Abstract
Systemic administration of hematopoietic growth factors such as granulocyte-colony stimulating factor (G-CSF) represents a novel approach for treatment of TBI. After mild controlled cortical impact (CCI), mice were treated with G-CSF (100 μg/kg) for 3 consecutive days. The primary behavioral end-point was performance on the radial arm water maze (RAWM) assessed before and after CCI (days 7 and 14). Secondary endpoints included a), motor performance on a rotating cylinder (rota-rod), b) measurement of microglial and astroglial response, c) hippocampal neurogenesis, and d) measures of neurotrophic factors (BDNF, GDNF) and cytokines in brain homogenates. G-CSF treated animals performed significantly better than vehicle-treated mice in the RAWM after one and two weeks, but not on the rota-rod. The cellular changes found in the G-CSF group included increased hippocampal neurogenesis as well as astrocytosis and microgliois in both striatum and hippocampus. Neurotrophic factors GDNF and BDNF, elaborated by activated microglia and astrocytes, were increased in G-CSF treated mice. These factors, along with G-CSF itself, are known to promote hippocampal neurogenesis, inhibit apoptosis and likely contributed to improvement in the hippocampal-dependent learning task. Ten cytokines that were modulated by G-CSF treatment following CCI were elevated on day 3, but only three of them remained altered by day 7 and all of them were no different than vehicle controls by day 14. The pro- and anti-inflammatory cytokines modulated by G-CSF administration interact in a complex and incompletely understood network involving both damage and recovery processes, underscoring the dual role of inflammation after TBI.
Keywords: Astrocytes, Microglia, Neurogenesis, Neuroinflammation, Neurotrophins, BDNF (brain-derived neurotrophic factor), Cytokines, brain repair, neurotrophic factors, astrocytosis, microgliosis, radial arm water maze, granulocyte-colony stimulating factor, traumatic brain injury
Visual Abstract
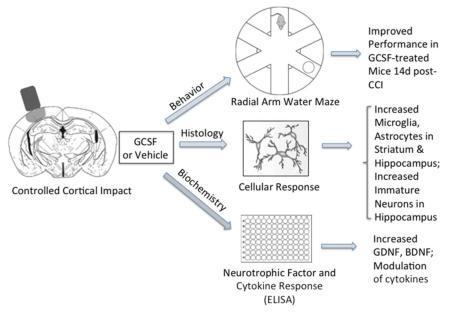
The effects of G-CSF in a mouse model of TBI were investigated at three levels of analysis: behavior, cellular response and impact on neurotrophic factors and cytokines. G-CSF or vehicle was administered for 3 days after mild CCI. Mice in the G-CSF treated group performed significantly better at 14 days after CCI. Along with the increased microgliosis and astrocytosis in striatum and hippocampus, there was a significant increase in expression of BDNF and GDNF which likely contributed to enhanced birth of new neurons in hippocampus.
Introduction
Traumatic brain injury (TBI) can be defined as an insult to the brain caused by an external physical force that may produce a diminished or altered state of consciousness and which results in an impairment of cognitive abilities or physical functioning(Cernak 2005). The pathophysiology of TBI is a complex process encompassing three overlapping phases: a) primary injury to brain tissue and/or the cerebral vasculature, b) the secondary injury which includes physiological, neuro-inflammatory and biochemical processes triggered by the primary insult and c) regenerative responses including enhanced proliferation of neural progenitor cells and endothelial cells (Cernak 2005). The secondary injury evolves over hours to days and may significantly contribute to chronic post-traumatic neurologic disability. Closely overlapping and following the secondary injury phase is the complex regenerative response that ultimately determines the extent of functional recovery from the injury. The overall goal of the present research was to investigate a new sub-acute therapeutic intervention in a rodent model of TBI that would impact the secondary injury processes and enhance recovery of cognitive and motor function.
Systemic administration of hematopoietic growth factors represents a novel approach to the treatment of TBI. Granulocyte-colony stimulating factor (G-CSF) is one of several colony-stimulating factors (CSFs) that control the production of circulating blood cells by the bone marrow. Although G- CSF has been used primarily to treat leukopenia, the agent has been studied in animal models of stroke where it has been reported to reduce brain damage and improve outcome (Schabitz et al. 2003; Shyu et al. 2006; Six et al. 2003; Solaroglu et al. 2006).
G-CSF treatment has also been reported to enhance recovery from TBI in rodent models. A course of intraperitoneal G-CSF administration (via osmotic minipump) initiated concomitantly with TBI in a rat model resulted in significantly better motor function recovery than the control group (Yang et al. 2010). The G-CSF group exhibited a greater increase in proliferative cells (BrdU+) and a significantly higher number of doublecortin expressing (DCX+) cells in the ipsilateral subventricular zone (SVZ) than the control group (Yang et al. 2010). A more recent study in rats reported that a single intravenous infusion of G-CSF (300 μg/kg) administered 7 days after controlled cortical impact (CCI) resulted in partial, short-lived motor benefits and modestly increased neurogenesis in hippocampus and sub- ventricular zone, along with dampening of microgliosis, indicated by estimated volumes of OX6 immunoreactivity at 8 weeks after the TBI (Acosta et al. 2014).
G-CSF has a myriad of effects including actions on the bone marrow to enhance proliferation and differentiation of hematopoietic stem cells and direct actions on neural cells of the brain. G-CSF readily passes the blood-brain barrier (Zhao et al. 2007) and interacts with G- CSF receptors, expressed by adult neural stem/progenitors, to promote neurogenesis (Jung et al. 2006; Sanchez-Ramos et al. 2009). In fact, both G-CSF and its receptor are widely expressed by neurons in the CNS, and their expression is induced by ischemia, which suggests an autocrine protective signaling mechanism (Schneider et al. 2005a). G-CSF is now recognized to possess several actions that contribute to longer-term CNS plasticity. G-CSF displays strong anti-apoptotic activity in mature neurons, stimulates neuronal differentiation of adult neural stem cells in the brain, and improves long-term recovery in more chronic stroke models (Schabitz et al. 2003; Schneider et al. 2005a; Schneider et al. 2005b).
The cellular and molecular mechanisms responsible for the beneficial effects of G-CSF in a rodent model of TBI require further exploration, especially during the sub-acute and regenerative phases of the injury. The overall goal of the present project is to mitigate the secondary injury that occurs hours to days after TBI and to enhance the recovery of hippocampal- dependent cognitive performance by administration of G-CSF in a mouse model of TBI. It is hypothesized that G-CSF will modulate the trauma-induced activation of microglia and astrocytes which in turn will stimulate production of neurotrophic factors that promote hippocampal neurogenesis and enhance recovery of behavior. To that end, G-CSF will be administered for 3 days immediately after mice undergo CCI to the right frontal cortex. The primary end-point will be the time-dependent recovery of performance in a radial arm water maze (RAWM), a hippocampal-dependent learning task. Secondary endpoints will be motor performance on a rotometer, changes in microglia and astroglia, hippocampal neurogenesis, neurotrophic factor expression and profiles of cytokines in the injured brai
Methods
Animals and Study Design
C57BL/6J male mice (30–40 g) were housed in standard laboratory cages and left undisturbed for 1 week after arrival at the animal facility. Animals had ad-libitum access to water and laboratory chow and were maintained in a temperature- and humidity-controlled room on a 12:12 light/dark cycle with light onset at 7:00 a.m. Three groups of 57BL/6J mice (n=60 total with n=20 for each of 3 time points: 3d, 7d, and 14d) underwent controlled cortical impact (CCI) (Pittsburgh Precision Instruments, Inc, USA) on the right side of brain on day 0, followed by administration of G-CSF 100 μg/kg sub-cutaneously (s.c.) daily X3 days or vehicle for 3 days (See Table 1). G-CSF (Neupogen™) was purchased from Amgen, Inc. (USA). A low dose of G-CSF (100 μg/kg ) was chosen for administration in the 3 days after injury because earlier studies revealed that a single high dose (300 μg/kg) administered 1 wk after CCI improved motor performance when tested 8 weeks later (Acosta et al. 2014). An objective of the present study was to determine if lower doses and earlier administration of G- CSF enhanced recovery of motor and cognitive function following a mild to moderate CCI.
Table 1.
Animal Treatment Groups
| GROUPS | TBI | Treatment | Time of Euthanasia |
Number of mice* |
|---|---|---|---|---|
| Group 1 | CCI on Right side, day 0 | Vehicle: daily injections ×3d | 3 days | 10 |
| CCI on Right side, day 0 | G-CSF 100ug/kg daily × 3d | 10 | ||
| Group 2 | CCI on Right side, day 0 | Vehicle: daily injections ×3d | 7 days | 10 |
| CCI on Right side, day 0 | G-CSF 100ug/kg daily × 3d | 10 | ||
| Group 3 | CCI on Right side, day 0 | Vehicle: daily injections × 3d | 14 days | 10 |
| CCI on Right side, day 0 | G-CSF 100ug/kg daily × 3d | 10 |
From each cohort of 10 mice, 6 were designated for Histology and 4 for cytokine analysis.
Behavioral testing included the rota-rod task and the radial arm water maze-(RAWM) at baseline and 7 and 14d after CCI. A sub-set of 4 mice from each group were euthanatized, brains dissected and brain regions frozen for determination of brain levels of trophic factors (BDNF, GDNF) and selected pro- and anti-inflammatory cytokines. The remaining sub-set of 12 mice from each group underwent histological analyses to determine extent of microgliosis and astrocytosis as well as alterations in hippocampal neurogenesis (n=6 of G-CSF treated and n=6 vehicle treated per time period)
This study was carried out in strict accordance with the recommendations from the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the University of South Florida.
Surgery and CCI
Animals underwent an experimental TBI using a controlled cortical impactor (Pittsburgh Precision Instruments, Inc, USA) as described previously (Yu et al. 2009). Animals initially received Buprenorphine (0.05 mg/kg, s.c.) at the time of anesthesia induction (with 125 mg/kg Ketamine, 12.5 mg/kg Xylazine). Once deep anesthesia was achieved (by checking for pain reflexes), individual animals were fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). After exposing the skull, craniectomy (approximately 3 mm), to accommodate the impactor tip) was performed over the right frontoparietal cortex (−0.5 mm anteroposterior and +0.5 mm mediolateral to bregma). All mice received a “mild” TBI. The pneumatically operated TBI device (with a convex tip diameter=2 mm ) impacts the brain at a velocity of 6.0 m/s reaching a depth of 0.5 mm below the dura mater layer and remains in the brain for 150 ms. The impactor rod was angled 15° to the vertical to maintain a perpendicular position in reference to the tangential plane of the brain curvature at the impact surface. A linear variable displacement transducer (Macrosensors, Pennsauken, NJ), connected to the impactor, measured velocity and duration to verify consistency. Bone wax was used to cover the craniectomized region and the skin incision sutured thereafter. A computer operated thermal blanket pad and a rectal thermometer allowed maintenance of body temperature within normal limits. All animals are closely monitored until recovery from anesthesia and over the next 3 consecutive days.
Radial Arm Water Maze (RAWM)
To study the cognitive effects of G-CSF in mice that had undergone mild to moderate CCI, a radial arm water maze (RAWM) task was employed. RAWM is a hippocampal-dependent, spatial learning task that is not rely on locomotor ability or swimming speed (Vorhees and Williams 2006). RAWM was started on day 7 post CCI. A six-arm radial arm maze was placed into a water tank of approximately 100 cm- diameter and a 25-cm-height, 5-cm-diameter platform was used. The platform was submerged 0.5 cm below the water surface. The temperature of the water was kept at 26 °C. Mice were placed in the start arm at the beginning of every trial, and the platform was located in the goal arm. Every animal had an assigned platform/arm location throughout acquisition of learning, yet the starting zone was randomly changed per trial. A spatial-training protocol was followed. Mice were given 2 blocks of 5 trials, each block separated by 30 minutes rest period per day, for a total of 10 trials a day for 2 days of acquisition of learning for both baseline and post-TBI training. Trials were only 60 seconds long. Once animals found their goal arm/platform, they were allowed to remain on the platform for 30 sec between trials. If mice were unable to find their goal arm/platform within 60 seconds, mice were guided to their goal arm and allowed to rest on the platform for 30 sec. On day 3, a probe trial was given; this was reversal training in which the mice were placed 180 degrees from the goal arm. Mice were given 5 trials to train for the new position (reversal training). RAWM performance analysis was done by averaging the trials per block, using 5 trials per block, then a total of 2 blocks per day (errors are scored every time mice do not enter the goal arm).
Motor, balance and coordination measured with rota-rod performance
The rota-rod (Ugo Basile S.R.L; 21025 Comerio VA Italy, Madel 47600, Rota-Rod for Mice) provided a motor balance and coordination assessment. Data were generated by averaging the scores (total time spent on treadmill divided by 3 trials) for each animal during training and testing days. Each animal was placed in a neutral position on a cylinder, then the rod was rotated with the speed accelerated linearly from 4 rpm to 40 rpm within 3 minutes, and the time spent on the rota-rod was recorded automatically. For training, animals were given 1 trial before testing. For testing, animals were given 3 trials and the average score on these 3 trials was used as the individual rota-rod score.
Immunohistochemistry
Mice were anesthetized with 150 mg/kg Ketamine, 15 mg/kg Xylazine and then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were stored in 4% paraformaldehyde, transferred to 25% sucrose solution in 4% paraformaldehyde, until the brains sank to the bottom. Then brains were slowly immersed into isopentane (cooled on dry-ice), left in isopentane for 20 seconds, removed, placed on a small piece of aluminum foil sitting on powdered dry-ice for 1-2 minutes (to let isopentane evaporate) and finally wrapped in the foil and stored at −80°C until sectioning. Brains slices were cut 30 μm thick, in a cryostat (Leica, Germany) set to −25° C. Every 6th coronal section, was taken from the corpus striatum (caudate/putamen) spanning 1.2 mm in the anterior-posterior direction (from Bregma +1.32 mm to Bregma=0 which corresponds to the beginning of the lateral ventricles to the anterior commissure). Serial sections were also cut from hippocampus, starting from Bregma −1.28 to Bregma −2.92. Every 6th section was kept for immunostaining.
Selective immunostaining of astrocytes and microglia was performed with antibodies to glial fibrillary acidic protein (GFAP) and (ionized calcium-binding adapter molecule-1 (Iba-1), respectively. Iba-1 is protein that is specifically expressed in macrophages / microglia and is upregulated during the activation of these cells. Antibodies to doublecortin (DBX) were used to label newborn neurons in the dentate gyrus of the hippocampus. Brain sections were preincubated in PBS containing 10% normal serum (goat or donkey; Vector) and 0.3% Triton X-100 (Sigma) for 30 min. The sections were then transferred to a solution containing primary antibodies in 1% normal serum, 0.3% triton X- 100/PBS and incubated overnight at 4°.
The specific antibodies used in each experiment were: rabbit anti-Iba1(Wako, Cat No 019-19741 RRID:nlx_152487), 1:500; rabbit anti-GFAP (Genemed Cat# 60-0032-7 RRID:AB_11203520) 1:50 in PBS; rabbit anti-DCX (Abcam, Cat #18723 RRID:nlx_152244), 1:1000 containing 1:100 normal serum without Triton X-100. After incubation with primary antibody, the sections were washed and incubated for 1 hour with Alexa Fluor 488 goat anti-rabbit IgG diluted 1:400 in PBS (Molecular Probes (Invitrogen) Cat# A11070 RRID:AB_142134) at room temperature. The sections were then rinsed in PBS three times and covered with a cover-glass. Green fluorescence signals from the labeled cells were visualized with fluorescence microscopy using appropriate filters.
Quantitative assessment of astrocytic, microglia responses and hippocampal neurogenesis
Quantitation of microgliosis and astrogliosis was made by computerized image analysis by an investigator blinded to the treatment. The method for quantitative image analyses has been previously described and used by members of our lab (Boyd et al. 2010; Sanchez-Ramos et al. 2009). A total of 12 mice were analyzed per time-point (3, 7 and 14 days). Images were acquired at a magnification of 200x as digitized tagged-image format files (TIFF) to retain maximum resolution using an Olympus BX60 microscope with an attached digital camera system (DP-70, Olympus, Tokyo Japan). Images of 6 randomly selected sections (each 25 microns thick) from each mouse were captured from serially sectioned striatum starting at beginning of the lateral ventricles to the anterior commissure) on both left and right side. Using Image J software (ImageJ, http://rsb.info.nih.gov/ij/index.html; RRID:nif-0000-30467), the green channel was selected and converted into a monochrome signal. Then, a threshold optical density was obtained that discriminated staining from background. Each anatomic region of interest was manually edited to eliminate artifacts. The thresholded signal was quantified as percent of the visual field for each image. Data were reported as the percentage of labeled area captured (positive pixels) divided by the full area captured (total pixels). Bias was eliminated by analyzing each entire region of interest represented by the sampling of 6 sections per mouse on right and 6 sections on the left side. The fluorescence signals from each mouse striatum or hippocampus (separate analyses for each side) were averaged and used to calculate the mean signal (% of the visual field) for each of the 3 Groups of mice (See Table 1 for number of mice per Group) at each time point (3, 7, 14 days after CCI).
As a surrogate index of neurogenesis, the expression of DCX, a marker of immature neurons (von Bohlen und Halbach 2007) was measured in the dentate gyrus of hippocampus using the approach described above. Bias was eliminated by analyzing each entire region of interest represented by the sampling of 6 sections per mouse dentate gyrus on right and 6 sections on the left side. Each analysis was done by a single examiner blinded to sample identities.
Cytokine Assay
After CCI on the right side, mice were euthanized at 3, 7 and 14 days (n=20 mice per time interval) followed by perfusion with saline. Frontal cortex, hippocampus and striatum of the left and right brains were dissected, and were placed in freezer at −70 C until assay for cytokines was undertaken. Mice Brain Samples were homogenized in T-PER Tissue Protein Reagent (Thermo Scientific CAT#PI-78510 ) with Protease and Phosphatase inhibitor cocktail (CAT# PI-78443) and each sample’s protein concentration was measured by a BCA kit (Fisher Scientific #23225). Levels of 23 cytokines were measured using a Bio-Rad Bio-Plex Pro Mouse Cytokine Grp I Panel 23-Plex assay kit (Bio-Rad, Catalogue # M60-009RDPD) and followed the kit’s instruction and read the 96- well plate by a Bio-Plex MAGPIX Mutiplate Reader. Each cytokine level was calculated based on its own standard curve. Data was expressed as the mean cytokine level on each side of brain at 3 time points (3, 7 and 14 days) following vehicle or G-CSF treatment.
BDNF and GDNF ELISA
Mice Brain Samples were homogenized in T-PER Tissue Protein Reagent (PI-#78510 ) with Protease and Phosphatase inhibitor cocktail ( PI-78443) and each brain sample’s protein concentration was measured by a BCA kit (Fisher Scientific #23225). Levels of Mouse BDNF and GDNF were measured using a Mouse BDNF and GDNF ELISA Kit from Boster Biological Technology (Fremont, CA. Cat # EK0309 and # EK0935) and followed the Kit instruction and Preparation. The ELISA 96-well plate was read at a Plate Reader (BioTek Synergy) at O.D absorbance at 450nm. The Cytokine concentration was expressed at Unit pg/ml with sample total protein concentration at 1mg/ml.
Data Analysis and Statistics
Neurohistologic measures, as well as measures of neurotrophic factors and cytokines were expressed as mean ± SEM and statistically evaluated using 2- way ANOVA followed by Dunnett’s or Sidak’s corrections for multiple comparisons (GraphPad version 5.01). Analysis of these data for each of the time intervals (3, 7, 14) utilized one-way ANOVA followed by corrections for multiple comparisons. Behavioral analysis (RAWM) of the reversal training also utilized one-way ANOVA followed by Bonferroni multiple comparison tests. All comparisons were considered significant at p < 0.05.
Results
The effects of CCI, followed by 3 days of G-CSF treatment on motor performance on the rota-rod, is shown in Table 2. Right-sided TBI significantly decreased performance indicated by the amount of time spent on the rod before falling off. By day 7, the performance in the G-CSF treated mice was 76% of baseline and 68% in the vehicle group. At 14 days the performance was 80% and 76% of pre-CCI levels, following vehicle or G-CSF treatment, respectively. Although both time and treatment contributed significantly to total variation (2- way ANOVA), comparison of vehicle and G-CSF groups showed no significant differences after correction for multiple comparisons (Table 2).
Table 2.
Rotometry (seconds on rota-rod before falling)
| Vehicle Rx Group | GCSF Rx Group | |||
|---|---|---|---|---|
| Before CCI | 128.20 ± 5.35 | n=10 | 109.20 ± 7.86 | n=10 |
| 7d post CCI | 87.85 ± 10.78 | n=10 | 83.41 ± 5.32 | n=10 |
| 14d post CCI | 102.10 ± 9.38 | n=10 | 82.77 ± 4.53 | n=10 |
2-way ANOVA of the rotometery data revealed time after CCI accounted for 26% of the variation (p<0.0001) and treatment with either G-CSF or vehicle contributed 6.4% of total variation (p=0.02). Comparison of row means (Sidak Multiple comparisons test) showed no significant differences between G-CSF and vehicle treated mice at each time point.
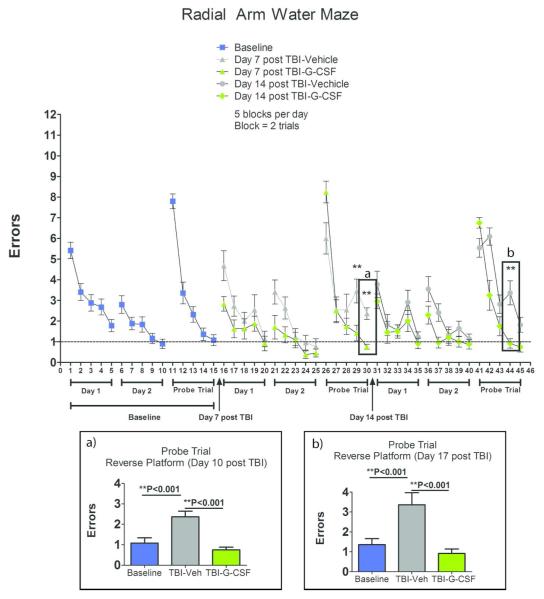
In contrast to the failure of G-CSF to significantly improve motor performance, G-CSF treated mice performed better than vehicle controls in a hippocampal-dependent learning task, the RAWM (Figure 1). During “probe trials”, in which the location of the platform was reversed, the G-CSF treated mice committed significantly less errors than vehicle-treated mice, both at 10 and 17 days after CCI (Figure 1a and 1b).
Figure 1.
Effects of CCI and G-CSF treatment on Performance in Radial Arm Water Maze. Summary data is plotted as mean number of errors on the y-axis and trials on the x-axis. RAWM training began on day 7 after CCI. Reversal training data analysis was performed on day 10 (Insert Box a) and day 17 (Insert Box b). Asterisks indicate significant differences in treatments compared to each other (vehicle vs G-CSF) and compared to baseline performance, based on one-way ANOVA followed by Bonferroni multiple comparison tests.
Since TBI triggers cellular and inflammatory responses in the sub-acute period, it was important to determine the extent to which G-CSF treatment impacted these cellular repair responses. Both vehicle-treated and G-CSF treated animals experienced significant astrocytosis and microgliosis in multiple brain regions (cortex, corpus callosum, striatum, hippocampus) on both sides of brain. Quantitative analysis of the cellular response was focused on striatum and hippocampus on both right and left sides. The striatum was chosen because of its important role in the automatic execution of learned motor programs (such as required for automatic running on a rotating cylinder) and the hippocampus for its role in spatial learning and memory, as in the RAWM.
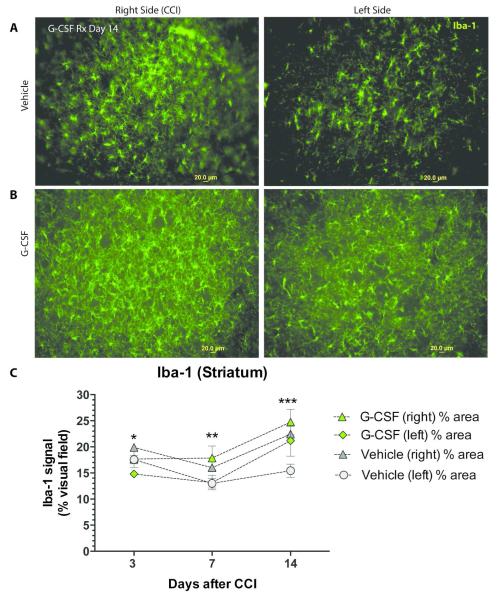
Immunohistological analysis of striatal Iba-1 immunoreactivity, a marker of activated microglia, revealed significant differences between G-CSF and vehicle-treated mice (Figure 2). The greatest number of Iba-1 cells was observed in the striatum of G-CSF treated group on days 7 and 14 on the side of the injury (right).
Figure 2.
- Iba-1 immunoreactive cells in striatum in mice treated with vehicle (day 14 after CCI). Left panel shows the right side of brain, which sustained CCI, and the right panel shows the contralateral (left) side. (scale bar = 20 μm)
- Iba-1 immunoreactive cells in striatum in mice treated with G-CSF (day 14 after CCI). Left panel is the right side and the right panel shows the contralateral (left) side. (scale bar = 20 μm)
- Summary data of striatal Iba-1 signal (mean % of visual field) plotted against days after CCI. Two-way ANOVA revealed that both time and treatment (G-CSF vs vehicle) contributed significantly to the variance (p<0.001). * One-way ANOVA of Day 3 data (followed by Dunnett’s multiple comparison test) indicates significant difference between G-CSF treatment (L side) compared to vehicle treatment (R side) (p<0.05). **Similar one-way ANOVA followed by Dunnett’s multiple comparison test of Day 7 data revealed significant differences between vehicle treatment (left side) and G-CSF treatment on right side (p<0.05). There was also a significant difference between vehicle treatment on the left side compared to right side (p<0.05). ***Day 14 data analysis indicated significant differences between vehicle (left) and GCSF treatments on both right and left as well. Vehicle treatment on the left side compared to right side was also significantly different (p<0.05).
Maximal microgliosis was also seen on day 14 in vehicle-treated mice on the right side. Two-way ANOVA of the summary data (Fig 2C) revealed that both time and treatment (G-CSF vs vehicle) contributed significantly to the variation (p<0.001).
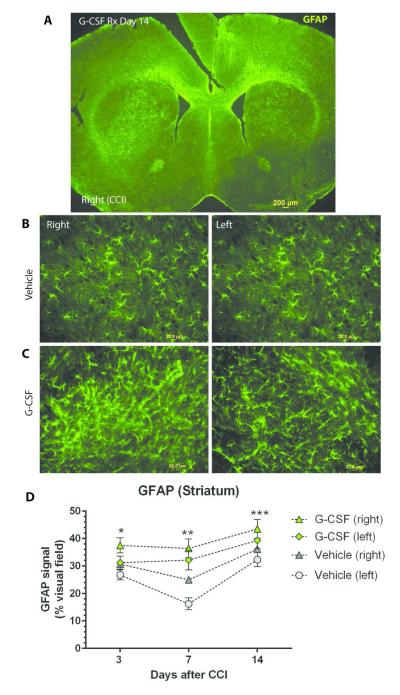
The impact G-CSF treatment following TBI on astrocytosis was similar to the microglial response (Figure 3). There was a time-dependent increase in GFAP+ cells on both sides of the striatum that peaked at 14 days following G- CSF treatment. Two-way ANOVA revealed that both time and treatment (G-CSF vs vehicle) contributed significantly to the variation (p<0.001). Analysis of Day 7 data revealed significant differences between vehicle treatment (left side) and G- CSF treatment on both left and right sides (p<0.05). On day 14, there were significant differences between vehicle (left) and GCSF treatments on right and left (p<0.05).
Figure 3.
- Low power photomicrograph (4x objective) of coronal section showing GFAP immunoreactive cells at 14 days after CCI. (scale bar= 200 μm)
- GFAP immunoreactive cells in striatum in mice treated with vehicle (day 14 after CCI). Left panel shows the right side of brain, which sustained CCI, and the right panel shows the contralateral (left) side. (scale bar = 20 μm)
- GFAP immunoreactive cells in striatum in mice treated with G-CSF (day 14 after CCI). Left panel is the right side of brain, and the right panel shows the contralateral (left) side. (scale bar = 20 μm)
- Summary data of striatal GFAP immunoreactivity (signal expressed as % of visual field area) over time. Two-way ANOVA revealed that both time and treatment (G-CSF vs vehicle) contributed significantly to the variance (p<0.001). * One-way ANOVA of Day 3 data (followed by Dunnett’s multiple comparison test) indicates significant difference between G-CSF treatment (R side) compared to vehicle treatment (L side) (p<0.05). ** Similar one-way ANOVA followed by Dunnett’s multiple comparison test of Day 7 data revealed significant differences between vehicle treatment (left side) and G-CSF treatment on both left and right sides (p<0.05). ***Day 14 data analysis indicated significant differences between vehicle (left) and GCSF treatments on right and left (p<0.05).
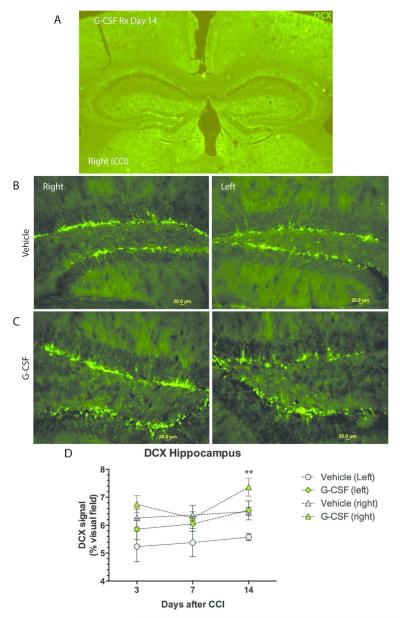
G-CSF treatment increased DCX expression in the dentate gyrus (Figure 4). At days 3 and 14, the greatest expression of DCX was seen on the right side in animals treated with G-CSF. Two-way ANOVA of data from day 14 (right vs left side and G-CSF treatment vs vehicle) showed that treatment comprised 21% of total variation and time comprised 19.4% of total variation (p=0.001). Further analysis revealed DCX expression was significantly higher in G-CSF treated right dentate gyrus compared to vehicle treated mice (p<0.05).
Figure 4.
- DCX immunoreactive neurons in dentate gyrus of hippocampus. Low power (4X objective) photomicrograph of hippocampus, both left and right sides, on day 14 after CCI.
- DCX expression in vehicle-treated mice, with panels depicting right and left dentate gyri of hippocampus on day 14 (scale bar=20 μm)
- Effects of G-CSF on DCX expression in dentate gyri on day 14, with panels depicting right and left sides. (scale bar=20 μm)
- Summary data on DCX expression with mean DCX signal plotted against
- time after CCI. * Two way ANOVA of data from day 14 (right vs left side and G-CSF treatment vs vehicle) showed that treatment comprised 21% if total variation and time comprised 19.4% of total variation (p=0.001). Tukey’s multiple comparison showed DCX expression was significantly higher in G-CSF treated right dentate gyrus compared to vehicle treated mice (p<0.05)
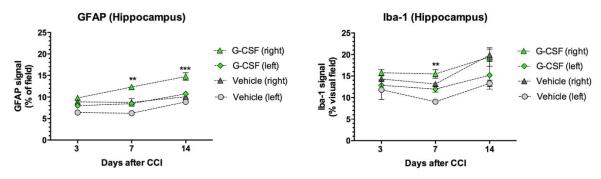
Hippocampal microgliosis was less extensive than the striatal microglial response. Conversely, striatal astrocytosis was quantitatively less than hippocampal astrocytosis (Figure 5).
Figure 5. Hippocampal astrocytosis and microgliosis.
The hippocampus exhibited time- and treatment-dependent increases in astrocytosis (left panel) and microgliosis (right panel). Two-way ANOVA revealed that G-CSF treatment and days after CCI each contributed significantly to the total variation in GFAP+ immunoreactivity (p<0.001). Comparison of the GFAP and Iba-1 signals (mean area of visual field) of in G-CSF vs vehicle groups on each side (with Sidak’s corrrections for multiple comparisons) indicated G-CSF treatment significantly increased hippocampal astrocytosis by days 7 (**) and 14 (***) with p<0.05. Two- way ANOVA of the microglial response (right panel) revealed that time and treatment each contributed significantly to total variation (p<0.05). Comparison of means of Iba-1 immunoreactivity in G-CSF vs vehicle groups with Sidak’s corrrections for multiple comparisons indicated G-CSF treatment significantly increased number of hippocampal microglia by days 7 (**)with p<0.05.
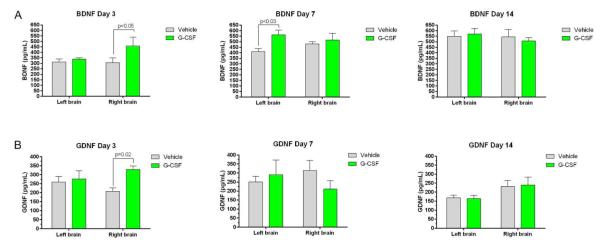
The protein levels of the neurotrophic factors BDNF and GDNF, were altered by G-CSF treatment in the injured brains (Figure 6). BDNF levels were increased on the right side of brain on day 3, but not days 7 and 14, in G-CSF treated mice (Figure 6A). On the left side, BDNF levels were increased only on day 7 in G-CSF treated mice.
Figure 6. Effects of G-CSF treatment on expression of BDNF and GDNF following right-sided CCI.
- BDNF levels (pg/mL) are plotted against time (days after CCI). Each panel shows results from each side of brain and compares effects of G-CSF to that of vehicle treatment. On the right side of brain, BDNF levels were significantly increased on day 3 after CCI compared to vehicle treatment. On the contralateral side (left) the BDNF levels took more timeto increase. By day 7, BDNF levels were significantly higher than vehicle controls.
- GDNF levels were plotted against days after CCI. On the right side of brain, GDNF levels were significantly greater than vehicle-treated mice on day 3, but dropped to levels below or equal to that measured in vehicle treated mice on days 7 and 14.
GDNF protein levels were significantly greater on the injured right side than vehicle-treated mice on day 3, but dropped to levels below, or equal, to that measured in vehicle treated mice on days 7 and 14 (Figure 6B). On the left side there were no difference between G-CSF and vehicle treated groups in levels of GDNF.
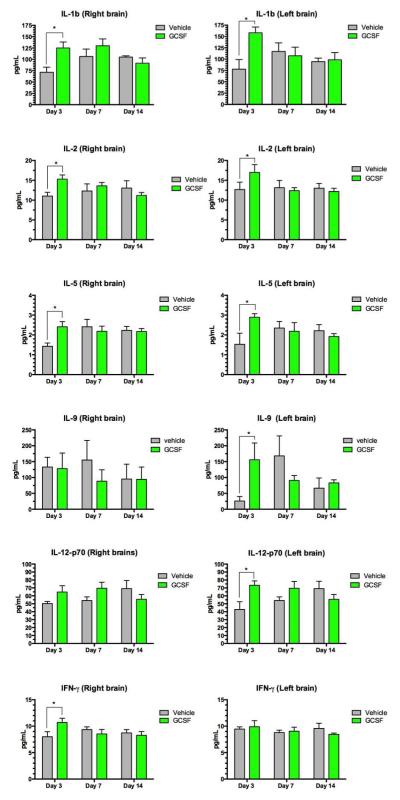
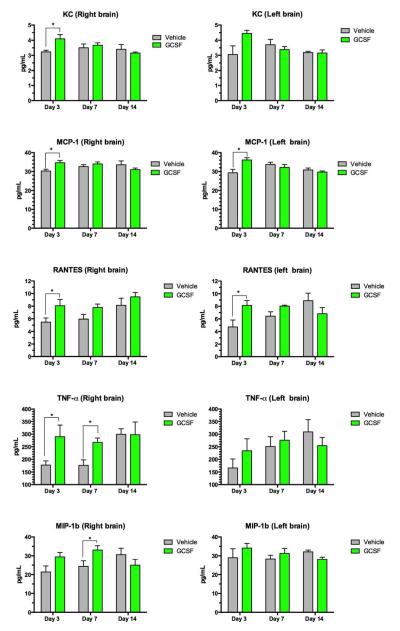
Eleven of the 23 cytokines measured were significantly increased by G- CSF treatment (Table 3). These cytokines (with the exception of MIP-1b) were elevated on day 3 but by day 14, G-CSF treated animals were no different than vehicle controls. (Quantitative data is shown in Figures 7 and 8).
Table 3.
Cytokines increased by G-CSF Treatment
| Day 3 | Day 7 | Day 14 | ||
|---|---|---|---|---|
| IL-1b | right | ↑ | - | - |
| left | ↑ | - | - | |
| IL-2 | right | ↑ | - | - |
| left | ↑ | - | - | |
| IL-5 | right | ↑ | - | - |
| left | ↑ | - | - | |
| IL-9 | right | - | - | - |
| left | ↑ | - | - | |
| IL-12(p70) | right | - | - | - |
| left | ↑ | - | - | |
| IFN-γ | right | ↑ | - | - |
| left | - | - | - | |
| KC | right | ↑ | - | - |
| left | - | - | - | |
| MCP-1 | right | ↑ | - | - |
| left | ↑ | - | - | |
| RANTES | right | ↑ | - | - |
| left | ↑ | - | - | |
| TNF- α | right | ↑ | ↑ | - |
| left | - | - | - | |
| MIP-1b CCL-4 | right | - | ↑ | - |
| left | - | - | - |
Upward arrows indicate significant increase elicited by G-CSF treatment compared to vehicle treatment (p<0.05); the (−) indicates no difference from vehicle control.
Figure 7.
Assay of a panel of cytokines and chemokines measured in brain homogenates. Each row shows measurements of a specific cytokine in the right and left side of brain. Each panel shows concentration of the cytokine protein in pg/mL and the y-axis indicates day 3, 7 and 14 after CCI. Two way ANOVA was performed followed by comparison of means for each time point, with correction for multiple comparisons. (* = statistical significance at p<0.05 in comparing mean levels in G-CSF vs vehicle groups.)
Figure 8.
Assay of a panel of cytokines and chemokines measured in brain homogenates. Each row shows measurements of a specific cytokine in the right and left side of brain. Each panel shows concentration of the cytokine protein in pg/mL and the y-axis indicates day 3, 7 and 14 after CCI. Two-way ANOVA was performed followed by comparison of means for each time point, with correction for multiple comparisons. (* = statistical significance at p<0.05 in comparing mean levels in G-CSF vs vehicle groups.)
Discussion
Our recently published report showed that a single dose of G-CSF (300 μg/kg) administered one week after moderate TBI in rats resulted in reduction of brain lesion volume, decreased cell death, improved motor behavior, dampened neuroinflammation and enhanced hippocampal neurogenesis, when measured 8 weeks after the injury (Acosta et al. 2014). The present study was designed to extend those findings by examining the sub-acute response (up to 2 weeks after mild CCI) to a lower dose of G-CSF (100 μg/kg) given 3 days in a row after the injury. Here the focus was on the time-dependent behavioral, cellular and inflammatory consequences. The primary behavioral end-point was performance on a RAWM task, a hippocampal-dependent spatial learning task assessed on day 7 and day 14 after CCI. Secondary endpoints included a) motor function, measured as the duration of time mice remain running on a rotating cylinder, b) quantitation of the microglial and astroglial responses in striatum and hippocampus, c) measures of hippocampal neurogenesis, and d) measures of neurotrophic factors (BDNF and GDNF) as well as a panel of cytokines at 3, 7 and 14 days in brain homogenates.
G-CSF treatment resulted in a significant improvement in performance on the RAWM on both the 7th and 14th day after CCI. More specifically, G-CSF treated mice performed better, (committed less errors) than vehicle controls in the “probe trials”, in which the location of the platform was reversed on days 10 and 17.
However, G-CSF treatment did not significantly impact performance on a rotating cylinder, a motor test of coordination and balance. By day 14, both vehicle and G-CSF treatment groups recovered to approximately 80% of pre-CCI performances. Although the G-CSF treatment group appeared to have better performances than the vehicle group on day 7, the difference did not reach statistical significance.
G-CSF was more effective in promoting recovery of a spatial memory task than a task of motor activity. One simple explanation spontaneous recovery of locomotor activity in vehicle-treated controls had already reached 70% of pre-CCI levels by day 7, leaving little room for further improvement to be promoted by G-CSF. However, it is also possible the deficit in spatial learning is much more sensitive to the direct actions of G-CSF on hippocampal neural circuitry than it is on motor circuits responsible for the automatic execution of learned motor programs like running on a rotating cylinder. For example, G-CSF is known to enhance hippocampal neurogenesis in models of TBI (Acosta et al. 2014; Yang et al. 2010), stroke (Schabitz et al. 2010; Schabitz and Schneider 2007) and Alzheimer’s disease (Boyd et al. 2010; Sanchez-Ramos et al. 2009). In the present report, enhanced expression of DCX, a surrogate index of hippocampal neurogenesis, was observed in G-CSF treated mice on both sides of brain. Furthermore, it is known that chemical ablation of neurogenesis impairs acquisition of hippocampal-dependent tasks (Shors et al. 2001; Shors et al. 2002), and so it is possible that increased neurogenesis triggered by direct actions of G-CSF on neural progenitor cells was responsible for enhanced recovery of performance in the RAWM. In addition to increasing neurogenesis, G-CSF has been shown to impact electrophysiological correlates of hippocampal neuroplasticity by altering synaptic activity. For example, a course of G-CSF treatment was reported to restore long-term depression (LTD) in ex vivo hippocampal slices in a mouse model of Alzheimer’s disease in which LTD was impaired by the disease process (Song et al. 2014).
G-CSF exerts both direct and indirect actions to influence the neuro-inflammatory response following injury. Direct interaction of G-CSF with its cognate receptor expressed on neural stem/progenitor cells, astrocytes and neurons results in proliferation and differentiation of neural progenitor cells and inhibition of neuronal apoptosis (Schneider et al. 2005a; Schneider et al. 2005b).
In addition to direct actions on neural cells, G-CSF triggers a complex cascade of peripheral effects including mobilization of white blood cells and modulation of pro-and anti-inflammatory cytokines (Hartung 1998). The traumatic injury to the right side of brain resulted in significant microgliosis and astrocytosis on both sides in vehicle treated mice, with side of the trauma showing the greatest increase. G-CSF treated mice exhibited a greater microglial and astrocytic response in both hippocampus and striatum, but with astrocytosis being more pronounced than microgliosis in the hippocampus. Along with the increase in astrocytes and activated microglia, concentrations of the neurotrophic factors BDNF and GDNF, were found to be increased by G-CSF treatment. This response is to be expected because these neurotrophic factors are elaborated by astrocytes and activated microglia (Batchelor et al. 1999). It is known that GDNF and BDNF stimulate hippocampal neurogenesis and possess anti-apoptotic properties (Boku et al. 2013; Chen et al. 2005). So the direct actions of G-CSF to stimulate neurogenesis, and its indirect actions to enhance BDNF and GDNF production by glial cells, resulted in an augmented neurogenic response to injury which translated into significant recovery from the TBI-induced deficits in the RAWM.
Microglia, macrophages and astrocytes release a plethora of inflammatory cytokines, chemokines and neurotrophic factors which sustain immune responses and contribute to both secondary tissue damage and repair (Morganti-Kossmann et al. 2001; Popovich et al. 1999). Chemokines are essential mediators of post-traumatic neuroinflammation because of their ability to induce directional migration of circulating leukocytes, especially monocytes. A significant proportion of microglia have been reported to be derived from blood-borne monocytes that migrate across the injured blood– brain barrier and contribute to the microglial/macrophage population at the site of injury (Song et al. 2013). In that study, a brain lesion was produced by insertion and immediate removal of a fine acupuncture needle through cortex to the hippocampus (Song et al. 2013). The lesion triggered a microglial response in which approximately 26% of Iba1+ cells were derived from circulating monocytes in hippocampus and frontal cortex.
In that same microlesion study, three of 17 cytokines were significantly elevated, peaking at 12 hrs and returning to normal by 48 hours, with the exception of MIP-1a which remained elevated (Song et al. 2013). By contrast, 10 cytokines that were modulated by G-CSF treatment following CCI in the present study were elevated on day 3, but only three of them remained altered by day 7 and all of them were no different than vehicle controls by day 14. MCP-1, elevated up to day 3, was notable because its potent chemotactic signaling for monocytes, macrophages, and microglia has been well-established (Chen et al. 2003; Fuentes et al. 1995). Prior research has demonstrated that TBI in transgenic mice that are MCP-1 deficient show acutely altered production of cytokines (within 2 to 24 hrs), but this did not affect lesion volume or cell death within the first week of injury (Semple et al. 2010). However, these MCP-1 deficient mice showed a delayed reduction in lesion volume, macrophage accumulation and astrogliosis as well as improved functional recovery (Semple et al. 2010). So clearly monocyte chemoattraction plays a significant role in mediating post-traumatic secondary brain injury.
In the present study, two of the chemokines that remained elevated by day 7 were MIP-1a and TNF-α. Like MCP-1, MIP-1a functions to draw monocytes to the site of injury. The effects of TNF-α range from induction of apoptotic and necrotic cell death to stimulation of cell growth and differentiation (Locksley et al. 2001). Hence, TNF-α is capable of both neurotoxic and neuroprotective activity. In fact, pro and anti-inflammatory cytokines interact in a complex and incompletely understood network involving both damage and recovery processes, underscoring the dual role of inflammation after TBI.
In summary, G-CSF treated animals recovered faster than vehicle-treated mice from a spatial learning deficit that was produced by mild CCI. Enhanced recovery in the hippocampal-dependent learning task was attributed to modulation by G-CSF of the sub-acute neuro-inflammatory response. The cellular changes found in the G-CSF group included increased hippocampal neurogenesis, as well as increased microgliosis and astrocytosis.
Going forward, it will be important to further investigate the role of blood- borne monocytes in mediating the beneficial effects of G-CSF in recovery from TBI. Even if the MCP-1 receptor on blood progenitor cells is blocked with a specific antagonist and interferes with monocyte recruitment to the site of the lesion, G- CSF may still have a range of beneficial actions because of its direct neurotrophic effects.
Significance.
Traumatic Brain Injury is a global public health problem and is recognized as the “signature injury” sustained by soldiers and civilians in foreign conflicts. The present research was designed to study if a medication commonly used to stimulate blood stem cell production could promote recovery from TBI in a mouse model. The administration of G-CSF for 3 days after mild TBI resulted in recovery of performance in a water maze, a test of learning and memory two weeks after injury. G-CSF treatment also stimulated astrocytosis and microgliosis, increased expression of neurotrophic factors, and generated new neurons in hippocampus.
Acknowledgements
Supported by Veterans Administration Merit Review Grant to S. Song and by the Helen Ellis Research Endowment Funds to J. Sanchez-Ramos
The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest: None of the authors have financial, personal or other relationships with organizations or other individuals that could inappropriately influence, or be perceived to influence, this submitted work.
References
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, Song S, Kaneko Y, Borlongan CV. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PloS one. 2014;9(3):e90953. doi: 10.1371/journal.pone.0090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JYF, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated Macrophages and Microglia Induce Dopaminergic Sprouting in the Injured Striatum and Express Brain-Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor. The Journal of Neuroscience. 1999;19(5):1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Takamura N, Kato A, Takebayashi M, Hisaoka-Nakashima K, Omiya Y, Inoue T, Kusumi I. GDNF facilitates differentiation of the adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3. Biochem Biophys Res Commun. 2013;434(4):779–784. doi: 10.1016/j.bbrc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. 2010;21(2):507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2(3):410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Experimental neurology. 2005;196(1):87–95. doi: 10.1016/j.expneurol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(6):748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155(12):5769–5776. [PubMed] [Google Scholar]
- Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Current opinion in hematology. 1998;5(3):221–225. doi: 10.1097/00062752-199805000-00013. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim SJ, Sinn DI, Kim SU, Kim M, Roh JK. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain research. 2006;1073-1074:190–201. doi: 10.1016/j.brainres.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16(3):165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Experimental neurology. 1999;158(2):351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, Cao C, Arendash GW. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience. 2009;163(1):55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2003;34(3):745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, Schneider D, Hamann GF, Rosenkranz M, Veltkamp R, Fiebach JB, Hacke W, Grotta JC, Fisher M, Schneider A. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2010;41(11):2545–2551. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schneider A. New targets for established proteins: exploring G-CSF for the treatment of stroke. Trends in pharmacological sciences. 2007;28(4):157–161. doi: 10.1016/j.tips.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005a;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005b;4(12):1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(4):769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. Cmaj. 2006;174(7):927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458(3):327–328. doi: 10.1016/s0014-2999(02)02785-1. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke; a journal of cerebral circulation. 2006;37(4):1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- Song S, Song S, Cao C, Lin X, Li K, Sava V, Sanchez-Ramos J. Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem cells international. 2013;2013:205878. doi: 10.1155/2013/205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Wang X, Sava V, Weeber EJ, Sanchez-Ramos J. In vivo administration of granulocyte colony-stimulating factor restores long-term depression in hippocampal slices prepared from transgenic APP/PS1 mice. Journal of neuroscience research. 2014;92(8):975–980. doi: 10.1002/jnr.23378. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DY, Chen YJ, Wang MF, Pan HC, Chen SY, Cheng FC. Granulocyte colony-stimulating factor enhances cellular proliferation and motor function recovery on rats subjected to traumatic brain injury. Neurological research. 2010;32(10):1041–1049. doi: 10.1179/016164110X12807570510013. [DOI] [PubMed] [Google Scholar]
- Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, Sanberg PR, Borlongan CV. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain research. 2009;1287:157–163. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Experimental neurology. 2007;204(2):569–573. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]