Abstract
Purpose
To examine changes in muscle strength and self-reported physical functioning in men receiving androgen deprivation therapy (ADT) for prostate cancer compared to matched controls.
Methods
Prostate cancer patients scheduled to begin ADT (n=62) were assessed within 20 days of starting ADT and 6 and 12 months later. Age and geographically-matched prostate cancer controls treated with prostatectomy only (n=86) were assessed at similar time intervals. Grip strength measured upper body strength, the Chair Rise Test measured lower body strength, and the SF-12 Physical Functioning scale measured self-reported physical functioning.
Results
As expected, self-reported physical functioning and upper body muscle strength declined in ADT recipients but remained stable in prostate cancer controls. Contrary to expectations, lower body muscle strength remained stable in ADT recipients but improved in prostate cancer controls. Higher Gleason scores, more medical comorbidities, and less exercise at baseline predicted greater declines in physical functioning in ADT recipients.
Conclusions
ADT is associated with declines in self-reported physical functioning and upper-body muscle strength as well as worse lower body muscle strength relative to prostate cancer controls. These findings should be included in patient education regarding the risks and benefits of ADT. Findings also underscore the importance of conducting research on ways to prevent or reverse declines in physical functioning in this patient population.
Keywords: prostate cancer, androgen deprivation, physical functioning, Antiandrogens, Physical Activity, Muscle Strength, Quality of Life
Introduction
Approximately 45% of men with prostate cancer will be treated with androgen deprivation therapy (ADT) as primary treatment or adjuvant to radiation or prostatectomy [9]. ADT significantly reduces the risk of mortality in prostate cancer[24], but despite its efficacy ADT it is associated with multiple side-effects, including symptoms of sarcopenia (i.e., loss of strength or mobility resulting from decreases in lean body mass) [26].
Previous research on decrements in physical functioning secondary to ADT has focused mostly on the extent of muscle loss. Studies suggest patients undergoing ADT demonstrate a 3–4% loss of lean body mass after beginning ADT [12, 23], in contrast, to no change in cancer-free older men and a loss of less than 0.5% in prostate cancer patients not treated with ADT [29]. Few studies have examined the functional consequences of ADT, such as declines in self-reported physical functioning. Some cross-sectional evidence has suggested that physical functioning is worse in men treated with ADT compared to controls [7], but other studies have failed to find an effect of ADT on physical functioning [27].
In cross-sectional studies, men receiving ADT demonstrated worse upper body strength [2], lower body strength [7, 18], self-reported physical functioning [14], and worse physical performance [4] than men who did not receive ADT. Others have not observed worse upper body strength or lower body strength in ADT recipients [15]. However, only one study has examined the impact of ADT on physical functioning longitudinally with a comparison group of men with prostate cancer not treated with ADT. In this study, Alibhai and colleagues found that ADT recipients reported worse physical functioning and exhibited worse upper body strength as well as less exercise tolerance than controls [1]. However, no differences were observed for lower body strength and predictors of worse physical functioning were not explored. These somewhat inconsistent results and the lack of data on predictors of the decline in physical functioning highlight the need for additional research.
In light of sparse and inconsistent evidence linking ADT with reduced lower body strength and physical functioning, the present study used a longitudinal design to determine the impact of ADT on upper and lower body muscle strength and self-reported physical functioning. It was hypothesized that prostate cancer patients treated with ADT would experience declines in physical functioning and muscle strength from the start of ADT to 12 months later, whereas prostate cancer patients treated with prostatectomy only would not. It was also hypothesized that ADT recipients would demonstrate worse physical functioning and muscle strength than controls at 6 and 12 months after initiation of ADT. No previous studies have examined longitudinal predictors of worsening physical functioning after ADT. Thus, additional exploratory analyses sought to identify predictors of individual differences of decline in outcomes that worsened significantly in ADT recipients.
Methods
Participants
Participants were recruited as part of a longitudinal study focused on cognitive side effects of ADT. The study included two samples: prostate cancer patients starting ADT (ADT recipients) and prostate cancer patients treated with prostatectomy only (prostate cancer controls). Eligibility criteria required that all participants be ≥ 18 years of age, be able to speak and read English, have ≥ eighth grade education, have no history of stroke, and be able to provide informed consent. Because the larger study focused on cognitive function, all participants were also free of cognitive impairment (Short Portable Mental Status Exam score < 3). Additional eligibility criteria for ADT recipients were that they be diagnosed with non-metastatic or asymptomatic metastatic prostate cancer, be disease-free or in remission for any other cancers, be scheduled to start ADT or have started ADT in past month for standard clinical purposes, and have a planned course of ADT treatment of at least 6 months. They also had to be free of: treatment for any other cancers in the 12 months prior to recruitment; any history of brain cancer; previous treatment with cranial irradiation; treatment with ADT in the 12 months prior to recruitment; and treatment with an anti-androgen agent (e.g., bicalutamide) in the 6 months prior to recruitment.
Additional eligibility criteria for the prostate cancer controls were that they be diagnosed with non-metastatic prostate cancer, be free of diagnoses of other cancers except non-melanoma skin cancer, have undergone prostatectomy, have no history of recurrent disease since undergoing prostatectomy, have no history of other forms of prostate cancer treatment (e.g., radiotherapy), not be scheduled for additional prostate cancer treatment, and not be receiving testosterone supplementation. Prostate cancer controls were matched to an ADT recipient on time since diagnosis (within 6 months), age (within 5 years), and education (i.e., ≤12 years, 13–16 years, or ≥17 years).
Procedure
Data collection began in September, 2008. The grip strength and chair rise tests were added in October, 2009. Written informed consent was obtained prior to initiation of study procedures. Participants were paid $80 at each evaluation. This study was approved by the Institutional Review Board at the University of South Florida.
ADT recipients and prostate cancer controls were recruited from Moffitt Cancer Center. ADT recipients were also recruited from the James A. Haley Veterans’ Hospital in Tampa, Florida. Baseline assessments were completed before or within one month of starting ADT and 6 and 12 months later. Individuals were contacted by telephone and those eligible and interested were scheduled for an appointment at which written informed consent was obtained and the baseline assessment conducted. Follow-up assessments were conducted 6 and 12 months after baseline.
Measures
Demographic and clinical factors were assessed at baseline. Age, education, race, ethnicity, and smoking history were assessed via self-report. Time since diagnosis, body mass index, Gleason score were assessed via medical chart review. Medical comorbidities were assessed using a self-report version of the Charlson Comorbidity Index (CCI) [17].
Self-reported physical functioning was examined at each assessment using the Physical Functioning subscale of the Medical Outcomes Study SF-12 scale (SF-12) [30]. Items in this subscale ask respondents to indicate the degree to which their health limits moderate activities (moving a table, playing golf) and more strenuous activity (climbing several flights of stairs). Scores range from 0 to 100, with higher scores indicating greater physical functioning. This scale has demonstrated adequate reliability and validity[30] and is widely used in studies of cancer patients [13, 31].
Upper body muscle strength was assessed at each assessment with the JAMAR 5030J1 Hand Dynamometer [21] in participants who reported no surgery in their hands or wrists in the previous 3 months and no history of arthritis. Dominant hand grip strength force measured in pounds was assessed twice at each visit; the mean score was used in statistical analyses. This instrument has demonstrated adequate reliability and validity in adults [22].
Lower body muscle strength was assessed at each assessment using the Chair Rise Test [16]. Participants were asked to rise as many times as possible within 30 seconds without using their arms. The number of rises was recorded. This test has demonstrated adequate reliability and validity in older adults [16] and is widely used in studies of cancer patients [3, 28].
Weekly exercise activity was assessed at baseline using the Godin Leisure-Time Exercise Questionnaire [10], which asks respondents to indicate the frequency with which they engaged in mild, moderate, or strenuous exercise in the previous week. Per standard scoring, a total weekly activity score was calculated [10]. This measure has demonstrated adequate reliability and validity in adults [11] and is widely used in studies of cancer patients [6].
Statistical Analysis
Analyses were limited to patients who completed the baseline assessment and at least one follow-up assessment for at least one physical functioning measure. Sixty-two ADT recipients and 86 controls were included based on this criterion. Chi-square and t-tests were conducted to examine differences between groups on demographic and clinical factors. Age was included as a covariate in all mixed models due to its strong association with physical functioning and muscle strength. Other demographic and clinical factors that demonstrated marginally-significant differences (p < .10) between groups were included as additional covariates.
Mixed model analyses were used to examine differences in change over time in physical functioning measures between groups. These analyses were conducted using PROC MIXED in SAS, Version 9.3 (Cary, NC). Unlike repeated-measures analysis of variance, mixed model analyses allow for the use of all available data at each time point without imputing missing data [25]. In order to control Type I error, omnibus analyses were conducted for each outcome to examine the group by time interaction for the ADT recipients compared to controls. For significant interactions, additional mixed models examined between-group differences at each assessment, and unadjusted mixed models examined within-group change over time. A two-sided alpha value of .05 was used as the criterion for statistical significance. Cohen’s d [5] was used to calculate effect sizes for group differences, and 0.5 standard deviations was used as a cutoff for clinically-significant differences between groups [20]. Unadjusted mixed models were used to produce estimated means of outcome variables for both groups. Exploratory linear regression analyses were conducted to identify baseline predictors of decline in physical functioning in ADT recipients. In these analyses, 12-month outcome measures were regressed on baseline scores as well as potential predictors.
Results
Demographic & Medical Characteristics
Among ADT recipients, 56 completed the baseline assessment on or before the day of their first ADT injection; 6 were assessed from 1 to 5 days after their first ADT injection. Twenty-five (40%) ADT recipients underwent radiation concurrently with ADT treatment. Demographic and clinical characteristics are shown in Table 1. ADT recipients reported more medical comorbidities, had higher Gleason scores, and were more likely to have metastatic disease than controls (ps ≤.004). In addition, ADT recipients had fewer years of education and were less likely to be White than controls (ps ≤.07). Thus, medical comorbidities, education, race, Gleason score, and prostate cancer infiltration were included as covariates in subsequent analyses. Because ADT is often prescribed for patients with higher Gleason score or metastatic disease (i.e., more advanced disease), separate analyses were also conducted without these covariates.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Characteristic | ADT Recipients (n = 62) | Controls (n = 86) | p |
|---|---|---|---|
|
| |||
| Age, years | .79 | ||
| Mean | 68.12 | 67.76 | |
| SD | 8.71 | 7.35 | |
|
| |||
| Time since diagnosis, years | .37 | ||
| Mean | 3.78 | 4.50 | |
| SD | 4.95 | 4.47 | |
|
| |||
| Body mass index, kg/m2 | .16 | ||
| Mean | 28.32 | 29.41 | |
| SD | 4.23 | 4.17 | |
|
| |||
| Comorbidity index score | .004 | ||
| Mean | 2.84 | 2.40 | |
| SD | 1.04 | 0.84 | |
|
| |||
| Characteristic | No. (%) | No. (%) | p |
|
| |||
| Education | .07 | ||
| ≤12 years | 23 (37) | 18 (21) | |
| 13 – 16 years | 32 (52) | 51 (59) | |
| ≥17 years | 7 (11) | 17 (20) | |
|
| |||
| Race | .03 | ||
| White | 53 (85) | 83 (97) | |
| Nonwhite | 9 (15) | 3 (4) | |
|
| |||
| Ethnicity | .64 | ||
| Hispanic | 1 (1) | 3 (3) | |
| Non-Hispanic | 60 (98) | 82 (96) | |
| Missing | 1 (1) | 1 (1) | |
|
| |||
| Gleason score | < .001 | ||
| 4–6 | 8 (13) | 41 (48) | |
| 7 | 16 (26) | 39 (45) | |
| 8 | 14 (23) | 2 (2) | |
| 9–10 | 7 (11) | 0 (0.0) | |
| Missing | 17 (27) | 4 (5) | |
|
| |||
| Prostate Cancer Infiltration | .01 | ||
| Regional | 43 (69) | 85 (99) | |
| Metastatic | 4 (6) | 0 (0) | |
| Missing | 15 (24) | 1 (1) | |
Note. N = 236. SD = standard deviation. p values calculated using Fisher’s exact test for categorical variables and t-tests for continuous variables. Missing levels were excluded from calculation of p values.
Changes in Physical Functioning
Figure 1 presents adjusted group means for self-reported physical functioning. There was a significant group by time interaction with and without controlling for Gleason score (ps<.001). Physical functioning worsened over time in ADT recipients (p < .001) but did not change over time in controls (p=.42). In both sets of analyses, there were no group differences at baseline (ps≥.31); however, ADT recipients reported worse physical functioning than controls at 6 months (ps≤.05, d=0.71), and 12 months (ps≤.002, d=0.92).
Figure 1.
Estimated means of physical functioning scores by group.
Figure 2 presents adjusted group means for grip strength. A significant group by time interaction was not observed when controlling for Gleason score or metastatic disease (p=.20). However, secondary analyses not controlling for disease severity found a significant group by time interaction (p=.01), such that grip strength worsened over time in ADT recipients (p=.04) but did not change in controls (p=.18). No group differences in grip strength were evident at baseline (p=.34) or 6 months (p=.06), but ADT recipients demonstrated significantly worse grip strength than controls at 12 months (p=.01, d=0.46).
Figure 2.
Estimated means of grip strength scores by group.
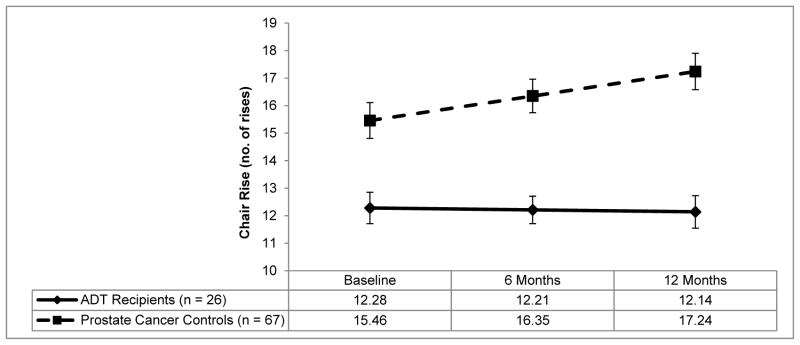
Figure 3 presents adjusted group means for chair rise performance. There were significant group by time interactions when controlling for disease severity (p=.05) and when not controlling for disease severity (p=.02). Chair rise scores did not change over time in ADT recipients (p=.81) but improved over time in controls (p<.001). When controlling for disease severity there were no group differences at any assessment (ps≥.16). Secondary analyses that did not control for disease severity found no group differences at baseline (p=.14), but ADT recipients demonstrated significantly worse chair rise scores than controls at 6 months (p=.02; d=.93) and 12 months (p=.003, d=1.05).
Figure 3.
Estimated means of chair rise scores by group.
Predictors of Changes in Physical Functioning
Analyses were conducted to examine predictors of decline in grip strength, chair rise, and physical functioning in ADT recipients. Greater declines in physical functioning were associated with higher Gleason scores at baseline, more medical comorbidities, and less exercise at baseline (ps≤.05); declines in physical functioning were not associated with disease infiltration, age, BMI, and concurrent radiotherapy (ps≥.15). Changes in grip strength and chair rise were not related to Gleason scores, disease infiltration, age, BMI, medical comorbidities, concurrent radiotherapy, and exercise (ps≥.06).
Discussion
This study examined changes in self-reported physical functioning and upper and lower body muscle strength over 12 months in men with prostate cancer receiving ADT and men with prostate cancer treated with prostatectomy only. Findings largely confirmed hypotheses that treatment with ADT would be associated with self-reported physical functioning and loss of muscle strength.
Self-reported physical functioning declined in ADT recipients but remained stable in the control group, with differences between groups evident at six months and 12 months. The direction and timing of changes in physical functioning are consistent with previous research [1, 14]. Differences between groups at 12 months reflected a large effect size and exceeded the cut-off [20] for clinically-significant change. Primary analyses controlling for disease severity found no group differences for change in upper body muscle strength; however, secondary analyses not controlling for disease severity did find a significant effect. Upper body muscle strength declined in ADT recipients but remained stable in controls. This is partially consistent with previous research. Alibhai and colleagues found that ADT recipients’ upper body strength scores decreased at 3 months and remained stable thereafter [1]. Although the magnitude of change was consistent in both studies, we found that ADT recipients demonstrated progressively worsening upper body strength over the 12 month follow-up period. We also found that ADT recipients demonstrated worse upper body strength than controls 12 months, reflecting a small to medium effect size that do not meet the cut-off [20] for clinically-significant differences. These findings suggest that ADT may have a gradual negative impact on upper body strength over the course of a year of treatment. This pattern of findings suggests that ADT is associated with clinically-meaningful declines in physical functioning which occur within six months after starting ADT and persist or worsen over time.
Lower body strength unexpectedly remained stable over time in ADT recipients but improved significantly in controls. The reason for the improvement in the control group is unclear. One possibility is that improvement may reflect practice effects, whereby positive changes in performance are due to familiarity with the task [19]. Improvements in controls’ chair rise scores may also reflect improved functioning over time after prostatectomy. Regardless of the reason, analyses that did not control for disease severity found that ADT recipients performed worse than controls at 6 months and continued to perform worse at 12 months. The difference at 12 months reflects a large effect size that exceeded the cut-off [20] for a clinically-significant difference. These findings are in contrast to previous longitudinal research which failed to find any differences in lower body functioning between ADT-treated patients and controls [1]; however, cross-sectional analyses have consistently demonstrated that ADT recipients perform worse on measures of lower body strength than controls [7, 18].
Exploratory analyses found that, among ADT recipients, those with higher Gleason scores, less exercise, and more medical comorbidities before starting ADT reported steeper declines in physical functioning over time. The exercise-related finding is in line with previous research in ADT recipients demonstrating the benefits of exercise for protecting against muscle loss [8]. Moreover, exercise in ADT recipients has demonstrated improvements in blood pressure, depression, and fatigue [6]. Together, these findings may aid clinicians in discussions regarding expected side-effects of ADT use in men with prostate cancer.
This study’s strengths include the use of a longitudinal design and the recruitment of prostate cancer control group. This study also has several limitations. First, because participants were followed for only 12 months, we are unable to determine whether the declines in physical functioning persist or worsen with continued administration beyond one year. Also, this study did not examine men who were scheduled to discontinue ADT (i.e., an intermittent therapy group), which would have permitted examinations of whether the declines in physical functioning are reversible. In addition, biological measures were not used to examine correspondence between loss of muscle mass and loss of muscle strength as well as physical functioning. The possibility that non-significant results were due to insufficient power cannot be ruled out. Osteoarthritis and musculoskeletal pain are potential confounders that were not assessed. Future studies should include extended follow-up for men receiving continuous ADT to determine its long-term impact and examine the possibility that declines may be reversible with discontinuation of ADT. There is also a need to examine a more comprehensive set of predictors of decline in physical functioning, including body composition and frailty. Because of the observational rather than experimental nature of this study, other factors that differed between groups besides ADT administration (e.g., disease severity) cannot be ruled out as contributors to group differences in physical functioning. Accordingly, the secondary analyses we conducted that did not control for disease severity must be interpreted with caution.
In conclusion, ADT was found to be associated with declines in self-reported physical functioning, upper-body muscle strength, and lower body muscle strength. These findings have important clinical implications and should be considered in discussions of the risks and benefits of ADT.
Acknowledgments
This work was supported by grants from the National Cancer Institute: R01CA132803 (PI: Jacobsen) & R25CA090314 (PI: Jacobsen). The authors wish to acknowledge the Biostatistics Core at the Moffitt Cancer Center (P30-CA076292; PI: Sellers).
Footnotes
Disclosures: None.
Compliance with Ethical Standards
The authors have no conflicts of interest to declare. The corresponding author is in full control of all primary data and agrees to allow the journal to review the data if requested. The data presented in this manuscript were collected through a protocol approved by the Institutional Review Board of the University of South Florida (Protocol #IRB 106381). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
References
- 1.Alibhai SM, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, Krahn M, Fleshner NE, Warde P, Canning SD. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. Journal of clinical oncology. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 2.Basaria S, Lieb J, Tang AM, DeWeese T, Carducci M, Eisenberger M, Dobs AS. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clinical endocrinology. 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertheussen GF, Kaasa S, Hokstad A, Sandmæl JA, Helbostad JL, Salvesen Ø, Oldervoll LM. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncologica. 2012;51:1070–1080. doi: 10.3109/0284186X.2012.699684. [DOI] [PubMed] [Google Scholar]
- 4.Clay CA, Perera S, Wagner JM, Miller ME, Nelson JB, Greenspan SL. Physical function in men with prostate cancer on androgen deprivation therapy. Physical Therapy. 2007;87:1325–1333. doi: 10.2522/ptj.20060302. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 6.Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, Kline G, Faris P. Physical activity for men receiving androgen deprivation therapy for prostate cancer: Benefits from a 16-week intervention. Supportive Care in Cancer. 2010;18:591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 7.Galvao D, Taaffe D, Spry N, Joseph D, Turner D, Newton R. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: a comprehensive cross-sectional investigation. Prostate Cancer and Prostatic Diseases. 2008;12:198–203. doi: 10.1038/pcan.2008.51. [DOI] [PubMed] [Google Scholar]
- 8.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. Journal of Clinical Oncology. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert SM, Kuo Y-f, Shahinian VB. Book Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Elsevier; City: 2011. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States; pp. 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godin G, Shephard R. Godin leisure-time exercise questionnaire. [Google Scholar]
- 11.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community Canadian journal of applied sport sciences. Journal canadien des sciences appliquees au sport. 1985;10:141–146. [PubMed] [Google Scholar]
- 12.Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. Journal of Cancer Survivorship. 2010;4:128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 13.Heckman JE, Chamie K, Maliski SL, Fink A, Kwan L, Connor SE, Litwin MS. The role of self-efficacy in quality of life for disadvantaged men with prostate cancer. The Journal of urology. 2011;186:1855–1861. doi: 10.1016/j.juro.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 14.Herr H, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. The Journal of urology. 2000;163:1743. [PubMed] [Google Scholar]
- 15.Joly F, Alibhai S, Galica J, Park A, Yi Q-L, Wagner L, Tannock I. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. The Journal of urology. 2006;176:2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 16.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research quarterly for exercise and sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 17.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Levy ME, Perera S, van Londen GJ, Nelson JB, Clay CA, Greenspan SL. Physical function changes in prostate cancer patients on androgen deprivation therapy: a 2-year prospective study. Urology. 2008;71:735–739. doi: 10.1016/j.urology.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Newell A, Rosenbloom PS. Mechanisms of skill acquisition and the law of practiceCognitive skills and their acquisition. Lawrence Earlbaum Associates; Hillsdale, NJ: 1981. pp. 1–55. [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 21.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- 22.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age and ageing: afr051. 2011 doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 23.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. The Journal of urology. 2013;189:S34–S44. doi: 10.1016/j.juro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. Doing data analysis with the multilevel model for changeApplied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. pp. 75–123. [Google Scholar]
- 26.Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, Leder BZ, Goessl C. Sarcopenia during androgen-deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2012;30:3271–3276. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. European Journal of Cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 28.Uth J, Schmidt JF, Christensen JF, Hornstrup T, Andersen LJ, Hansen PR, Christensen KB, Andersen LL, Helge EW, Brasso K. Effects of recreational soccer in men with prostate cancer undergoing androgen deprivation therapy: study protocol for the’FC Prostate’randomized controlled trial. BMC cancer. 2013;13:595. doi: 10.1186/1471-2407-13-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Critical reviews in oncology/hematology. 2008;68:172–177. doi: 10.1016/j.critrevonc.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]