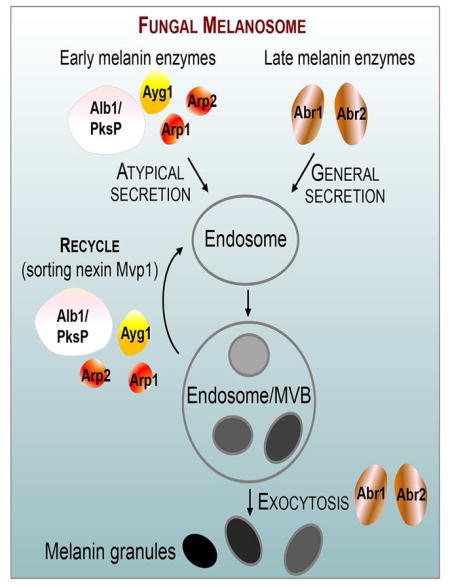
SUMMARY
Protection by melanin depends on its subcellular location. Although most filamentous fungi synthesize melanin via a polyketide synthase pathway, where and how melanin biosynthesis occurs, and how it is deposited as extracellular granules, remain elusive. Using a forward genetic screen in the pathogen Aspergillus fumigatus, we find that mutations in an endosomal sorting nexin abolish melanin cell wall deposition. We find that all enzymes involved in the early steps of melanin biosynthesis are recruited to endosomes through a non-conventional secretory pathway. In contrast, late melanin enzymes accumulate in the cell wall. Such subcellular compartmentalization of the melanin biosynthetic machinery occurs in both A. fumigatus and A. nidulans. Thus, fungal melanin biosynthesis appears to be initiated in endosomes with exocytosis leading to melanin extracellular deposition, much like the synthesis and trafficking of mammalian melanin in endosomally-derived melanosomes.
Graphical Abstract
INTRODUCTION
Melanins are resilient biopigments formed by oxidative polymerization of phenolic or indolic precursors. The contribution off melanization to species survival goes beyond colorization and protection against UV. Melanins are used by the host to defend against microbes and conversely by microbes to invade their host. In insects, the formation of melanotic capsules is a key immune defense mechanism against microbial invasions (Lavine and Strand, 2002). In humans, melanins are involved in anti-microbial defense and immune-modulation (Burkhart and Burkhart, 2005). On the other hand, melanins protect pathogens from host immune responses or enable them to penetrate host barriers. The importance of melanin for microbial pathogenesis is illustrated in the human fungal pathogen Aspergillus fumigatus. This fungus causes a spectrum of diseases, including allergic bronchopulmonary aspergillosis, aspergilloma, chronic pulmonary aspergillosis, and the fatal invasive aspergillosis (Latgé and Steinbach, 2009). Melanin coated on spores plays a multifaceted protective role for A. fumigatus during infection: it promotes attachment to host tissues, helps evade host recognition by masking various pathogen-associated molecular patterns, scavenges ROS generated by phagocytes, prevents phagolysosome acidification, and inhibits macrophage apoptosis (Chotirmall et al., 2014).
The effectiveness of melanin host protection depends on its subcellular location. However, how melanin is synthesized and contained in fungal cells, and how it gets deposited to the cell wall remains unclear. It is known that the majority of filamentous fungi, including A. fumigatus, synthesize melanin via the polyketide pathway, which requires endogenous substrates (e.g. acetyl-CoA), a polyketide synthase (PKS), laccases, and sometimes additional modification enzymes. As melanin, a negatively charged macromolecule, is found in the fungal cell wall as layers of globular particles (Eisenman et al., 2005; Hambleton et al., 2003; Walker et al., 2010), the cytoplasmic location of the synthases and substrates presents a conundrum for melanin’s trafficking across the plasma membrane.
Here, a forward genetic screen in A. fumigatus uncovered an endosomal sorting mutant that lacks melanin deposition in the cell wall. We find compartmentalization of fungal melanin biosynthetic machinery to the endosomal system. This theme shows striking resemblance with melanosome biogenesis and trafficking in mammals, implicating a unified cellular principle governing melanization in eukaryotes. The importance of endosomes in compartmentalization and trafficking of the melanin biosynthetic machinery may apply to other fungal secondary metabolism pathways.
RESULTS
Mutations in the Endosomal Sorting Nexin Mvp1 Abolish Melanin Deposition to the Cell Wall
In Aspergillus, melanization occurs only during conidiation (Figure S1A). We previously performed a T-DNA insertional mutagenesis screen in A. fumigatus to identify mutations that cause specific defects in conidial pigmentation (Jackson et al., 2009). Most color mutants harbored mutations in the melanin biosynthetic genes and showed conidial colors identical to the melanin gene deletion mutants. However, one insertional mutant, mvp1Tn [referred to as #12(Jackson et al., 2009)], displayed a unique light conidial color (Figure 1A). In this mvp1Tn mutant, no mutation was detected in the melanin gene cluster and all six melanin biosynthesis genes were induced during conidiation (Figure S1B), similar to wild type (WT) (Upadhyay et al., 2013). The results suggest that the pigmentation defect was not due to impaired expression of melanin biosynthetic genes.
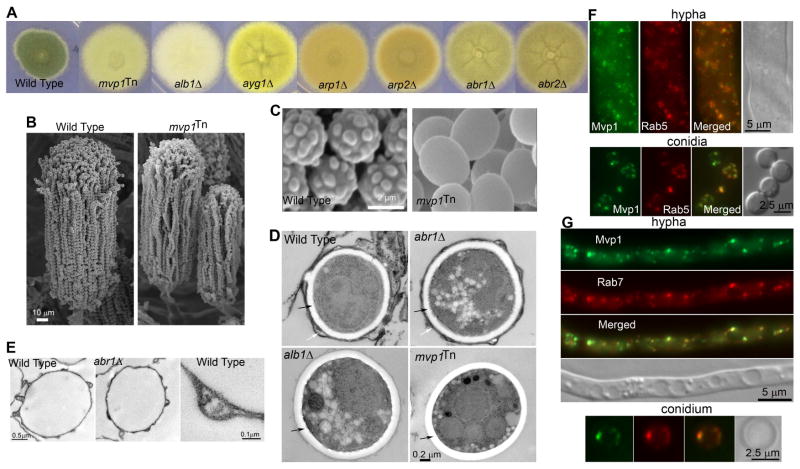
Figure 1. Mutations in the endosomal sorting nexin Mvp1 cause lack of melanin deposition in the cell wall.
(A) Colony images of WT, mvp1Tn, and the melanin gene mutants (alb1Δ, ayg1Δ, arp1Δ, arp2Δ, abr1Δ & abr2Δ ).
(B) SEM images of mature conidiophores of WT and the mvp1Tn mutant.
(C) SEM images of WT and mvp1Tn conidia.
(D) TEM images of intact conidia of WT, alb1Δ, abr1Δ, and mvp1Tn mutants. White arrows point to the electron-dense melanin layer. Black arrows point to the electron-transparent cell wall layer.
(E) TEM images of melanin ghosts extracted from WT and abr1Δ conidia.
(F) Localization of Mvp1 and the endosomal marker Rab5.
(G) Localization of Mvp1 and the endosomal marker Rab7.
See also Figure S1.
It is known that deficiencies in conidial differentiation or maturation caused by mutations in AbaA or WetA also yield lighter color (Tao and Yu, 2011) (Figure S1C). However, abaAΔ produces aberrant conidiophores with no spores, while wetAΔ produces conidia with defective spore walls that results in characteristic cell lysis and deflation (Boylan et al., 1987; Tao and Yu, 2011) (Figure S1D). In contrast, the mvp1Tn mutant developed normal conidiophores with long chains of conidia with no cell lysis (Figure 1B). Accordingly, no mutations were identified in the abaA or the wetA locus in this mutant.
Examination of the mvp1Tn mutant by electron microscopy revealed a surprisingly smooth conidial surface (Figure 1C), similar to the albino mutant (alb1Δ or pksPΔ ) that completely lacks melanin (Bayry et al., 2014; Tsai et al., 1998). Known mutants with lighter color, such as wetAΔ, still have an echinulated conidial surface (Figure S1C–D). This suggests that melanin is likely absent from the cell surface of the mvp1Tn mutant. Consistently, after digestion with lysing enzymes and hot acid treatment that melanin is resistant to, the mvp1Tn conidia showed no dark outer shell that was obvious in WT (Figure S1E), similar to the ayg1Tn melanin mutant. We then extracted melanin ghosts from conidia of WT (positive control), the ayg1Tn melanin mutant (negative control), and the mvp1Tn mutant. No difference was observed in the dry biomass of the mvp1Tn and the ayg1Tn melanin mutant. Thus, it appears that the mvp1Tn mutant has very little, if at all, mature melanin in the cell wall.
Under transmission electron microscope, melanin is the electron dense layer outside of the electron-transparent cell wall in wild-type conidia, and it correlates with spikes that give rise to the echinulated conidial surface (Figure1C–D). A similar electron-dense outer layer was observed in abr1Δ conidia, probably due to its ability to generate late melanin intermediates that can rectify cell-wall integrity (Bayry et al., 2014). By contrast, this electron-dense layer was absent in alb1Δ as well as mvp1Tn conidia (Figure 1D). Furthermore, WT and abr1Δ conidia yielded sphere-shaped melanin ghosts that are similar in size to the original conidia (Figure 1E). By contrast, the alb1Δ and mvp1Tn mutants failed to yield any discernable structures. Collectively, our data indicate that the mvp1Tn mutant does not have mature melanin or late melanin intermediates in the cell wall.
We found that this insertional mutant had the Ti plasmid inserted into the mvp1 gene that encodes a conserved endosomal sorting nexin (SNX8 in mammals) (Figure S1F, I). Deletion of mvp1 in the model filamentous fungus A. nidulans likewise rendered a strain with a smooth conidial surface and defective pigmentation (Figure S1H). Introducing A. nidulans Mvp1- GFP into A. nidulans mvp1Δ mutant restored these phenotypes (Figure S1H). The A. fumigatus version of Mvp1- GFP also compensated for the loss of mvp1 in A. nidulans, indicating cross-species conservation in Mvp1’s function.
Like many other sorting nexins, Mvp1 contains a Bin-Amphiphysin-Rvs (BAR) domain that can oligomerize and curve membranes, and a PHOX (PX) domain that recognizes phosphatidylinositol 3-phosphate, a lipid enriched in endosomal membranes (Figure S1F). In yeast and mammals, Mvp1 is broadly located in the endosomal system and participates in retrograde trafficking (Dyve et al., 2009; van Weering et al., 2012). In A. fumigatus, Mvp1-GFP was expressed at all developmental stages (Figure S1G). When co-expressed with the fluorescence-tagged early or late endosome marker Rab5 or Rab7, we found that Mvp1 largely matched the pattern of these endosomal markers (Figure 1F–G). It is notable that Mvp1 and Rab5/Rab7 also have distinct signals, as revealed by their fluorescence intensity profiles plotted along the cells (Figure S2A–B). Such localization pattern of Mvp1 is consistent with the behaviors of the retromer SNX subcomplex observed in yeast and mammals, where a retromer could bulge from endosomes forming tubule-like structures that are not decorated by Rab5 or Rab7 (Liu et al., 2012; Rojas et al., 2008). Some Mvp1-labeled puncta displayed rapid and long distance movement (Video S1), similar to Rab5-labeled early endosomes. The findings indicate that Mvp1 localizes broadly to the endosomal system in Aspergillus.
The Melanin Biosynthetic Machinery Shows a Stage-Specific Localization Pattern
The evidence above indicates that Mvp1 is important for Aspergillus melanization. As export of certain cargo is deficient in mvp1Δ yeast cells (Chi et al., 2014), we hypothesize that one of this sorting nexin’s cargos in Aspergillus could be melanin biosynthetic enzymes. If true, melanin enzymes should be localized to endosomes.
In A. fumigatus, the polyketide-derived melanin is synthesized by six enzymes encoded by a gene cluster (Langfelder et al., 1998; Sugareva et al., 2006; Tsai et al., 2001; Tsai et al., 1999) (Figure 2A). The foundation PKS enzyme Alb1 initiates melanin production from the substrates acetyl-CoA and malonyl-CoA. A series of subsequent enzymatic reactions carried out by Ayg1, Arp2, Arp1, and Arp2 lead to the production of vermelone, which is then oxidized and polymerized by the copper oxidase Abr1 and the laccase Abr2 to form mature melanin. The production of vermelone or the later melanin intermediate rectifies phenotypical defects caused by the lack of mature melanin, including cell surface structures and fungal virulence (Bayry et al., 2014). Consistently, we observed normal conidial structure in abr1Δ (Figure 1D–E). Therefore, we classify enzymes that function prior to the vermelone production as early enzymes (Alb1/Ayg1/Arp1/Arp2) and enzymes that function afterwards as late enzymes (Abr1/Abr2).
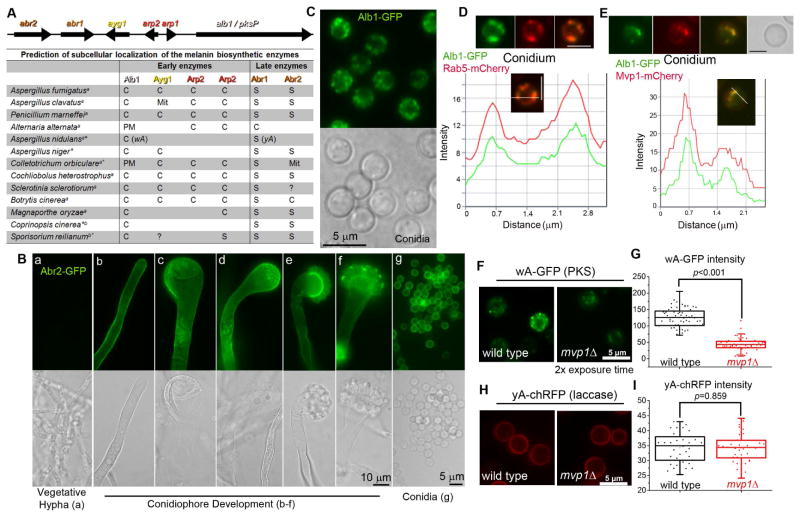
Figure 2. Distinct localization patterns for the early and late melanin enzymes.
(A) The melanin gene cluster in A. fumigatus and the predicted localization of melanin enzymes in diverse fungal species. S: Secreted protein; C: Cytosolic protein; PM: Plasma membrane; Mit: Mitochondria; a: Ascomycetes; b: Basidiomycetes; *: melanin genes not arranged in a cluster; ?: the presence of an ortholog is uncertain.
(B) The expression and localization of Abr2 during development. [a] vegetative hypha; [b] young stalk; [c–d] mature stalk; [e–f] young conidiophore; [g] conidia.
(C) Localization of Alb1 in conidia.
(D) Localization of Alb1-GFP and the endosomal marker Rab5-mCherry, and a fluorescence intensity plot along a cellular axis indicated with a white line.
(E) Localization of Alb1-GFP and Mvp1-mCherry, and a fluorescence intensity plot showing co-localization.
Localization(F) and fluorescence intensity (G) of the early melanin enzyme wA-GFP in WT and mvp1Δ in A. nidulans.
Localization (H) and fluorescence intensity (I) of the late melanin enzyme yA-chRFP in WT and mvp1Δ. Scale bars in panels D–F: 2.5 μm.
See also Figure S2
To our surprise, bioinformatic prediction indicates that only the late enzymes Abr1 and Abr2 are secretory proteins. All the early enzymes are cytosolic proteins, consistent with their lack of a secretion signal or transmembrane domain (Figure 2A). Further analyses of additional species that synthesize melanin via the PKS pathway predicted that early enzymes are generally cytosolic while late enzymes are generally secretory (Figure 2A). This pattern is found across all the fungal species examined, irrespective of their evolutionary distance (ascomycetes or basidiomycetes), the cluster organization of melanin genes, or the number of enzymes involved.
The predicted subcellular localization challenges our hypothesis that melanin enzymes traffic through endosomes. To interrogate our hypothesis, we decided to verify experimentally the subcellular localization of the melanin biosynthetic machinery in A. fumigatus. We fluorescence-tagged all six melanin enzymes and used their native promoters to drive their expression. As expected, melanin enzymes were not expressed during vegetative hyphal growth, but were produced in conidiophores during conidiation (Figure 2B, a–g; Figure S2).
Both the late enzymes Abr1 (Upadhyay et al., 2013) and Abr2, as predicted, predominantly delineated the cell’s outline, with some proteins localized to intracellular puncta (Figure 2B, Figure S2G). However, the PKS enzyme Alb1 did not show diffuse cytoplasmic localization as predicted. Rather, Alb1-GFP was localized to small puncta that resemble endosomes (Figure 2C). This multi-modular PKS enzyme (2146 aa) with the fluorescence tag is functional based on its ability to restore alb1Δ ’s pigmentation defect (Figure S2C). Similarly, other early enzymes, despite their predicted cytosolic nature, were localized to small puncta (Figure S2D–F). Thus the melanin enzymes displayed two distinct localization patterns: the early enzymes are located to intracellular punctate structures while the late enzymes are prominently located to cell periphery.
To examine if the two localization patterns also apply to other fungi, we tagged two known melanin enzymes in A. nidulans. The evolutionary distance between A. fumigatus and A. nidulans is comparable to that between mammals and fish. Unlike A. fumigatus that uses six enzymes encoded by a gene cluster, A. nidulans uses two unlinked enzymes for melanization: the PKS wA and the laccase yA (Aramayo and Timberlake, 1990; Tsai et al., 1999) (Figure 2A). Nonetheless, we found that the early enzyme wA was localized to intracellular puncta (Figure 2F) and the late enzyme yA mostly outlined the cells (Figure 2H). Thus, it appears that irrespective of the number of enzymes involved in melanin biosynthesis or the cluster arrangement, the early enzymes are in intracellular punctate structures while the late enzymes are secreted to the cell periphery.
Early Melanin Biosynthetic Enzymes Co-localized with Endosome Markers
To examine if the intracellular puncta highlighted by the early enzymes are endosomes, we introduced the fluorescently labeled endosomal marker Rab5 into these strains. Alb1 localized to the same structures as Rab5 (Figure 2D). Similar co-localization with Rab5 was also observed for two other early enzymes tested (Arp1& Arp2) (Figure S2I–J). Moreover, these early enzymes largely match the localization of the endosomal sorting nexin Mvp1 (Figure 2E; Figure S2H).
As Mvp1 is known to be involved in retrograde trafficking, we examined the impact of the mvp1 disruption on the melanin enzymes. For this purpose, we compared the localization and intensity of the early enzyme wA-GFP and the late enzyme yA-RFP in WT and mvp1Δ in A. nidulans. In mvp1Δ, wA-GFP was still localized to puncta, but the fluorescence intensity was much lower than that in WT (Figure 2F–G). By contrast, there was no apparent difference in yA-RFP localization or fluorescence intensity with or without Mvp1 (Figure 2H–I). The observations indicate that Mvp1 helps maintain the protein level of the early melanin enzyme, possibly through recycling the proteins for repetitive usage. We reasoned that if early melanin enzymes are drastically reduced in the mvp1 mutant, the level of early melanin intermediates would accordingly be decreased. To test this hypothesis, we treated WT and the ayg1 and mvp1Tn mutants with tricyclazole. Tricyclazole inhibits Arp2 and causes accumulation of the shunt product flaviolin derived from the intermediate produced by Ayg1 (Tsai et al., 2001). As expected, tricyclazole treatment altered the mvp1Tn conidial color only slightly (Figure S2O) and resulted in no detectable accumulation of the predicted flaviolin by thin layer chromatography (Figure S2P). The results are consistent with the predicted low level of melanin intermediates in mvp1 mutants.
Copper is an obligate cofactor for melanin enzymes (Chang, 2009). In A. fumigatus, the P-type Cu-transporter CtpA is required for melanization under copper-limiting conditions (Upadhyay et al., 2013) (Figure S2K). P-type transporters typically deliver copper from the cytosol to the lumen of a secretory compartment (Banci et al., 2010). Consistently, CtpA was detected in small vesicles when copper was limiting and in vacuoles when copper was excessive (Figure S2L). Under normal growth conditions, the distribution of CtpA in the cell largely overlapped with Mvp1 and the early enzyme Alb1 (Figure S2M). Collectively, the findings suggest that early melanin enzymes and CtpA (to supply the copper cofactor) are recruited to endosomes to facilitate melanogenesis during conidiation.
Early Melanin Enzymes Showed Co-localization with MVB Markers
Given that none of the early melanin enzymes have a secretion signal or a transmembrane domain, how they become associated with endosomes and whether they function in the cytosolic side or in the lumen of the organelle are unknown. Because previous observations in other fungi indicate melanin or melanin intermediates in intracellular and extracellular vesicles, and mature melanin as granules in the cell wall (Alviano et al., 1991; Eisenman et al., 2005; Hambleton et al., 2003; Walker et al., 2010), we conjecture that melanin enzymes likely function in the lumen of the endomembrane system, which could be achieved through membrane invagination, as in the formation of multivesicular bodies (MVBs).
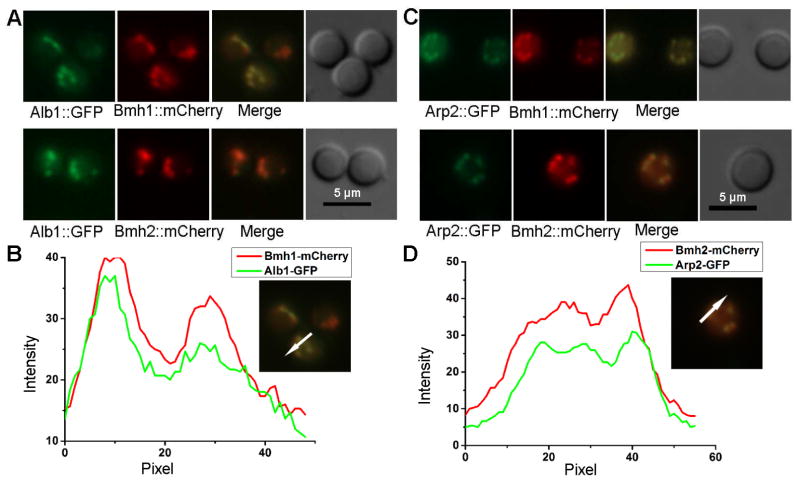
14-3-3 is a marker for MVBs and extracellular vesicles in the fungus Cryptococcus neoformans (Li et al., 2015). 14-3-3 proteins are also found in extracellular vesicles in mammals (Pisitkun et al., 2004). A. fumigatus has two 14-3-3 isoforms, Bmh1 and Bmh2. We fluorescently tagged both Bmh1 and Bmh2, and compared their subcellular localization with early melanin enzymes Alb1 and Arp2. Both 14-3-3 isoforms co-localized with the two melanin enzymes tested (Figure 3A–D), suggesting that endosome-derived MVBs might be an important cellular compartment for these melanin enzymes. Consistent with this idea, the activity of laccase, the enzyme responsible for Cryptococcus melanization, is drastically reduced in a strain with low level of the 14-3-3 protein (Li et al., 2015).
Figure 3. Co-localization of two 14-3-3 isoforms, Bmh1 and Bmh2, with melanin enzymes Alb1 and Arp2.
(A) Images of Alb1-GFP with either Bmh1-mCherry (top) or Bmh2-mCherry (bottom) in conidia.
(B) The fluorescence intensity profiles of Alb1-GFP and Bmh1-mCherry plotted against the conidial cellular axis in the direction shown in the images at right.
(C) Images of Arp2-GFP with either Bmh1-mCherry (top) or Bhm2-mCherry (bottom) in conidia.
(D) The fluorescence intensity profiles of Arp2-GFP and Bmh2-mCherry plotted against the conidial cellular axis in the direction shown in the images at right.
Late Melanin Enzymes Are Secreted and Accumulated in the Cell Wall
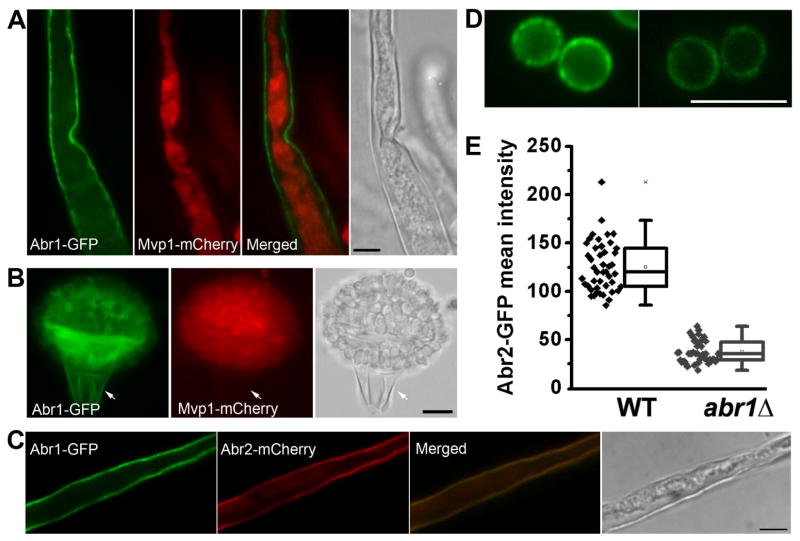
Different from early enzymes, both late enzymes Abr1 and Abr2 are mostly located to the cell periphery (Figure 2B; Figure S2G). To ascertain whether they are accumulated in the plasma membrane or the cell wall, we designed two approaches to separate the cell wall from cytoplasm. We first tested the classic plasmolysis approach that compresses cytoplasm using the A. nidulans strain with GFP-SsoA, a known plasma membrane t-SNARE (Figure S3A) (Taheri-Talesh et al., 2008). As expected, the GFP-SsoA signal stayed with cytoplasm that was separated from the cell wall during plasmolysis (Figure S3B). By contrast, Abr1-GFP was mostly retained in the cell wall while intracellular Mvp1-mCherry was compressed with cytoplasm away from the cell wall (Figure 4A). Abr2 was also primarily retained in the cell wall during plasmolysis, similar to Abr1 (Figure 4C). In abr1Δ, Abr2-GFP was still secreted, but the fluorescence intensity was much reduced (Figure 4D–E). This suggests that Abr1 is needed for Abr2’s stability, but not its secretion. In the second approach, we mechanically severed the cell. Upon wounding, septum pores will be sealed to prevent excessive cytoplasmic loss in the neighboring cell. As expected, GFP-SsoA was retained with the cytoplasm only in the hyphal cell adjacent to the damaged cell (Figure S3C). Similarly, intracellular Mvp1-mCherry was absent from the damaged conidiophore stalk cell but it was present in the neighboring intact cells on the conidiophore head (Figure 4B) due to the protection by sealed septa. By contrast, Abr1 was present in both the adjacent intact cells and the severed stalk cell (Figure 4B), indicating that Abr1 was retained in the cell wall of the damaged cell. Taken together, the findings demonstrate that Abr1 and Abr2 accumulate in the cell wall.
Figure 4. The late enzymes accumulate in the cell wall.
(A) Plasmolysis reveals the cell wall localization of Abr1 and intracellular localization of Mvp1.
(B) Mechanic severing reveals Abr1’s association with the cell wall in the damaged stalk cell. Intracellular Mvp1 is retained in the intact conidia but is lost in the severed stalk cell.
(C) Cells expressing Abr1-GFP and Abr2-mCherry were treated for plasmolysis. The separation of cytoplasm from the cell wall (DIC) reveals the localization of both Abr2 and Abr1 in the cell wall. Scale bar: 5 μm
Localization (D) and fluorescence intensity (E) of Abr2-GFP in WT and abr1Δ (p <0.001). Scale bars: 5 μm.
See also Figure S3
DISCUSSION
Our findings of stage-specific subcellular compartmentalization of melanin biosynthetic machinery and previous observations of melanin in intracellular and extracellular vesicles lead us to propose that melanin biosynthesis and trafficking occur in the endomembrane system, and the late enzymes and mature melanin are exported through exocytosis. In many aspects, endosomes with melanin produced by Aspergillus during conidiation resemble the well-recognized tissue- and physiologically specific lysosome-related organelles (LROs) that are derived from the endosomal system in higher eukaryotes. One prominent LRO is melanosomes that confer color to the skin, eyes, and hair. In mammals, melanin is synthesized in melanosomes by melanocytes, which get transferred to neighboring keratinocytes, likely via shedding or exocytosis by melanocytes followed by internalization by keratinocytes (Wu and Hammer, 2014). Thus, intracellular transport and intercellular transfer of melanosomes are as important for pigmentation as the biosynthesis of melanin itself in mammals.
In both Aspergillus and mammals, pigmentation requires three elements: melanin biosynthetic machinery, melanosome structure, and intracellular and extracellular trafficking. Mutations in any of the three aspects result in diseases such as hypopigmentation or albinism. Examples in mammals include defects in targeting the melanin biosynthetic machinery to melanosomes (enzymes TYR/TYRP1 and the copper transporter ATP7A) (Setty et al., 2008; Sitaram and Marks, 2012) or in melanosome trafficking (Barral and Seabra, 2004). The human melanin enzymes TYR/TYRP1are both classical secreted proteins, similar to some yeasts that only use classically secreted laccases to polymerize exogenous precursors for melanization (Eisenman et al., 2005). By contrast, Aspergillus early melanin enzymes have no secretion signal and are thus recruited to the endosome via non-conventional secretion pathway. In both systems, having membrane-delimited organelles for melanization could confer several advantages: it concentrates substrates/precursors and multiple enzymes that need to function sequentially (metabolic channeling); sequestration of toxic intermediates minimizes potential damage to other cytoplasmic machineries; and the endosomal system provides mobility and effective exportation of the negatively charged macromolecules across the plasma membrane. Thus, despite the diversity in enzymes and substrates involved in making melanin, the remarkable resemblance between fungal and mammalian melanosomes suggests the conserved cellular principles evolved for melanization in eukaryotes.
Melanins are unique in that they are macro-polymers that act as a structural component. Besides melanin, fungal PKS pathways produce various small polyketides that have great economic and health impact. One well-characterized polyketide, aflatoxin, is synthesized and trafficked in aflatoxisomes (Chanda et al., 2010), which were established as endosomes (Ehrlich et al., 2012). Aflatoxins and its intermediate are presumably exported in endosome-derived MVBs, as evidenced by the detection of these compounds in discrete round patches on the intact cell surface (Chanda et al., 2010). Certain enzymes of some other PKS or NRPS pathways are known to localize to vesicles, including some involved in synthesizing penicillin (Muller et al., 1992), cyclosporin (Hoppert et al., 2001), or trichothecene (Menke et al., 2013). The first complete gene cluster to be characterized for their enzymes’ subcellular location is the fumiquinazoline cluster, where all four enzymes are localized by GFP-tagging using overexpressing strains (Lim et al., 2014). Interestingly, the enzyme involved in the last step of fumiquinazoline production is localized to the cell wall (Lim et al., 2014), similar to the late melanin enzymes that we reported here. It is conceivable that compartmentalization of all or some biosynthetic enzymes is a common mechanism to control the biogenesis and trafficking of various secondary metabolites (Keller, 2015; Kistler and Broz, 2015).
Compartmentalization in secretory vesicles might also apply to other biosynthetic machineries. For example, chitin, the defining fungal cell wall component, is synthesized in membrane-delimited structures called chitosomes (Bartnicki-Garcia, 2006). The polysaccharide capsule materials in Cryptococcus are found in extracellular vesicles derived from MVBs (Rodrigues et al., 2008) and they are likely both synthesized and trafficked in endosomes. Thus, using vesicles might be a unified cellular principle to direct biosynthesis of macromolecules in a defined area and to provide the mobility for enzymes and secreted products.
The finding of endosomal/MVB localization of all four early melanin enzymes was unexpected, given that none of them are considered conventional secretory proteins. The discovery provides an excellent example of trafficking to endosomes/MVBs of biosynthetic machinery that is composed of largely “atypical” secretory proteins. The requirement of a classic endosomal sorting nexin suggests that non-canonical secretion pathway utilizes the conventional endomembrane system. Thus, the critical step regarding the atypical protein secretion might lie at the step of recruitment.
These discoveries raise many questions that are not limited to fungal biology. LROs are possibly used to synthesize and/or sort cargoes by diverse organisms for various functions, as in the delivery virulence factors by fungi (Yi and Valent, 2013), in the facilitation of host entry by trypanosomes (Andrews, 2002), or in the generation/delivery of cytolytic granules by cytotoxic T cells and natural killer cells (Bonifacino, 2004). Do other LROs share common characteristics with melanosomes? Studying fungal melanosomes could yield powerful insights into these questions that are fundamental to our understanding of eukaryotic biology.
EXPERIMENTAL PROCEDURES
Strains, gene expression, and western blot
Strains and primers are listed in Table S1 and Table S2 respectively. Details of gene deletion, transcript analyses, protein tagging, sequencing, prediction of subcellular localization, protein extraction, and western blotting can be found in the Supplemental Experimental Procedures.
Microscopy
The expression of all fluorescence-tagged proteins was driven by their own promoters. Details for their construction, the measurement of fluorescence intensity, and the fluorescence intensity profiling can be found in the Supplemental Experimental Procedures. Procedures of sample preparation and analyses for scanning and transmission electron microscopy are also included in the supplemental data.
Plasmolysis and mechanic severing
The aerial conidiophore structures of A. fumigatus are highly resistant to osmolytes such as glycerol or sorbitol. Thus we used ethanol to render the membrane leaky to small molecules and the cytoplasm compressed under such a condition. Severing the conidiophore stalk or hypha by forceps caused loss of cytoplasm, rendering a ghost cell with only cell wall remaining. Other cells connected to the ghost cell remained intact, due to the protection by septa. In Fig. 4B, the cell wall signal from Abr1 from the conidiophore that included the stalk cell, phialides, and conidia appeared overwhelming due to the clustering of these many cells. Images of individual conidia or the stalk clearly showed cell wall localization of Abr1 and Abr2.
Supplementary Material
HIGHLIGHTS.
Endosomal trafficking is critical for melanization in fungi
There is stage-specific subcellular localization of the melanin biosynthetic enzymes
Early melanin enzymes have no secretion signal and are atypical secretory proteins
There is a unified cellular principle for melanogenesis in mammals and fungi
Acknowledgments
We thank Sven Krappmann, June Kwon-Chung, Jae-hyuk Yu, and Berl Oakley for their generous gifts of strains and plasmids, Alex Idnurm for helpful suggestions, and TAMU MIC for technical assistance. The work was supported by NIH (R21 AI088266 to XL) and TAMU Biology Department. Dr. Lin holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Supplemental information includes Supplemental Experimental Procedures, three figures, two tables, and one video.
AUTHOR CONTRIBUTIONS
S.U., X.X., and X.L. conceived and designed the experiments; S.U., X.X., J.J., D.L., and X.L. performed the experiments; S.U., X.X., J.J., D.L., R.R., and X.L. analyzed the data; S.U., X.X., D.L., and X.L. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alviano CS, Farbiarz SR, De Souza W, Angluster J, Travassos LR. Characterization of Fonsecaea pedrosoi melanin. J Gen Microbiol. 1991;137:837–844. doi: 10.1099/00221287-137-4-837. [DOI] [PubMed] [Google Scholar]
- Andrews NW. Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol. 2002;158:389–394. doi: 10.1083/jcb.200205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramayo R, Timberlake WE. Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nucleic Acids Res. 1990;18:3415. doi: 10.1093/nar/18.11.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci L, Bertini I, McGreevy KS, Rosato A. Molecular recognition in copper trafficking. Natural product reports. 2010;27:695–710. doi: 10.1039/b906678k. [DOI] [PubMed] [Google Scholar]
- Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2004;17:111–118. doi: 10.1111/j.1600-0749.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Chitosomes: past, present and future. FEMS yeast research. 2006;6:957–965. doi: 10.1111/j.1567-1364.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Bayry J, Beaussart A, Dufrene YF, Sharma M, Bansal K, Kniemeyer O, Aimanianda V, Brakhage AA, Kaveri SV, Kwon-Chung KJ, et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infection and immunity. 2014;82:3141–3153. doi: 10.1128/IAI.01726-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann N Y Acad Sci. 2004;1038:103–114. doi: 10.1196/annals.1315.018. [DOI] [PubMed] [Google Scholar]
- Boylan MT, Mirabito PM, Willett CE, Zimmerman CR, Timberlake WE. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol Cell Biol. 1987;7:3113–3118. doi: 10.1128/mcb.7.9.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart CG, Burkhart CN. The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection) International journal of dermatology. 2005;44:340–342. doi: 10.1111/j.1365-4632.2004.02556.x. [DOI] [PubMed] [Google Scholar]
- Chanda A, Roze LV, Linz JE. A possible role for exocytosis in aflatoxin export in Aspergillus parasiticus. Eukaryotic cell. 2010;9:1724–1727. doi: 10.1128/EC.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS. An updated review of tyrosinase inhibitors. International journal of molecular sciences. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi RJ, Liu J, West M, Wang J, Odorizzi G, Burd CG. Fission of SNX-BAR-coated endosomal retrograde transport carriers is promoted by the dynamin-related protein Vps1. J Cell Biol. 2014;204:793–806. doi: 10.1083/jcb.201309084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotirmall SH, Mirkovic B, Lavelle GM, McElvaney NG. Immunoevasive Aspergillus virulence factors. Mycopathologia. 2014;178:363–370. doi: 10.1007/s11046-014-9768-y. [DOI] [PubMed] [Google Scholar]
- Dyve AB, Bergan J, Utskarpen A, Sandvig K. Sorting nexin 8 regulates endosome-to-Golgi transport. Biochem Biophys Res Commun. 2009;390:109–114. doi: 10.1016/j.bbrc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- Ehrlich KC, Mack BM, Wei Q, Li P, Roze LV, Dazzo F, Cary JW, Bhatnagar D, Linz JE. Association with AflR in endosomes reveals new functions for AflJ in aflatoxin biosynthesis. Toxins. 2012;4:1582–1600. doi: 10.3390/toxins4121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Nosanchuk JD, Webber JB, Emerson RJ, Camesano TA, Casadevall A. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry. 2005;44:3683–3693. doi: 10.1021/bi047731m. [DOI] [PubMed] [Google Scholar]
- Hambleton S, Tsuneda A, Currah RS. Comparative morphology and phylogenetic placement of two microsclerotial black fungi from Sphagnum. Mycologia. 2003;95:959–975. [PubMed] [Google Scholar]
- Hoppert M, Gentzsch C, Schorgendorfer K. Structure and localization of cyclosporin synthetase, the key enzyme of cyclosporin biosynthesis in Tolypocladium inflatum. Arch Microbiol. 2001;176:285–293. doi: 10.1007/s002030100324. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PloS one. 2009;4:e4224. doi: 10.1371/journal.pone.0004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP. Translating biosynthetic gene clusters into fungal armor and weaponry. Nature chemical biology. 2015;11:671–677. doi: 10.1038/nchembio.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler HC, Broz K. Cellular compartmentalization of secondary metabolism. Front Microbiol. 2015;6:68. doi: 10.3389/fmicb.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder K, Jahn B, Gehringer H, Schmidt A, Wanner G, Brakhage AA. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Medical microbiology and immunology. 1998;187:79–89. doi: 10.1007/s004300050077. [DOI] [PubMed] [Google Scholar]
- Latgé J-P, Steinbach WJ. Aspergillus fumigatus and aspergillosis. Washington, D.C: ASM Press; 2009. [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect biochemistry and molecular biology. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Li J, Chang YC, Wu CH, Liu J, Kwon-Chung KJ, Huang SH, Shimada H, Fante R, Fu X, Jong A. The 14-3-3 gene function of Cryptococcus neoformans is required for its growth and virulence. J Microbiol Biotechnol. 2015 doi: 10.4014/jmb.1508.08051. Epub: Oct. 6, 2015. [DOI] [PubMed] [Google Scholar]
- Lim FY, Ames B, Walsh C, Keller N. Coordination between BrlA regulation and secretion of the oxidoreductase FmqD directs selective accumulation of fumiquinazoline C to conidial tissues in Aspergillus fumigatus. Cellular microbiology. 2014;18:1267–1283. doi: 10.1111/cmi.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG. Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Molecular biology of the cell. 2012;23:2505–2515. doi: 10.1091/mbc.E11-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke J, Weber J, Broz K, Kistler HC. Cellular development associated with induced mycotoxin synthesis in the filamentous fungus Fusarium graminearum. PloS one. 2013;8:e63077. doi: 10.1371/journal.pone.0063077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WH, Bovenberg RA, Groothuis MH, Kattevilder F, Smaal EB, Van der Voort LH, Verkleij AJ. Involvement of microbodies in penicillin biosynthesis. Biochimica et biophysica acta. 1992;1116:210–213. doi: 10.1016/0304-4165(92)90118-e. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling 428 of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram A, Marks MS. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology (Bethesda) 2012;27:85–99. doi: 10.1152/physiol.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugareva V, Hartl A, Brock M, Hubner K, Rohde M, Heinekamp T, Brakhage AA. Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch Microbiol. 2006;186:345–355. doi: 10.1007/s00203-006-0144-2. [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani SA, Oakley BR. The tip growth apparatus of Aspergillus nidulans. Molecular biology of the cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Yu JH. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology (Reading, England) 2011;157:313–326. doi: 10.1099/mic.0.044271-0. [DOI] [PubMed] [Google Scholar]
- Tsai HF, Chang YC, Washburn RG, Wheeler MH, Kwon-Chung KJ. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. Journal of bacteriology. 1998;180:3031–3038. doi: 10.1128/jb.180.12.3031-3038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HF, Fujii I, Watanabe A, Wheeler MH, Chang YC, Yasuoka Y, Ebizuka Y, Kwon-Chung KJ. Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J Biol Chem. 2001;276:29292–29298. doi: 10.1074/jbc.M101998200. [DOI] [PubMed] [Google Scholar]
- Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. Journal of bacteriology. 1999;181:6469–6477. doi: 10.1128/jb.181.20.6469-6477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Torres G, Lin X. Laccases involved in 1,8-dihydroxynaphthalene melanin biosynthesis in Aspergillus fumigatus are regulated by developmental factors and copper homeostasis. Eukaryotic cell. 2013;12:1641–1652. doi: 10.1128/EC.00217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Verkade P, Cullen PJ. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic. 2012;13:94–107. doi: 10.1111/j.1600-0854.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- Walker CA, Gomez BL, Mora-Montes HM, Mackenzie KS, Munro CA, Brown AJ, Gow NA, Kibbler CC, Odds FC. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryotic cell. 2010;9:1329–1342. doi: 10.1128/EC.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29C:1–7. doi: 10.1016/j.ceb.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Valent B. Communication between filamentous pathogens and plants at the biotrophic interface. Annual review of phytopathology. 2013;51:587–611. doi: 10.1146/annurev-phyto-081211-172916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.