (14.9)1,2 | 43.5 (15.6)1,2,3 |
| Systolic blood pressure (mmHg) | 119.0 (15.6) | 120.6 (17.5)1 | 127.1 (17.1)1,2 | 128.5 (16.4)1,2 |
| Diastolic blood pressure (mmHg) | 73.2 (9.5) | 74.5 (10.1)1 | 77.7 (10.4)1,2 | 78.0 (9.6)1,2 |
| C-reactive protein >0.3 mg/dL | 21.5 | 25.51 | 36.11,2 | 37.71,2 |
NHANES III, third National Health and Nutrition Examination Survey; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase; MET, metabolic equivalent; BMI, body mass index; HDL, high-density lipoprotein.
p<0.05 compared with normal liver
p<0.05 compared with mild hepatic steatosis
p<0.05 compared with moderate hepatic steatosis.
Statistics are mean (standard deviation) or percentage.
Among 14,432 participants 20 years or older without viral hepatitis or iron overload, those with liver enzyme activities in the highest decile were more likely to be Mexican American, heavier drinkers, and diabetic, and had higher levels of BMI, waist-to-hip circumference ratio, total cholesterol, diastolic blood pressure, C-reactive protein, and hepatic steatosis, and lower caffeine intake compared with the middle (4th-9th) deciles for ALT and AST or deciles 1-9 for GGT (Table 2). Compared with the reference deciles, participants with ALT activity in the highest decile were younger and less likely to be non-Hispanic black and those with GGT activity in the highest decile were older and more likely to be non-Hispanic black, while AST activity was unrelated to age or non-Hispanic black ethnicity.
Table 2.
Characteristics* of NHANES III examination participants by ALT, AST or GGT decile, United States, 1988-1994
| ALT decile† | AST decile‡ | GGT decile§ | ||||||
|---|---|---|---|---|---|---|---|---|
| 1-3 (n=4,634) | 4-9 (n=8,356) | 10 (n=1,464) | 1-3 (n=4,384) | 4-9 (n=8,437) | 10 (n=1,633) | 1-9 (n=9,822) | 10 (n=1464) | |
| Hepatic steatosis | ||||||||
| Normal | 76.21 | 65.1 | 39.81 | 71.61 | 66.0 | 46.21 | 67.8 | 44.71 |
| Mild | 13.7 | 14.6 | 9.31 | 14.5 | 13.8 | 11.4 | 14.2 | 11.8 |
| Moderate | 8.21 | 13.5 | 30.11 | 10.61 | 13.4 | 25.11 | 11.9 | 25.21 |
| Severe | 1.91 | 6.8 | 20.81 | 3.41 | 6.8 | 17.31 | 6.1 | 18.41 |
| ALT (IU/L) | 8.9 (2.4)1 | 17.0 (5.5) | 44.2 (23.6)1 | 11.5 (4.5)1 | 16.7 (7.7) | 38.2 (26.0)1 | 15.7 (9.4) | 31.9 (24.8)1 |
| AST (IU/L) | 16.7 (4.0)1 | 20.5 (5.8) | 36.1 (23.2)1 | 14.7 (2.1)1 | 20.9 (3.4) | 40.3 (21.8)1 | 19.7 (7.3) | 31.3 (22.1)1 |
| GGT (IU/L) | 18.9 (14.1)1 | 27.2 (26.1) | 63.8 (77.8)1 | 20.3 (15.5)1 | 26.7 (24.5) | 63.5 (80.8)1 | 20.9 (10.2) | 95.7 (79.6)1 |
| Age (years) | 46.7 (19.3)1 | 44.8 (16.4) | 41.2 (14.4)1 | 42.6 (16.5)1 | 46.1 (17.6) | 46.4 (16.7) | 44.5 (17.4) | 48.5 (15.7)1 |
| Women | 52.0 | 53.1 | 55.2 | 51.5 | 54.3 | 49.6 | 52.9 | 52.4 |
| Race-ethnicity | ||||||||
| Non-Hispanic white | 77.7 | 78.0 | 71.91 | 78.7 | 77.3 | 72.71 | 77.6 | 65.51 |
| Non-Hispanic black | 13.71 | 8.9 | 7.21 | 10.6 | 10.0 | 10.4 | 9.9 | 18.01 |
| Mexican American | 3.21 | 5.2 | 10.21 | 4.31 | 4.9 | 8.51 | 4.4 | 6.91 |
| Education (years) | 12.1 (3.2)1 | 12.5 (3.1) | 12.2 (3.4) | 12.3 (3.1) | 12.5 (3.2) | 12.2 (3.4) | 12.5 (3.2) | 11.7 (3.6)1 |
| Alcohol intake | ||||||||
| Never | 14.2 | 12.6 | 14.5 | 11.41 | 14.0 | 14.5 | 13.8 | 13.4 |
| Former | 33.4 | 33.0 | 32.3 | 34.7 | 32.4 | 32.0 | 33.5 | 30.8 |
| >0-<1 drink/day | 38.3 | 40.7 | 35.41 | 41.3 | 39.4 | 33.81 | 40.0 | 32.41 |
| 1-2 drinks/day | 8.9 | 8.3 | 8.4 | 8.1 | 8.5 | 10.0 | 7.8 | 12.01 |
| >2 drinks/day | 5.2 | 5.4 | 9.31 | 4.41 | 5.7 | 9.81 | 4.9 | 11.41 |
| Cigarette smoking | ||||||||
| Never | 42.11 | 48.3 | 51.7 | 39.61 | 50.1 | 50.4 | 47.8 | 44.6 |
| Former | 25.2 | 27.0 | 25.8 | 25.7 | 26.5 | 27.7 | 25.8 | 28.7 |
| >0-<1 pack/day | 13.41 | 10.9 | 13.5 | 12.9 | 11.3 | 12.1 | 11.6 | 13.6 |
| ≥1 pack/day | 19.21 | 13.8 | 9.01 | 21.91 | 12.1 | 9.8 | 14.8 | 13.2 |
| Caffeine intake (mg/day) | 247.5 (305.8) | 223.5 (273.4) | 193.4 (210.2)1 | 257.6 (322.4)1 | 217.8 (257.7) | 193.0 (238.8)1 | 230.8 (278.8) | 194.7 (235.5)1 |
| Physical activity (METs/month) | 108.5 (127.4) | 114.0 (135.1) | 101.7 (120.6)1 | 99.2 (120.1)1 | 115.9 (135.2) | 121.4 (141.6) | 112.8 (133.5) | 95.8 (119.7)1 |
| BMI (kg/m2) | 25.1 (5.0)1 | 27.0 (5.6) | 29.7 (6.5)1 | 26.3 (5.6)1 | 26.6 (5.5) | 28.4 (6.5)1 | 26.5 (5.5) | 29.0 (6.3)1 |
| Waist-to-hip ratio | 89.8 (9.2)1 | 91.2 (9.1) | 93.5 (8.5)1 | 90.5 (9.5) | 90.9 (9.0) | 93.2 (8.5)1 | 90.4 (9.1) | 94.8 (8.0)1 |
| Diabetes | 5.51 | 7.0 | 13.51 | 7.51 | 6.2 | 11.81 | 6.0 | 16.61 |
| Total cholesterol (mg/dL) | 199.7 (42.6)1 | 206.9 (42.0) | 212.9 (46.6)1 | 199.4 (41.1)1 | 207.4 (43.0) | 211.9 (45.6)1 | 202.8 (41.9) | 219.3 (44.8)1 |
| HDL cholesterol (mg/dL) | 52.2 (15.7)1 | 50.3 (15.1) | 46.7 (15.6)1 | 49.0 (14.3)1 | 51.5 (15.4) | 49.9 (18.4)1 | 50.4 (14.9) | 50.0 (18.2) |
| Systolic blood pressure (mmHg) | 122.8 (19.1) | 122.5 (17.5) | 123.0 (16.1) | 120.3 (17.2)1 | 123.3 (18.0) | 126.5 (18.3)1 | 122.1 (17.9) | 128.8 (18.1)1 |
| Diastolic blood pressure (mmHg) | 72.7 (9.9)1 | 74.5 (9.8) | 77.0 (10.4)1 | 73.0 (9.9)1 | 74.4 (9.8) | 76.9 (10.5)1 | 73.9 (9.9) | 77.7 (10.5)1 |
| C-reactive protein >0.3 mg/dL | 26.8 | 24.8 | 35.61 | 27.91 | 24.4 | 33.61 | 26.7 | 48.61 |
| Estimated glomerular filtration rate <60 ml/min/1.73m2 | 7.91 | 4.1 | 2.21 | 4.6 | 5.2 | 5.4 | 5.0 | 5.6 |
NHANES III, third National Health and Nutrition Examination Survey; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase; MET, metabolic equivalent; BMI, body mass index; HDL, high-density lipoprotein.
p<0.05 compared with 4th-9th deciles for ALT and AST or compared with 1st-9th deciles for GGT.
Statistics are mean (standard deviation) or percentage.
ALT cut-points were 13 and 34 IU/L for men and 9 and 22 IU/L for women.
AST cut-points were 18 and 30 IU/L for men and 15 and 26 IU/L for women.
GGT cut-point was 58 IU/L for men and 40 IU/L for women.
Mortality follow-up
The median follow-up time among participants 20 years or older without viral hepatitis or iron overload was 19.3 years (interquartile range, 17.4-21.1 years). The cumulative mortality from all causes was 36.2% (4,661 deaths) at 23 years of follow-up. The cause-specific cumulative mortality (underlying or other cause) was 16.3% (1,933 deaths) from cardiovascular disease, 10.8% (1,192 deaths) from cancer, 5.4% (576 deaths) from diabetes, and 1.1% (116 deaths) from liver disease.
Hepatic steatosis and liver disease mortality
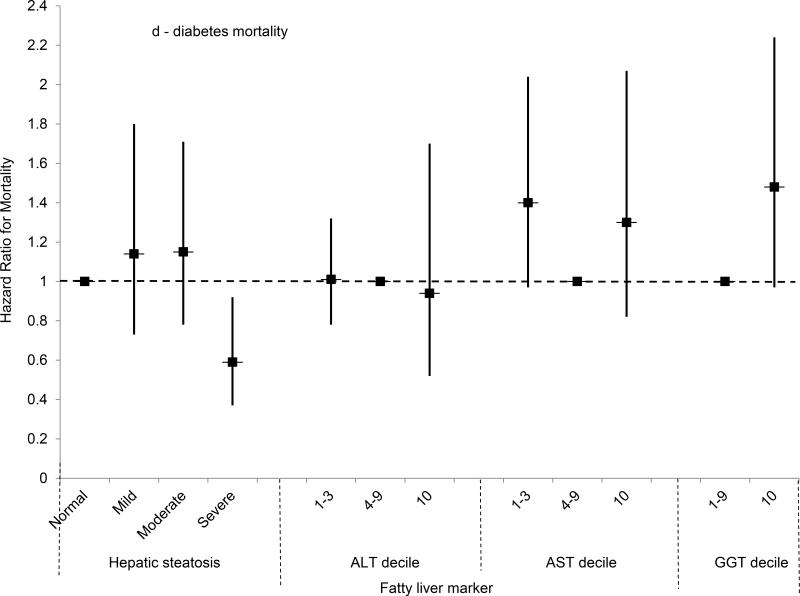
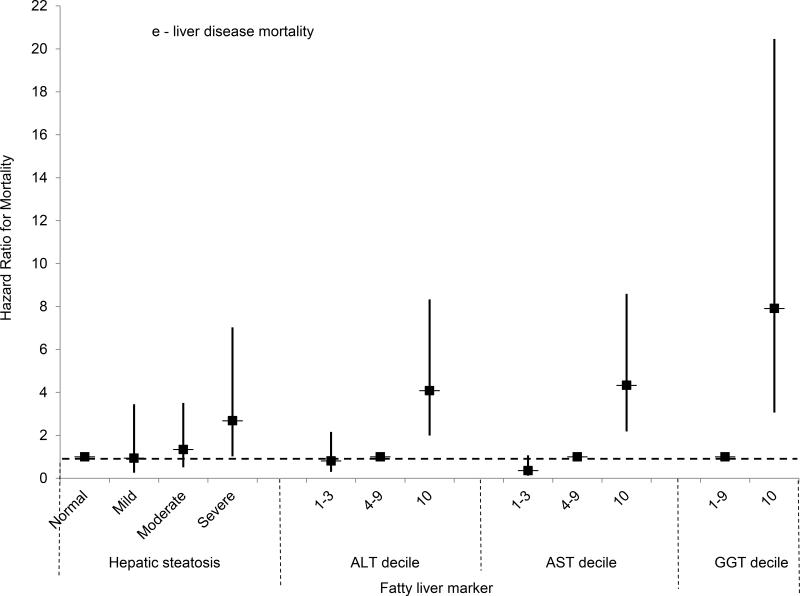
For mortality both overall and from each specific cause, the unadjusted cumulative mortality rates rose with increasing hepatic steatosis on ultrasound (Table 3). For liver disease mortality, severe hepatic steatosis was associated with almost four times the risk in age-adjusted analysis (HR, 3.92; 95% CI, 1.49-10.27, p=0.006) and a test for trend was significant (p=0.011). In multivariate-adjusted analysis, liver disease mortality remained over two and a half times as high among persons with severe steatosis (HR, 2.68; 95% CI, 1.02-7.03; p=0.046); however, a test for trend missed statistical significance (p=0.072) (Figure 2e). We were unable to evaluate liver disease mortality from underlying cause of death alone among persons with severe steatosis because of the small number of deaths; however, a test for trend was significant in age-adjusted analysis (p=0.037) and missed significance in multivariate-adjusted analysis (p=0.072).
Table 3.
Cumulative probability of mortality (unadjusted) over 23 years and age-adjusted hazard ratios for mortality by category of hepatic steatosis on ultrasound (N=12,216), United States, 1988-2011
| Mortality outcome Hepatic steatosis category* | No. of deaths | Unadjusted cumulative mortality† | Age-adjusted |
||
|---|---|---|---|---|---|
| HR‡ | 95% CI | p-value | |||
| All-cause | |||||
| Normal | 1,617 | 24.8 | 1.0 | ||
| Mild | 388 | 28.5 | 1.17 | 0.98 – 1.41 | 0.082 |
| Moderate | 577 | 35.5 | 1.20 | 1.06 – 1.35 | 0.005 |
| Severe | 296 | 38.1 | 1.26 | 1.06 - 1.49 | 0.011 |
| Ordinal | -- | -- | 1.09 | 1.04 – 1.14 | <0.001 |
| Cardiovascular disease | |||||
| Normal | 577 | 9.5 | 1.0 | ||
| Mild | 146 | 11.7 | 1.16 | 0.86 – 1.57 | 0.31 |
| Moderate | 246 | 16.0 | 1.41 | 1.19 – 1.68 | <0.001 |
| Severe | 95 | 14.5 | 1.30 | 0.97 – 1.73 | 0.078 |
| Ordinal | -- | -- | 1.13 | 1.04 – 1.22 | 0.003 |
| Cancer | |||||
| Normal | 485 | 7.8 | 1.0 | ||
| Mild | 111 | 8.8 | 1.19 | 0.84 – 1.69 | 0.33 |
| Moderate | 147 | 11.7 | 1.08 | 0.83 – 1.41 | 0.54 |
| Severe | 99 | 13.8 | 1.13 | 0.81 – 1.59 | 0.46 |
| Ordinal | -- | -- | 1.05 | 0.96 – 1.14 | 0.31 |
| Diabetes | |||||
| Normal | 196 | 3.2 | 1.0 | ||
| Mild | 57 | 5.1 | 2.01 | 1.25 – 3.23 | 0.005 |
| Moderate | 111 | 8.0 | 2.80 | 1.98 – 3.96 | <0.001 |
| Severe | 59 | 10.1 | 2.16 | 1.27 – 3.69 | 0.006 |
| Ordinal | -- | -- | 1.41 | 1.23 – 1.61 | <0.001 |
| Liver disease | |||||
| Normal | 50 | 0.82 | 1.0 | ||
| Mild | 14 | 1.1 | 0.96 | 0.29 – 3.15 | 0.94 |
| Moderate | 21 | 1.4 | 1.58 | 0.69 – 3.65 | 0.27 |
| Severe | 10 | 1.4 | 3.92 | 1.49 – 10.27 | 0.006 |
| Ordinal | -- | -- | 1.50 | 1.10 – 2.03 | 0.011 |
HR, hazard ratio; CI, confidence interval.
N=7,729 with normal liver and 1,657 with mild, 1,908 with moderate, and 922 with severe hepatic steatosis.
Estimated using Kaplan-Meier analysis.
Estimated using Cox proportional hazards regression analysis.
Figure 2.
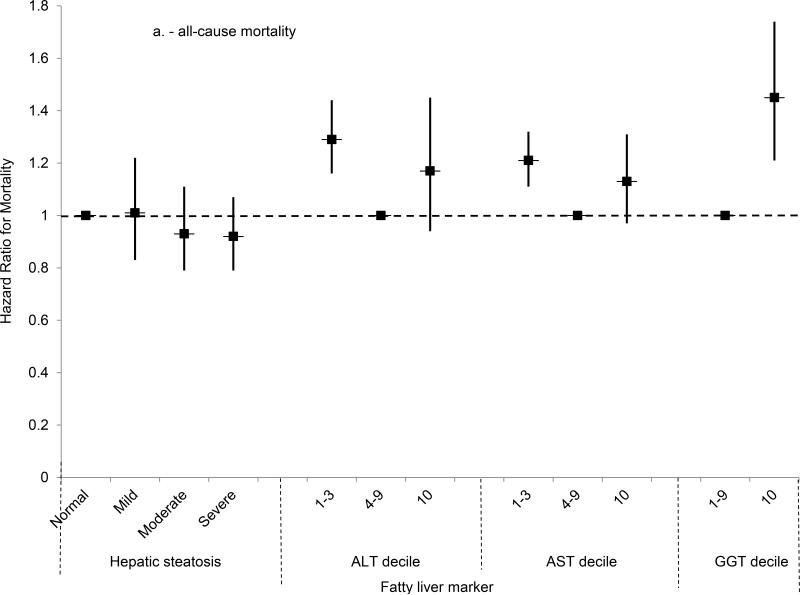
(A) Multivariate-adjusted hazard ratios and 95% confidence intervals for all-cause mortality by category of hepatic steatosis on ultrasound or AST, ALT or GGT decile, United States, 1988-2011.
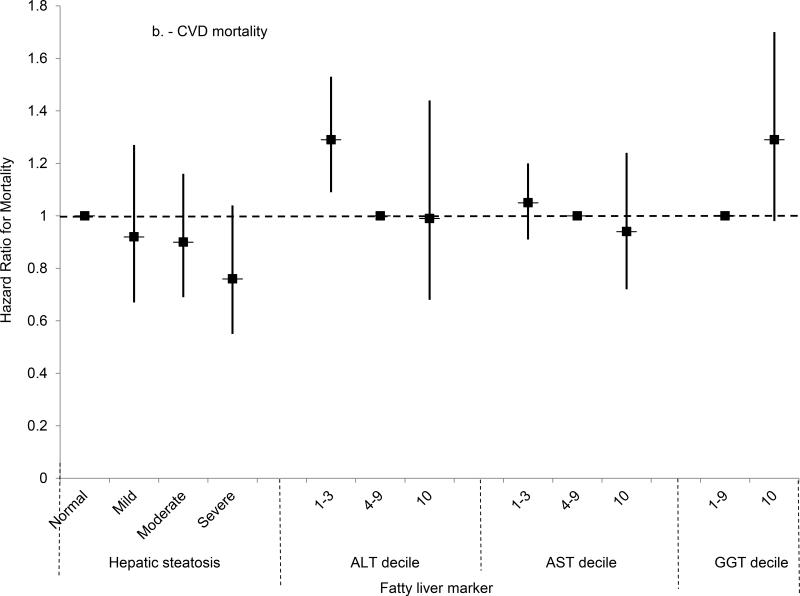
(B) Multivariate-adjusted hazard ratios and 95% confidence intervals for cardiovascular disease mortality by category of hepatic steatosis on ultrasound or AST, ALT or GGT decile, United States, 1988-2011.
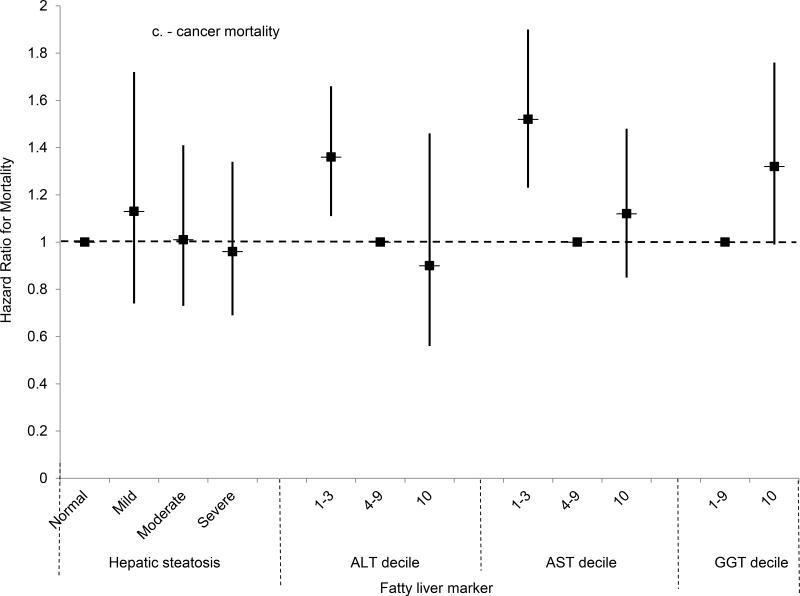
(C) Multivariate-adjusted hazard ratios and 95% confidence intervals for cancer mortality by category of hepatic steatosis on ultrasound or AST, ALT or GGT decile, United States, 1988-2011.
(D) Multivariate-adjusted hazard ratios and 95% confidence intervals for diabetes mortality by category of hepatic steatosis on ultrasound or AST, ALT or GGT decile, United States, 1988-2011.
(E) Multivariate-adjusted hazard ratios and 95% confidence intervals for liver disease mortality by category of hepatic steatosis on ultrasound or AST, ALT or GGT decile, United States, 1988-2011.
Footnotes for Figure 2:
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase.
For hepatic steatosis, N=7,729 with normal liver and 1,657 with mild, 1,908 with moderate, and 922 with severe steatosis.
For ALT, N=4,627 in deciles 1-3, 8,343 in deciles 4-9, and 1,462 in decile 10. Cut-points were 13 and 34 IU/L for men and 9 and 22 IU/L for women.
For AST, N=4,375 in deciles 1-3, 8,428 in deciles 4-9, and 1,629 in decile 10. Cut-points were 18 and 30 IU/L for men and 15 and 26 IU/L for women.
For GGT, N=9,804 in deciles 1-9 and 1,460 in decile 10. Cut-points were 58 IU/L for men and 40 IU/L for women.
Hazard ratios were estimated using Cox proportional hazards regression analysis and adjusted for age, sex, race-ethnicity, education, alcohol intake, cigarette smoking, caffeine intake from beverages, physical activity, BMI, waist-to-hip ratio, diabetes, total and HDL cholesterol, systolic and diastolic blood pressure, C-reactive protein, and estimated glomerular filtration rate.
Mortality from all causes was higher among persons with moderate or severe steatosis, and a test for trend was significant, in analyses adjusted only for age (Table 3). However, after adjusting for multiple factors, no statistically significant association was found (Figure 2a). For cardiovascular disease mortality, moderate hepatic steatosis was associated with higher mortality and a test for trend was significant in analysis adjusted only for age, but no statistically significant association was found after multivariate-adjustment (Figure 2b). For cancer, mortality was not increased in analyses adjusted only for age or in multivariate-adjusted analyses (Figure 2c). For diabetes, mortality was over twice as high for each hepatic steatosis category and a test for trend was significant in analysis adjusted only for age, but no statistically significant association was found after multivariate-adjustment (Figure 2d). Results for cardiovascular disease, cancer, and diabetes mortality were similar if limited to underlying cause of death alone (data not shown).
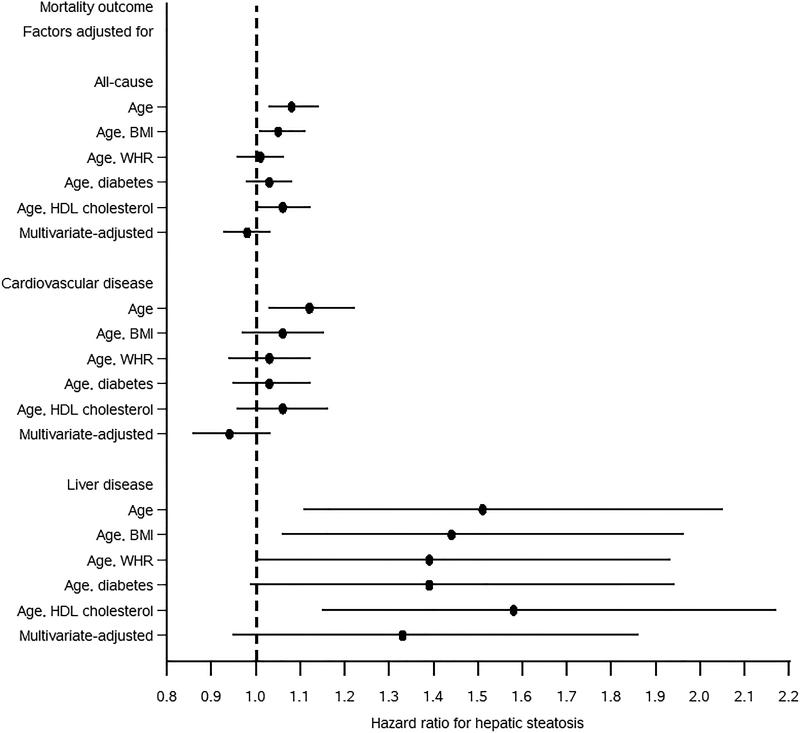
The increased all-cause and cardiovascular disease mortality with hepatic steatosis that was found in analyses adjusted only for age, but not with multivariate adjustment was further evaluated to identify risk factors accounting for the difference. We conducted analyses adjusting for age and each of the following risk factors individually: waist-to-hip circumference ratio, diabetes, and HDL cholesterol. These analyses included 11,715 participants with complete data on all of these risk factors of interest. Hepatic steatosis was categorized as an ordinal variable with four levels, i.e., normal, mild, moderate, and severe. For all-cause mortality adjusting for waist-to-hip ratio, diabetes, or HDL cholesterol in addition to age decreased the risk of higher mortality with increasing hepatic steatosis, and with adjustment for waist-to-hip ratio or diabetes, this relationship was no longer statistically significant (Figure 3). Likewise, for cardiovascular disease mortality the higher risk in age-adjusted analysis was no longer statistically significant with the addition of any of the other three factors. In contrast, for liver disease mortality, the age-adjusted HR was only modestly decreased with addition of each of the other factors
Figure 3.
Hazard ratios and 95% confidence intervals for the relationship of hepatic steatosis on ultrasound with all-cause, cardiovascular disease, or liver disease mortality adjusted for risk factors, United States, 1988-2011 (N=11,715).
WHR, waist-to-hip ratio; HDL, high-density lipoprotein.
Hepatic steatosis was included in models as an ordinal variable with 4 levels, i.e., normal, mild, moderate, and severe. Hazard ratios were estimated using Cox proportional hazards regression analysis. Multivariate-adjusted hazard ratios were adjusted for age, sex, race-ethnicity, education, alcohol intake, cigarette smoking, caffeine intake from beverages, physical activity, BMI, waist-to-hip ratio, diabetes, total and HDL cholesterol, systolic and diastolic blood pressure, and C-reactive protein.
Because the relationship of severe hepatic steatosis on ultrasound with mortality has not been previously studied in the general U.S. population, we conducted 2 supplementary analyses to examine: 1) mortality outcomes among participants with severe hepatic steatosis and additional risk factors (ALT and/or AST elevated, overweight or obese, and diabetes), and 2) factors associated with mortality among the subgroup of persons with severe hepatic steatosis. Additional description and results of these analyses are presented in Supplementary Online Tables 1 and 2.
Liver enzymes and liver disease mortality
We also studied the relationship of liver enzyme elevation with mortality outcomes. Because persons with ALT in the highest decile were younger (Table 2), they had lower unadjusted cumulative mortality rates for all outcomes except liver disease for which cumulative mortality was higher (Table 4). After adjusting for age, ALT in the highest decile was associated with over 5 times the risk of liver disease mortality (Table 4). The risk remained increased over fourfold with adjustment for additional factors (HR, 4.08; 95% CI, 1.99-8.33; p<0.001) (Figure 2e). If liver disease mortality was defined based on underlying cause of death alone, rather than from any of the multiple causes recorded on the death certificate, there were 57 deaths. The cumulative mortality was increased among persons with ALT in the highest decile (1.6%) compared to those in the 4th-9th deciles (0.48%). In age-adjusted analysis, persons with ALT in the highest decile had six times the risk of liver disease mortality (HR=6.06, 95% CI=2.42-15.17, p<0.001), and the risk remained increased in multivariate-adjusted analysis (HR=3.78, 95% CI=1.71-8.34, p=0.002).
Table 4.
Cumulative probability of mortality (unadjusted) over 23 years and age-adjusted hazard ratios for mortality by ALT or AST decile (N=14,432), United States, 1988-2011
| ALT* | AST† | |||||||
|---|---|---|---|---|---|---|---|---|
| Mortality outcome ALT or AST decile | No. of deaths | Unadjusted cumulative mortality‡ | Age-adjusted |
No. of deaths | Unadjusted cumulative mortality | Age-adjusted |
||
| HR§ | 95% CI | HR | 95% CI | |||||
| All-cause | ||||||||
| 1-3 | 1,985 | 46.1 | 1.37 | 1.24 – 1.52 | 1,391 | 35.0 | 1.46 | 1.35 – 1.58 |
| 4-9 | 2,356 | 33.0 | 1.0 | 2,708 | 36.3 | 1.0 | ||
| 10 | 277 | 22.5 | 1.30 | 1.07 – 1.58 | 519 | 38.6 | 1.20 | 1.06 – 1.37 |
| Cardiovascular disease | ||||||||
| 1-3 | 880 | 22.7 | 1.38 | 1.16 – 1.64 | 586 | 15.8 | 1.41 | 1.22 – 1.62 |
| 4-9 | 944 | 14.2 | 1.0 | 1,151 | 16.6 | 1.0 | ||
| 10 | 95 | 8.8 | 1.19 | 0.83 – 1.72 | 182 | 16.1 | 1.04 | 0.83 – 1.30 |
| Cancer | ||||||||
| 1-3 | 503 | 14.7 | 1.44 | 1.19 – 1.74 | 402 | 12.3 | 1.79 | 1.48 – 2.17 |
| 4-9 | 611 | 9.7 | 1.0 | 658 | 10.0 | 1.0 | ||
| 10 | 64 | 5.4 | 0.94 | 0.63 – 1.40 | 118 | 10.2 | 1.13 | 0.89 – 1.43 |
| Diabetes | ||||||||
| 1-3 | 183 | 5.6 | 0.84 | 0.61 – 1.16 | 223 | 6.6 | 1.89 | 1.37 – 2.60 |
| 4-9 | 336 | 5.4 | 1.0 | 274 | 4.5 | 1.0 | ||
| 10 | 52 | 4.9 | 2.12 | 1.24 – 3.61 | 74 | 7.2 | 1.98 | 1.37 – 2.86 |
| Liver disease | ||||||||
| 1-3 | 28 | 0.82 | 0.90 | 0.38 – 2.14 | 19 | 0.55 | 0.44 | 0.17 – 1.17 |
| 4-9 | 56 | 0.93 | 1.0 | 49 | 0.83 | 1.0 | ||
| 10 | 31 | 2.6 | 5.18 | 2.82 – 9.52 | 47 | 3.8 | 5.73 | 2.83 – 11.59 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HR, hazard ratio; CI, confidence interval.
N=4,627 in deciles 1-3, 8,343 in deciles 4-9, and 1,462 in decile 10. Cut-points were 13 and 34 IU/L for men and 9 and 22 IU/L for women.
N=4,375 in deciles 1-3, 8,428 in deciles 4-9, and 1,629 in decile 10. Cut-points were 18 and 30 IU/L for men and 15 and 26 IU/L for women.
Estimated using Kaplan-Meier analysis.
Estimated using Cox proportional hazards regression analysis.
All-cause mortality was increased with an ALT in the highest decile after adjusting for age (Table 4); however, with adjustment for multiple factors, the relationship was no longer statistically significant (Figure 2a). For cardiovascular disease, an ALT in the highest decile was not associated with a significantly increased risk of mortality after age-adjustment, and this was unchanged in multivariate-adjusted analysis (Figure 2b). A negative result was, likewise, found for cancer (Figure 2c). For diabetes, ALT in the highest decile was associated with over twice the risk of mortality in age-adjusted analysis, but not after multivariate adjustment (Figure 2d). The increased diabetes mortality among persons with ALT in the highest decile with adjustment only for age was explained by the higher prevalence of diabetes at baseline among persons with elevated ALT. With adjustment for age and baseline diabetes alone, ALT in the highest decile was no longer associated with increased diabetes mortality (HR=1.08; 95% CI, 0.66-1.75; p=0.77). Adjusting for other factors included in the multivariate-adjusted model had limited effect. If mortality was limited to underlying cause of death alone, results were similar to those for underlying or other cause of death for cardiovascular disease and cancer and for diabetes mortality there was no longer a statistically significant association in age-adjusted analysis (data not shown).
For AST, unadjusted cumulative mortality rates from all-causes and from diabetes and liver disease were higher with AST in the highest decile (Table 4). Age- and multivariate-adjusted relationships were similar to those for ALT (Table 4, Figures 2a-e). For liver disease, the mortality risk was increased over five-fold in age-adjusted analysis, and remained over four times higher with multivariate adjustment (HR, 4.33; 95% CI, 2.18-8.59; p<0.001).
GGT elevation was more likely to be related to mortality compared with ALT or AST. For both all-cause mortality and each specific cause, the cumulative mortality was higher among participants with GGT in the highest decile (Table 5). For liver disease mortality, GGT in the highest decile was associated with over 10 times the risk in age-adjusted analysis (Table 5). The risk remained increased almost eight-fold with adjustment for additional factors (HR=7.91, 95% CI=3.06-20.46, p<0.001) (Figure 2e). If liver disease mortality was defined based on underlying cause of death alone, there were 39 deaths. The cumulative mortality was higher among persons with GGT in the highest decile (2.0%) compared to those in the 4th-9th deciles (0.27%). In age-adjusted analysis, persons with GGT in the highest decile had over eleven times the risk of liver disease mortality (HR=11.71, 95% CI=4.28–32.07, p<0.001), and an over eight-fold higher risk remained in multivariate-adjusted analysis (HR=8.19, 95% CI=2.78–24.18).
Table 5.
Cumulative probability of mortality (unadjusted) over 23 years and age-adjusted hazard ratios for mortality by GGT decile (N=11,264), United States, 1988-2011
| Mortality outcome GGT decile* | No. of deaths | Unadjusted cumulative mortality† | Age-adjusted |
||
|---|---|---|---|---|---|
| HR‡ | 95% CI | p-value | |||
| All-cause | |||||
| 1-9 | 2,915 | 32.5 | 1.0 | ||
| 10 | 522 | 40.5 | 1.66 | 1.46 – 1.88 | <0.001 |
| Cardiovascular disease | |||||
| 1-9 | 1,229 | 14.6 | 1.0 | ||
| 10 | 196 | 17.2 | 1.51 | 1.27 – 1.80 | <0.001 |
| Cancer | |||||
| 1-9 | 743 | 9.4 | 1.0 | ||
| 10 | 135 | 13.9 | 1.54 | 1.12 – 2.11 | 0.008 |
| Diabetes | |||||
| 1-9 | 336 | 4.3 | 1.0 | ||
| 10 | 84 | 9.1 | 2.75 | 2.08 – 3.63 | <0.001 |
| Liver disease | |||||
| 1-9 | 40 | 0.55 | 1.0 | ||
| 10 | 40 | 3.5 | 10.75 | 5.26 – 21.96 | <0.001 |
GGT, gamma glutamyltransferase; HR, hazard ratio; CI, confidence interval.
N=9,804 in deciles 1-9 and 1,460 in decile 10. Cut-point was 58 IU/L for men and 40 IU/L for women.
Estimated using Kaplan-Meier analysis.
Estimated using Cox proportional hazards regression analysis.
The risk of all-cause mortality was over 60% higher among persons with GGT in the highest decile in age-adjusted analysis (Table 5), and an increased risk remained after adjusting for multiple factors (HR, 1.45; 95% CI, 1.21-1.74; p<0.001) (Figure 2a). For both cardiovascular disease and cancer, the mortality risk for persons with GGT in the highest decile was increased over 50% in age-adjusted analysis, but diminished with multivariate adjustment and no longer reached statistical significance (Figures 2b-c). For diabetes mortality, GGT in the highest decile was associated with over two and a half times the risk in age-adjusted analysis, but the association diminished and no longer reached statistical significance after multivariate adjustment (Figure 2d). Results for cardiovascular disease, cancer, and diabetes mortality were similar if mortality was limited to underlying cause of death alone.
DISCUSSION
In this large, national, U.S. population-based study with up to 23 years of follow-up, ultrasound-documented fatty liver among persons without viral hepatitis or iron overload was associated with mortality from liver disease. This relationship was limited to persons with severe hepatic steatosis. Although the number of liver disease deaths among persons with severe steatosis was not large, the relationship was consistent in age-adjusted and multivariate-adjusted analyses. Higher liver disease mortality in association with fatty liver was not found in previous analyses of the NHANES population which had shorter follow-up and did not look at severe steatosis on ultrasound as a separate category.(10, 11) In contrast, fatty liver disease was not independently associated with mortality overall or from cardiovascular disease, cancer, or diabetes. In analyses adjusted only for age, mortality from all-causes, cardiovascular disease, and diabetes was higher with fatty liver and the risk rose with increasing steatosis. However, adjusting for metabolic factors such as obesity, diabetes, and dyslipidemia, eliminated these statistically significant associations. These findings are consistent with those of previous studies in the U.S. population with shorter follow-up and varying definitions of fatty liver disease.(10, 11)
In analyses of liver enzymes, higher liver disease mortality was found with elevation of all three enzymes, consistent with previous studies with shorter follow-up.(12, 14) For GGT, which is less specific to the liver compared with ALT or AST, an association was also found with overall mortality, also consistent with a previous study.(12) Positive relationships of GGT elevation with cardiovascular disease, cancer, and diabetes mortality just missed statistical significance in the current analysis which used higher cut-points than in the previous study which found associations with these outcomes.(12)
NAFLD contributes to premature death and is believed to accompany cardiovascular disease leading to increased mortality.(8, 9) Despite up to 23 years of follow-up (median 19.3), we were unable to confirm such an association with cardiovascular disease mortality in the general U.S. population. Fatty liver disease is a complex trait influenced by both genetic variation and environmental factors. It shares many important risk factors with cardiovascular disease and NAFLD is considered to be the liver manifestation of the metabolic syndrome.(31, 32) However, the variation in hepatic steatosis prevalence among racial-ethnic groups(1, 4) and the incomplete correlation between metabolic and other risk factors and the presence of fatty liver suggest an important contribution for genetic factors. Recently, significant advances have taken place in understanding the genetics of NAFLD. Genetic variation in the patatin-like phospholipase domain-containing protein 3 (PNPLA3) was found to be associated with differences in proton magnetic resonance spectroscopy measured hepatic triglyceride content.(33) This finding was subsequently confirmed in other populations and extended to include associations with the histological severity of NAFLD and the occurrence of NAFLD-associated hepatocellular carcinoma.(34-38) Additional NAFLD-associated loci have been identified including variants in or near the genes GCKR, LYPLAL1, PPP1R3B, and the NCAN/TM6SF2/CILP2/PBX4 region on chromosome 19.(39) Interestingly, gene variants conferring increased risk for NAFLD did not uniformly result in abnormalities in lipids or glycemic and anthropometric traits.(35, 39, 40) More recently, a variant of the transmembrane 6 superfamily member 2 (TM6SF2) gene conferring susceptibility to hepatic steatosis was identified.(41) This finding has been independently validated and extended to include associations with hepatic fibrosis progression and a lower risk of cardiovascular disease.(42-44) TM6SF2 has been determined to play a role in VLDL secretion from liver into serum and normal gene activity results in increased serum lipid and risk of myocardial infarction and decreased risk of liver steatosis, while the reverse is found with the genetic variant.(40, 41) These findings suggest a genetic heterogeneity in the etiology of NAFLD with the existence of multiple metabolic subtypes.(39, 40) Further understanding of the complex genetic basis of fatty liver disease may help to explain why mortality associations found in studies of selected patient samples have not been confirmed in the general U.S. population.
The term ‘NAFLD’ refers to hepatic steatosis by imaging or histology in persons without secondary causes for hepatic fat accumulation, such as significant alcohol intake (>21 drinks on average per week for men or >14 drinks on average per week for women), medications, or hereditary disorders.(45) However, because over two thirds of U.S. adults are now overweight or obese, mixed forms of NAFLD and alcoholic fatty liver disease are increasingly likely.(46, 47) Furthermore, the accuracy of self-reported alcohol consumption is unknown and clinical diagnosis based on patient-reported intake remains a potential source of misclassification between modest and significant alcohol use. Therefore, we treated fatty liver disease holistically and chose not to exclude persons based on their reported alcohol consumption in this integrated noninvasive fatty liver disease marker study. Instead, we adjusted for alcohol intake in multivariate analyses to present a more comprehensive and informative picture of the fatty liver disease epidemic in the U.S.
As previously reported, a limitation of using NHANES to study liver injury is the reliance on ultrasound-detected hepatic steatosis or single serum liver enzyme activities as markers, whereas the criterion standard for clinical management is a histological diagnosis. However, liver biopsies cannot be conducted on the general population. Magnetic resonance imaging is highly accurate and correlates well with histology,(48) but is expensive, requires expert interpretation and is not easily performed in large populations. In NHANES III, ultrasounds were performed in 1988 to 1994 to evaluate gallbladder disease and archived video tapes were reviewed in 2009-2010 to assess hepatic steatosis.(49, 50) Ultrasound may be less sensitive in detecting mild hepatic steatosis and may not differentiate moderate from severe steatosis. Another limitation of the current study was the lack of validation of cause of death. Although ascertainment of vital status using the National Death Index is very high (>99%), assigning cause of death based on death certificate diagnoses can lead to misclassification. Thirdly, because participants were not reevaluated for hepatic steatosis or liver enzyme elevation during over two decades of follow-up, some may have developed them leading to misclassification; however, we hypothesize that such an increase in the prevalence of noninvasive liver disease markers would lead to a stronger association with liver disease mortality. Finally, despite up to 23 years of follow-up, the number of liver disease deaths was limited among persons with severe hepatic steatosis. These limitations are balanced by the benefits of a large, national, population-based sample, particularly the avoidance of ascertainment bias that occurs in clinical studies of selected patients and the ability to generalize the results to the U.S. population. Other important strengths of our study were over two decades of mortality follow-up and the availability of data on numerous known predictors of liver disease mortality measured at baseline.
In conclusion, in the U.S. population severe hepatic steatosis on ultrasound was associated with increased liver disease mortality; however, there was no independent relationship with deaths from all-causes, cardiovascular disease, cancer, or diabetes after accounting for other known risk factors. ALT and AST elevation were, likewise, associated only with liver disease mortality, while elevated GGT was positively related to liver disease deaths and to overall mortality. Complementary noninvasive liver injury markers identified through comprehensive genetic and epigenetic analyses will further enhance our understanding of the risk of premature death attributable to fatty liver disease in the general population.
Supplementary Material
ACKNOWLEDGMENTS
The National Center for Health Statistics (NCHS) was the source for the National Health and Nutrition Examination Survey III Linked Mortality Files. All analyses, interpretations, and conclusions are those of the authors and not NCHS. The authors thank Patricia Barnes for assistance in using the NCHS Research Data Center.
Financial support: The work was supported by a contract from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN276201200161U).
Abbreviations
- NAFLD
non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma glutamyltransferase
- HR
hazard ratio
- CI
confidence interval
- BMI
body mass index
- HDL
high-density lipoprotein
- ICD
International Classification of Diseases
Contributor Information
Aynur Unalp-Arida, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, aynur.unalp-arida@nih.gov
Constance E. Ruhl, Social & Scientific Systems, Inc.
REFERENCES
- 1.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 10.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. e411. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Ruhl CE, Everhart JE. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol. 2013;178:1702–1711. doi: 10.1093/aje/kwt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCHS . Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. National Center for Health Statistics; Hyattsville, MD: 1994. [June 2015]. p. 321. Vital and health statistics, series 1: programs and collection procedures, no. 32. DHHS publication no. (PHS) 94-1308. http://www.cdc.gov/nchs/data/series/sr_01/sr01_032.pdf. [Google Scholar]
- 16.Gunter EW, Lewis BG, Koncikowski SM. Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. National Center for Environmental Health, Center for Disease Control and Prevention; Atlanta, GA: 1966. [June 2015]. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. [Google Scholar]
- 17.NCHS [June 2015];Third National Health and Nutrition Examination Survey: hepatic steatosis ultrasound images assessment procedure manual. http://www.cdc.gov/nchs/data/nhanes/nhanes3/Hepatic_Steatosis_Ultrasound_Procedures_Manu al.pdf.
- 18.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124:1821–1829. doi: 10.1016/s0016-5085(03)00395-0. [DOI] [PubMed] [Google Scholar]
- 20.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Michels KB, Willett WC, Fuchs CS, Giovannucci E. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst. 2005;97:282–292. doi: 10.1093/jnci/dji039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCHS . National Health and Nutrition Examination Survey III; Body Measurements (Anthropometry) Westat; Rockville, MD: 1988. [June 2015]. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf. [Google Scholar]
- 24.NCHS [June 2015];National Health and Nutrition Examination Survey III Cycle 2: pulse and blood pressure procedures for household inteviewers. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/bpqc.pdf.
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics . Office of Analysis and Epidemiology, NCHS 2011 Linked Mortality Files Matching Methodology. Hyattsville, Maryland: Sep, 2013. [June 2015]. Available at the following address: http://www.cdc.gov/nchs/data/datalinkage/2011_linked_mortality_file_matching_methodology.pdf. [Google Scholar]
- 27.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 28.NCHS NCHS Research Data Center (RDC). [June, 2015]; http://www.cdc.gov/rdc/.
- 29.Kleinbaum DG. Survival Analysis: A Self-Learning Text. Springer; New York: 1996. [Google Scholar]
- 30.Breslow NE, Day NE. Statistical Methods in Cancer Research: the Design and Analysis of Cohort Studies. International Agency for Research on Cancer; Lyon, France: 1987. pp. 48–79. [PubMed] [Google Scholar]
- 31.Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513–1517. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 32.Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, et al. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. Qjm. 1999;92:73–79. doi: 10.1093/qjmed/92.2.73. [DOI] [PubMed] [Google Scholar]
- 33.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–912. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 37.Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183–1190. e1182. doi: 10.1016/j.cgh.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahali B, Liu YL, Daly AK, Day CP, Anstee QM, Speliotes EK. TM6SF2: catch-22 in the fight against nonalcoholic fatty liver disease and cardiovascular disease? Gastroenterology. 2015;148:679–684. doi: 10.1053/j.gastro.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 41.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 44.Sookoian S, Castano GO, Scian R, Mallardi P, Fernandez Gianotti T, Burgueno AL, et al. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61:515–525. doi: 10.1002/hep.27556. [DOI] [PubMed] [Google Scholar]
- 45.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 46.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260–1268. doi: 10.1016/s1542-3565(05)00743-3. [DOI] [PubMed] [Google Scholar]
- 48.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 50.NCHS Third National Health and Nutrition Examination Survey. Gallbladder ultrasonography procedure manual. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/gallblad.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.