Abstract
Genetically-engineered pigs are increasingly recognized as valuable models for the study of human disease. Immunohistochemical study of cellular markers of disease is an important tool for the investigation of these novel models so as to evaluate genotype and treatment differences. Even so, there remains a lack of validated markers for pig tissues that can serve as a translational link to human disease in organs such as the lung. Herein, we evaluate markers of cellular inflammation (CD3, CD79a, BCL6, IBA1, and myeloperoxidase) and those that may be involved with tissue remodeling (alpha-smooth muscle actin, beta-tubulin-III, lactoferrin, MUC5AC, MUC5B, and CFTR) for study of lung tissues. We compare the utility of these markers between pig and human lungs to validate translational relevance of each marker. Our results suggest these markers can be a useful addition in the pathological evaluation of porcine models of human disease.
Keywords: Immunohistochemistry, markers, pig, human, lung, leukocytes, mucus
INTRODUCTION
Pigs have traditionally been a useful species to model pathophysiology to better understand human biology (Swindle et al., 2012). In particular, the pig lung has been valuable to study normal physiology (Sommerer et al., 2004) as well as developmental (Glenny et al., 2007), infectious (Rajao and Vincent, 2015), and environmental/toxicological diseases (Gushima et al., 2001; Grainge et al., 2010). The similar lung size, anatomy, and physiology to humans along with the longevity of pigs compared to smaller rodents make the pig a useful species to model human lung diseases (Rogers et al., 2008a; Aigner et al., 2010).
In recent years, development of genetically modified pigs has been reported for several human diseases including cystic fibrosis (Ostedgaard et al., 2011; Rogers et al., 2008b; Stoltz et al., 2010; Stoltz et al., 2013; Olivier et al., 2015), cancer (Sieren et al., 2014), muscular dystrophy (Klymiuk et al., 2013), atherosclerosis (Davis et al., 2014), and diabetes mellitus (Renner et al., 2010). The success of these models has spurred further interest in pigs as a model species for translational studies; however, one potential limitation, compared to small rodent models, is the relative lack of available and validated reagents, assays and techniques for translational use in pigs with comparison to humans (Olivier et al., 2012). Antibodies and validated immunohistochemistry techniques are useful for pathological evaluation.
In some cases, human markers have been successfully applied to pig tissues (Meyerholz et al., 2010) and these techniques can serve as a guide for use in investigational studies. For antibodies, commercial vendors will often list species that are compatible for immunohistochemistry; however, these endorsements are frequently based upon predicted sequence identity or on anecdotal reports from the vendor's clients – both of these approaches lack robust substantiation. Importantly, there is currently a lack of direct comparison of lung tissue markers between pigs and humans. To this end, we have begun optimizing several immunohistochemical techniques for direct translational use in pig and human tissues.
Investigational studies of the lung often evaluate cellular inflammation (Gauger et al., 2012; Mirakaj et al., 2014; Posa et al., 2013) and tissue remodeling as parameters (Aguayo, 1994; Wright et al., 2014) for endpoint assessment. Accordingly, we used optimized markers of cellular inflammation (CD3, CD79a, BCL6, IBA1, and myeloperoxidase) and remodeling (alpha-smooth muscle actin, beta-tubulin-III, lactoferrin, MUC5AC, MUC5B, CFTR) in the pig lung to evaluate their utility for translational applications to human lung.
MATERIALS AND METHODS
Tissues
All pig and human tissues were collected from archival formalin fixed paraffin-embedded blocks. Pig tissues came from studies that had received University of Iowa Institutional Animal Care and Use Committee (IACUC) approval. Human tissues were acquired through the Cell Culture Core Repository (University of Iowa), which has received institutional approval from the University of Iowa Institutional Review Board (IRB #:199507432) for collection of human tissues. Comparisons for each cellular marker were made from at least 3 samples from respective porcine (2-6 months of age) and human tissues (adults, 3-4th decade). Unless otherwise specified, tissues came from healthy individuals lacking overt clinical disease. Tissues were selected for each marker that was deemed most useful to demonstrate efficacy (e.g. tonsil to highlight CD3 staining) and when possible the “healthy” lung (lacking clinical evidence of overt or chronic disease) was chosen as tissue of choice. CF lung tissues immunostained for neutrophils came from a 2 month old pig that had a homozygous null mutation and from a human that was homozygous for the ΔF508 mutation.
Immunohistochemistry
All tissues included in the study were routinely fixed in 10% neutral buffered formalin for ~ 4-7 days with the first few days on a rotating plate to better fix the samples (Olivier et al., 2012). Tissues were routinely processed and paraffin embedded and then sectioned (~4 μm) onto glass slides (Superfrost Plus Microscope Slides, Fisher Scientific Co. Pittsburgh, PA) for antigen retrieval and application of IHC reagents (Supplemental Table 1). Tissue chromogen (DAB, brown color) and counter stain (Surgipath Harris Hematoxylin, Leica Biosystems, Richmond, IL) were applied and then followed by routine dehydration and coverslipping. Tissues were evaluated and scored following principles of histopathological scoring and by using an ordinal scoring system (Gibson-Corley et al., 2013).
RESULTS
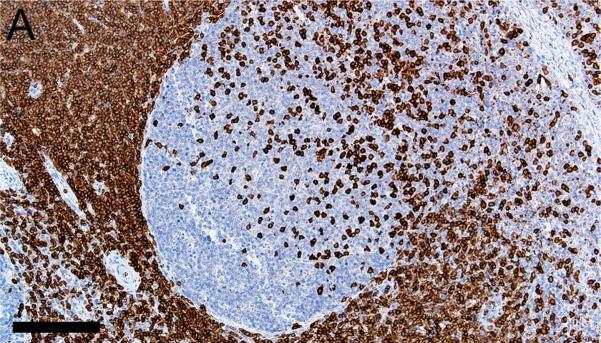
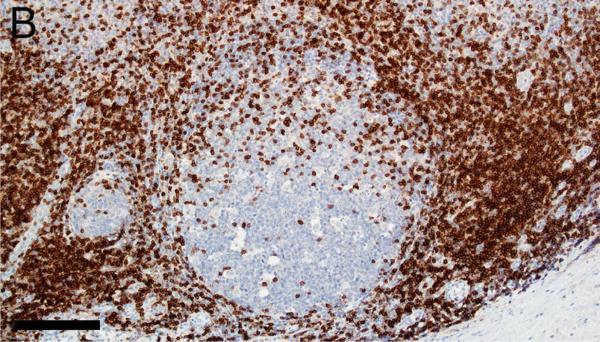
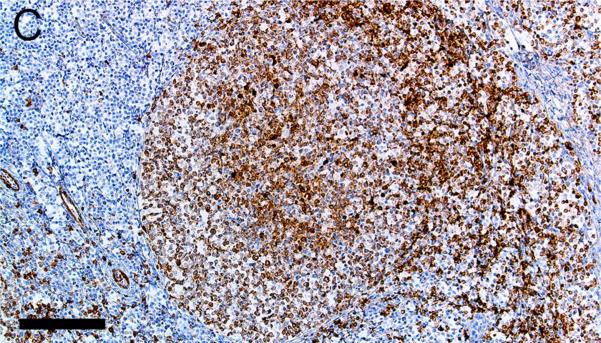
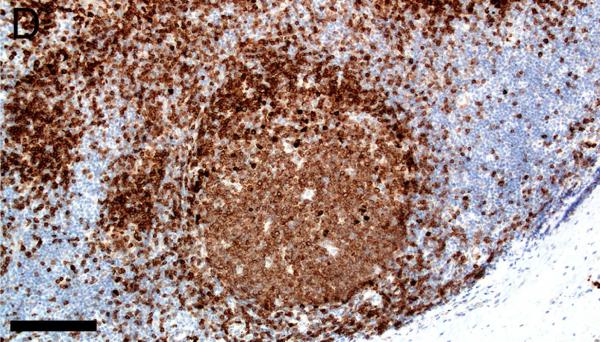
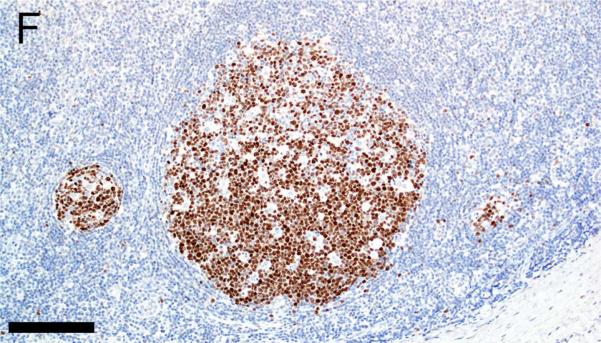
Evaluation of lymphoid markers in lymphoid tissues showed CD3+ cells in T-cell rich zones, CD79a+ cells in B-cell rich and germinal center zones, and BCL6+ cells exclusively in germinal centers (Figures 1A-F). These markers appeared to be very similar between species with the exception of slightly less-intense staining by CD79a in porcine B cells (Table 1).
Figure 1.
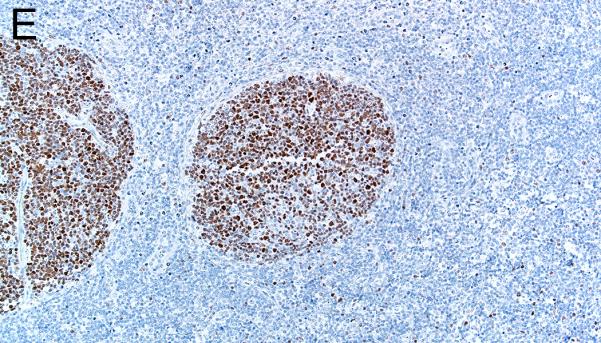
Immunostaining of healthy pig lymph node (A,C,E) and human tonsil (B,D,F) for CD3 (A,B), CD79a (C,D) and BCL6 (E,F), bars = 160 μm.
Table 1.
Immunohistochemical markers, tissues, immunostaining scores and localization.
| Marker | Tissue | Immunostaining scores | Localization |
|---|---|---|---|
| CD3 | Healthy Tonsil (h), lymph node (p) | +++ (h, p) | L: T-cell rich zones N: Nominal |
| CD79a | Healthy Tonsil (h), lymph node (p) | +++ (h); ++ (p) | L: B-cell rich zones including germinal centers N: Smooth muscle (p) |
| BCL6 | Healthy Tonsil (h), lymph node (p) | +++ (h); ++/+++ (p) | L: Germinal center B-cells N: Nominal |
| IBA1 | Healthy lung (h,p) | ++/+++ (h, p) | L: Macrophages (alveolar, interstitial and intravascular) N: Nominal |
| Myeloperoxidase | CF lung (h,p) | +++ (h); ++ (p) | L: Neutrophils > macrophages N: Nominal |
| MUC5AC | Healthy lung (h,p) | +++ (h, p) | L: Goblet cells of surface epithelium N: Nominal |
| MUC5B | Healthy lung (h,p) | +++ (h, p) | L: Mucous cells of submucosal gland and goblet cells of surface epithelium N: Nominal |
| Lactoferrin | Healthy lung (h,p) | +++ (h); ++ (p) | L: Serous cells of submucosal gland, scattered surface epithelial cells, scattered macrophages N: Nominal |
| Beta Tubulin III | Healthy lung (h,p) | +++ (h, p) | L: Axon within surface epithelium, submucosa and in nerves N: Nominal |
| CFTR | Healthy lung (h,p) | ++/+++ (h, p) | L: Apical surface of serous cells in submucosal glands and scattered surface epithelial cells N: Nominal |
| alpha-Smooth Muscle Actin | Heathy lung (h,p) | +++ (h, p) |
L: Smooth muscle of airways, vessels, and glands.
N: Nominal |
Scoring of Immunostaining: + weak/mild; ++ moderate; +++ robust/strong
Abbreviations: (h), human; (p), pig; CF, cystic fibrosis; L, Localization; N, Nonspecific staining
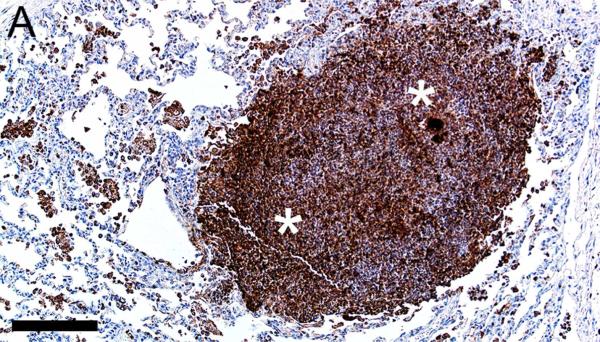
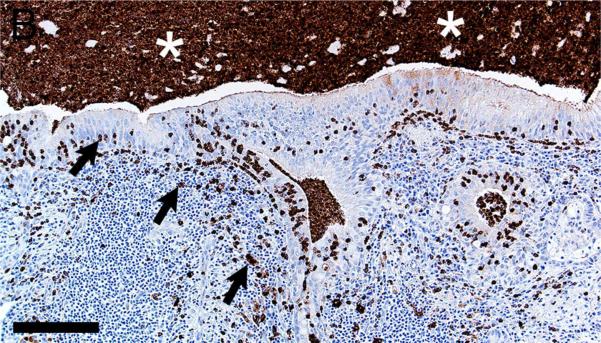
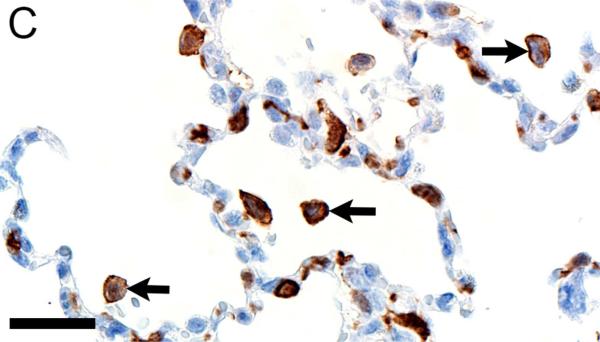
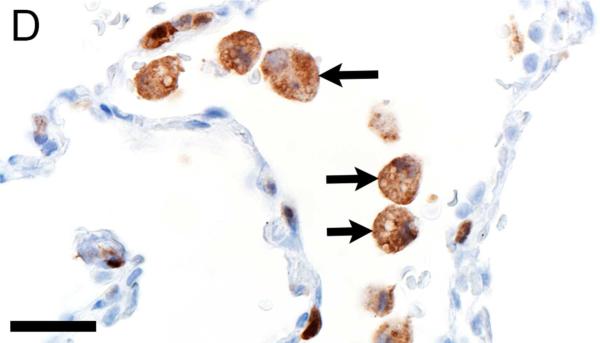
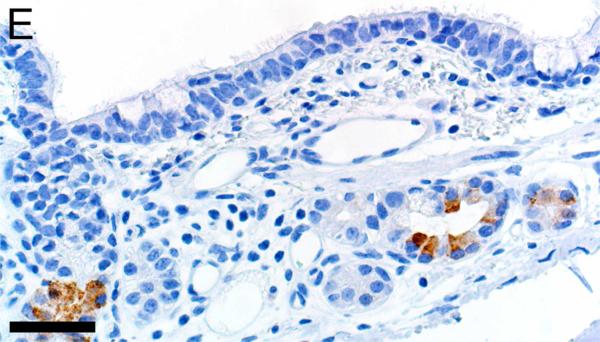
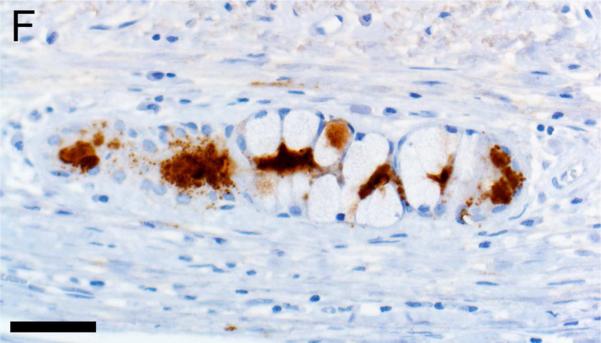
In CF lungs, myeloperoxidase had robust staining at sites of neutrophilic inflammation (Figures 2A-B) and in areas of macrophages, findings consistent with both being myeloid lineage leukocytes (Table 1). IBA1 had moderate to intense immunostaining of alveolar macrophages within the airspaces (Figures 2C-D), and staining within the septal walls consistent with interstitial or intravascular macrophages (Table 1) (Cai et al., 2014; Winkler, 1988).
Figure 2.
Immunostaining of lung from pig (A,C) and human (B,D). A,B) Aggregates of myeloperoxidase+ [MPO+] neutrophils were detected within airspaces (asterisks, A & B) of CF lungs. Note the MPO+ neutrophils exocytosing across the surface epithelium and airway wall (arrows, B), bars = 160 μm. C,D) IBA1+ alveolar macrophages (arrows) were localized within airspaces and IBA+ cellular staining was also detected within the septal walls of healthy lungs, bar = 26 μm.
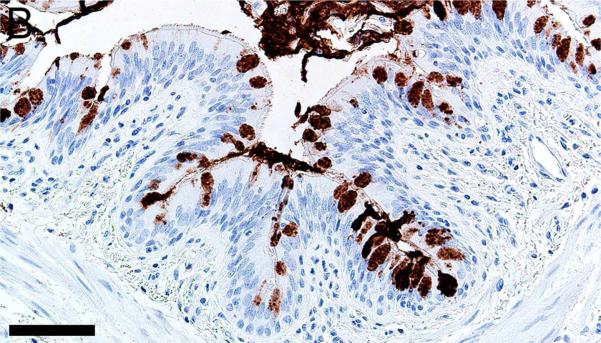
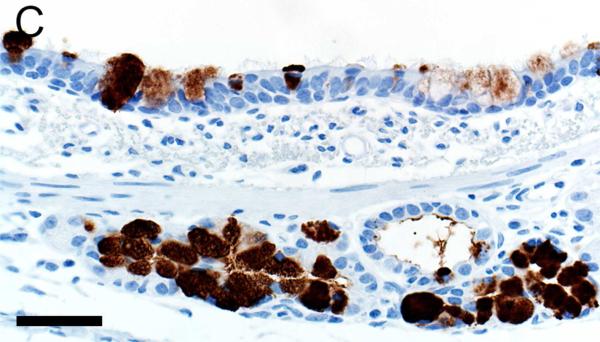
Mucus and submucosal gland secretions are common contributors to several airway diseases including CF, asthma, chronic obstructive pulmonary disease (COPD) and chronic mucus hypersecretion associated with smoking (Rajavelu et al., 2015; Stoltz et al., 2015; Dijkstra et al., 2015). MUC5AC immunostaining was found in goblet cells of the surface epithelium (Figures 3A-B, Table 1) while MUC5B was seen in mucous cells of submucosal gland and goblet cells of the surface epithelium (Figures 3C-D). In contrast, lactoferrin was detected principally in serous cells of the submucosal glands (Figures 3E-F) and in scattered epithelial cells of the surface epithelium.
Figure 3.
Immunostaining of healthy lung from pig (A,C,E) and human (B,D,F). A,B) MUC5AC immunostaining of goblet cells of the surface epithelium, bars = 80 μm. C,D) MUC5B immunostaining of mucous cells in the submucosal gland (C,D) and goblet cells in the surface epithelium (C), bars = 40 μm. E,F) Lactoferrin [LTF] immunostaining of serous cells in the submucosal gland, bars = 40 μm.
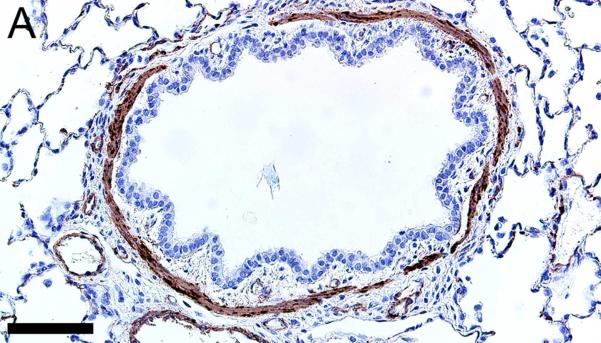
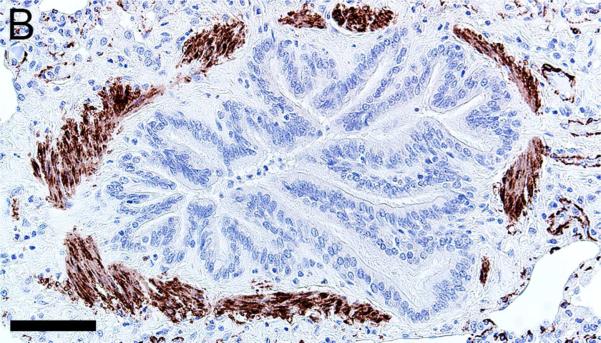
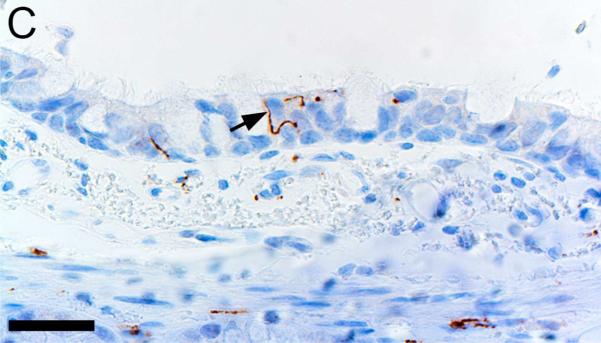
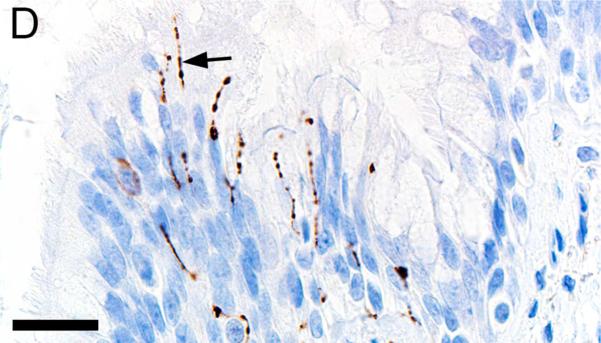
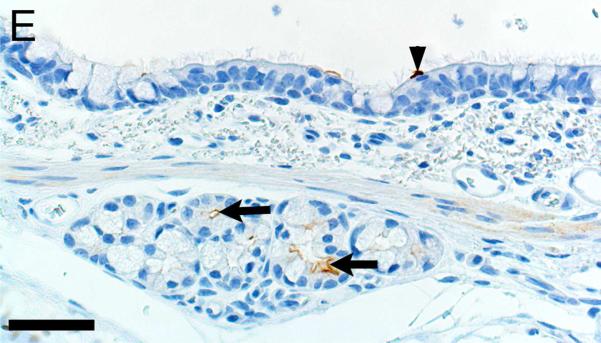
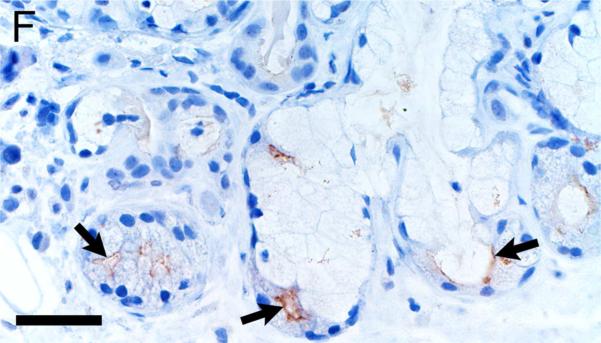
Alpha-smooth muscle actin was detected in airway smooth muscle (Figures 4A-B, Table 1) as well as the tunica media of vessels of both pig and human tissue. Beta-tubulin-III a marker of axons, was detected in axons within nerves and scattered within submucosal tissues and within the epithelium (Figures 4C-D) where it extended to near the luminal surface. CFTR, an anion channel, was detected on the apical surface of scattered surface epithelial cells and apically on serous cells of the submucosal glands (Figures 4E-F).
Figure 4.
Immunostaining of healthy lung from pig (A,C,E) and human (B,D,F). A,B) Airway smooth muscle and tunica media of vessels were immunostained by alpha-smooth muscle actin [SMA], bars = 80 μm. C,D) Beta-tubulin-III [BT] immunostaining detects axons in the surface epithelium (arrows) and in the submucosa, bars = 26 μm. E,F) CFTR immunostaining was detected in submucosal glands on the apical cytoplasm/membrane of serous cells (arrows, E & F) and scattered surface epithelial cells (arrowhead, E), bars = 40 μm.
DISCUSSION
Most of the immunohistochemistry markers that were optimized in the pig gave comparable immunostaining in human tissues. Lymphoid leukocyte subsets composed of T-cells (CD3+), B-cells (CD79a+) and germinal center B-cells (BCL6+) can contribute to the inflammatory changes within the lung, BALT and pulmonary lymph nodes following lung injury or disease (Chvatchko et al., 1996; Pabst and Gehrke, 1990; Valheim et al., 2011). Lymphoid markers were very comparable in localization and staining intensity (Table 1). Neutrophils and macrophages are important leukocytes to assess for lung injury and disease (Richeldi et al., 2004). Myeloperoxidase, a myeloid leukocyte marker, immunostained neutrophils as well as macrophages as might be predicted. Neutrophils and activated macrophages appeared to have more robust immunostaining than unstimulated macrophages. While neutrophils and macrophages can often be distinguished by their morphologic appearance, the presence of overlapping staining in these cells types can be problematic when doing morphometry or when examining sites with degenerate cells. In contrast, IBA1, while more commonly used in the brain, was surprisingly useful to detect many macrophage/monocyte lineage cells in pigs and humans; further investigations are ongoing.
Submucosal glands can serve as a significant site of airway remodeling through excessive mucus production, defective mucus release into airways, or by gland hypertrophy in chronic disease (Hoegger et al., 2014; Stoltz et al., 2013; Stoltz et al., 2015). Thus, evaluation of the submucosal glands can be important. Lactoferrin is produced by serous cells of the submucosal glands and some surface epithelial cells, but is not expressed by macrophages (Vareille et al., 2011). However, lactoferrin is bound and taken up by macrophages which are speculated to hold a recirculating pool of lactoferrin (Britigan et al., 1991); this could explain apparent macrophage immunostaining. MUC5AC and MUC5B are mucins found in distinct compartments of the airway wall (Meyerholz et al., 2010) with MUC5AC in surface epithelium, and MUC5B in mucous cells of submucosal glands and variably in surface epithelium. The mucus and serous markers were effective in both pig and human tissues.
Environmental exposure to toxicants or airway diseases like asthma, COPD and CF can lead to remodeling of airway smooth muscle (e.g. hypertrophy) and decreased lung function (McCuaig and Martin, 2013; Stoltz et al., 2015; Wylam et al., 2015). Alpha-smooth muscle immunostaining worked well for detecting smooth muscle around airways. Additionally, tunica media of vessels was also immunostained in both pigs and humans – which could possibly confound evaluation of smooth muscle in the walls of distal airspaces.
Activation of autonomic and sensory nerves innervating the airway can elicit cough, bronchoconstriction, mucus secretion, and apnea (Canning, 2006; Undem et al., 1999). Several studies have found increased neural innervation to the airway in experimental models of asthma in both mouse and non-human primates (Aven et al., 2014; Kajekar et al., 2007; Yu et al., 2008). Beta-tubulin-III effectively identified pig and human axons within nerves and clearly demonstrated axons extending towards the lumen within the surface epithelium.
CFTR encodes an anion channel that when mutated causes cystic fibrosis (Stoltz et al., 2015). However, recent evidence suggests that acquired CFTR dysfunction can be observed in COPD and following exposure to cigarette smoke (Courville et al., 2014; Rasmussen et al., 2014). CFTR is often found in the apical membrane of serous cells in submucosal glands and also in scattered surface epithelial cells, similar to what we observed in pig and human tissues.
In conclusion, this study provides direct validation for use of several cellular markers in pathological assessment of porcine tissues for translational investigations. Limitations of this study include the relatively small sample size for each group. Also, we lacked control of the time interval from death to receipt of the fresh human lung tissue – even so, the tissues lacked evidence of autolysis. Advantages of this study included consistent methodology in fixation of tissues for a more standardized final product. Also, the optimization of staining methods in pig tissues as a preliminary step worked well because many of the markers were made for use in humans and so application to the human tissue was often straightforward. Lastly, we were able to directly compare tissues in the same experimental setting and showed that there is remarkable overlap in localization and appearance of tissue markers in pigs and humans. These findings complement and expand upon existing studies showing that the pig is a useful model for study of human pathobiology.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by the National Institutes of Health (P01 HL051670, P01 HL091842, P30 DK054759, 1K99HL119560, DP2 HL117744), American Asthma Foundation, Cystic Fibrosis Foundation Research and Development Program and Cystic Fibrosis Foundation Mucociliary Clearance Consortium.
Abbreviations
- BALT
bronchus-associated lymphoid tissue
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- HIER
heat induced epitope retrieval
- h
human
- L
localization
- N
nonspecific staining
- p
pig
REFERENCES
- Aguayo SM. Determinants of susceptibility to cigarette smoke. Potential roles for neuroendocrine cells and neuropeptides in airway inflammation, airway wall remodeling, and chronic airflow obstruction. Am J Respir Crit Care Med. 1994;149:1692–1698. doi: 10.1164/ajrccm.149.6.7911710. [DOI] [PubMed] [Google Scholar]
- Aigner B, Renner S, Kessler B, et al. Transgenic pigs as models for translational biomedical research. J Mol Med (Berl) 2010;88:653–664. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- Aven L, Paez-Cortez J, Achey R, et al. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J. 2014;28:897–907. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan BE, Serody JS, Hayek MB, et al. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J Immunol. 1991;147:4271–4277. [PubMed] [Google Scholar]
- Cai Y, Sugimoto C, Arainga M, et al. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985) 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- Chvatchko Y, Kosco-Vilbois MH, Herren S, et al. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med. 1996;184:2353–2360. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville CA, Tidwell S, Liu B, et al. Acquired defects in CFTR-dependent beta-adrenergic sweat secretion in chronic obstructive pulmonary disease. Respir Res. 2014;15:25. doi: 10.1186/1465-9921-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BT, Wang XJ, Rohret JA, et al. Targeted disruption of LDLR causes hypercholesterolemia and atherosclerosis in Yucatan miniature pigs. PLoS One. 2014;9:e93457. doi: 10.1371/journal.pone.0093457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra AE, Boezen HM, van den Berge M, et al. Dissecting the genetics of chronic mucus hypersecretion in smokers with and without COPD. Eur Respir J. 2015;45:60–75. doi: 10.1183/09031936.00093314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger PC, Vincent AL, Loving CL, et al. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol. 2012;49:900–912. doi: 10.1177/0300985812439724. [DOI] [PubMed] [Google Scholar]
- Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny RW, Bernard SL, Luchtel DL, et al. The spatial-temporal redistribution of pulmonary blood flow with postnatal growth. J Appl Physiol (1985) 2007;102:1281–1288. doi: 10.1152/japplphysiol.00632.2006. [DOI] [PubMed] [Google Scholar]
- Grainge C, Jugg BJ, Smith AJ, et al. Delayed low-dose supplemental oxygen improves survival following phosgene-induced acute lung injury. Inhal Toxicol. 2010;22:552–560. doi: 10.3109/08958370903571831. [DOI] [PubMed] [Google Scholar]
- Gushima Y, Ichikado K, Suga M, et al. Expression of matrix metalloproteinases in pigs with hyperoxia-induced acute lung injury. Eur Respir J. 2001;18:827–837. doi: 10.1183/09031936.01.00049201. [DOI] [PubMed] [Google Scholar]
- Hoegger MJ, Fischer AJ, McMenimen JD, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R, Pieczarka EM, Smiley-Jewell SM, et al. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol. 2007;155:55–63. doi: 10.1016/j.resp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Klymiuk N, Blutke A, Graf A, et al. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum Mol Genet. 2013;22:4368–4382. doi: 10.1093/hmg/ddt287. [DOI] [PubMed] [Google Scholar]
- McCuaig S, Martin JG. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir Med. 2013;1:137–147. doi: 10.1016/S2213-2600(12)70058-9. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Stoltz DA, Namati E, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Mutz C, Vagts D, et al. Rosiglitazone dampens pulmonary inflammation in a porcine model of acute lung injury. Inflammation. 2014;37:1102–1110. doi: 10.1007/s10753-014-9834-0. [DOI] [PubMed] [Google Scholar]
- Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2015;308:G459–471. doi: 10.1152/ajpgi.00146.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier AK, Naumann P, Goeken A, et al. Genetically modified species in research: Opportunities and challenges for the histology core laboratory. J Histotechnol. 2012;35:63–67. doi: 10.1179/2046023612y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Meyerholz DK, Chen JH, et al. The DeltaF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- Posa R, Magyar T, Stoev SD, et al. Use of computed tomography and histopathologic review for lung lesions produced by the interaction between Mycoplasma hyopneumoniae and fumonisin mycotoxins in pigs. Vet Pathol. 2013;50:971–979. doi: 10.1177/0300985813480510. [DOI] [PubMed] [Google Scholar]
- Rajao DS, Vincent AL. Swine as a model for influenza a virus infection and immunity. ILAR J. 2015;56:44–52. doi: 10.1093/ilar/ilv002. [DOI] [PubMed] [Google Scholar]
- Rajavelu P, Chen G, Xu Y, et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JE, Sheridan JT, Polk W, et al. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J Biol Chem. 2014;289:7671–7681. doi: 10.1074/jbc.M113.545137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner S, Fehlings C, Herbach N, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, Mariani M, Losi M, et al. Triggering receptor expressed on myeloid cells: role in the diagnosis of lung infections. Eur Respir J. 2004;24:247–250. doi: 10.1183/09031936.04.00014204. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008a;295:L240–263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008b;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieren JC, Meyerholz DK, Wang XJ, et al. Development and translational imaging of a TP53 porcine tumorigenesis model. J Clin Invest. 2014;124:4052–4066. doi: 10.1172/JCI75447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerer D, Suss R, Hammerschmidt S, et al. Analysis of the phospholipid composition of bronchoalveolar lavage (BAL) fluid from man and minipig by MALDI-TOF mass spectrometry in combination with TLC. J Pharm Biomed Anal. 2004;35:199–206. doi: 10.1016/j.jpba.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle MM, Makin A, Herron AJ, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49:344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- Undem BJ, McAlexander M, Hunter DD. Neurobiology of the upper and lower airways. Allergy 54 Suppl. 1999;57:81–93. doi: 10.1111/j.1398-9995.1999.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Valheim M, Gamlem H, Gjerset B, et al. Pathological Findings and Distribution of Pandemic Influenza A (H1N1) 2009 Virus in Lungs from Naturally Infected Fattening Pigs in Norway. Influenza Res Treat. 2011;2011:565787. doi: 10.1155/2011/565787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareille M, Kieninger E, Edwards MR, et al. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181:217–234. doi: 10.1002/aja.1001810302. [DOI] [PubMed] [Google Scholar]
- Wright DB, Meurs H, Dekkers BG. Integrins: therapeutic targets in airway hyperresponsiveness and remodelling? Trends Pharmacol Sci. 2014;35:567–574. doi: 10.1016/j.tips.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Wylam ME, Sathish V, VanOosten SK, et al. Mechanisms of Cigarette Smoke Effects on Human Airway Smooth Muscle. PLoS One. 2015;10:e0128778. doi: 10.1371/journal.pone.0128778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Zheng X, Peake J, et al. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J Allergy Clin Immunol. 2008;122:640–647. e641. doi: 10.1016/j.jaci.2008.04.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.