Abstract
Purpose
The majority (95%) of lung cancer patients report stigma, with 48% of lung cancer patients specifically reporting feeling stigmatized by their medical providers. Typically associated with the causal link to smoking and the historically poor prognosis, lung cancer stigma can be seen as a risk factor for poor psychosocial and medical outcomes in the context of lung cancer diagnosis and treatment. Thus, modifiable targets for lung cancer stigma-reducing interventions are needed. The present study sought to test the hypothesis that good patient-provider communication is associated with lower levels of lung cancer stigma.
Methods
Lung cancer patients (n=231) across varying stages of disease, participated in a cross-sectional, multi-site study designed to understand lung cancer stigma. Patients completed several survey measures, including demographic and clinical characteristics, a measure of patient-provider communication (Consumer Assessment of Healthcare Providers and Systems Program or CAHPS), and a measure of lung cancer stigma (Cataldo Lung Cancer Stigma Scale).
Results
As hypothesized, results indicated that good patient-provider communication was associated with lower levels of lung cancer stigma (r=-.18, p<.05). These results remained significant, even when controlling for relevant demographic and clinical characteristics (Stan. Β = −.15, p<.05).
Conclusions
Results indicate that good patient-provider communication is associated with lower levels of lung cancer stigma, suggesting that improving patient-provider communication may be a good intervention target for reducing lung cancer stigma.
Keywords: lung cancer, lung cancer stigma, patient-provider communication
Introduction
Many lung cancer patients (both smokers and non-smokers) report experiencing lung cancer stigma. 1-3 Lung cancer patients with a smoking history are often seen as responsible, and sometimes deserving, of this type of cancer.4 Unfortunately, lung cancer stigma is linked to several adverse psychosocial consequences,5-13 including depression and withdrawal.5,12 Lung cancer patients report experiencing stigma from external sources such as friends and family (perceived or felt stigma)6 as well as internalized (self) stigma.7 Surprisingly, a large number of lung cancer patients (48%) report experiencing perceived stigma specifically from medical providers.6,7,14 Despite the large number of patients reporting perceived stigma from medical care providers, most interventions designed to reduce health-related stigma target the individual patient,15 rather than considering the potential contextual benefits of reducing stigma by focusing on improving patient-provider communication. Evidence supporting a link between patient-provider communication and lung cancer stigma could illuminate a potential mechanism to target for reducing patients’ perceptions of stigma.
There are several reasons why gaining a better understanding of the relationship between patient-provider communication and lung cancer stigma is critical. First, although essential for clinical management, assessment of smoking history may inadvertently insinuate that patients are to be blamed for their cancer.7 Second, there is evidence that clinicians may go into encounters with lung cancer patients with preconceptions that negatively affect their interactions with these patients. For instance, clinicians have been shown to perceive lung cancer patients as more difficult to treat, having a lower quality of life, and higher symptom reports than other solid tumor patients.16 This view of lung cancer patients as being more difficult to treat, termed “therapeutic nihilism,” has been shown to negatively affect management of lung cancer patients’ care17-19 and may similarly affect communication regarding lung cancer. Finally, perceived stigma from medical providers has been associated with patients delaying20 and underreporting21,22 symptoms as well as misreporting their smoking behaviors, believing that disclosure may negatively affect their treatment and access to medical care.6 There is mounting evidence that stigmatizing communication may occur within clinical encounters with lung cancer patients.
Providing support for the hypothesis that good patient-provider communication may reduce lung cancer stigma, prior research indicates that empathic physician communication increases patients’ satisfaction and adherence23 and lowers psychological distress.24 Clinical encounters with lung cancer patients provide a large number of opportunities for thoracic oncologists and surgeons to respond to patient concerns.25 For instance, when patients express certain negative emotions (e.g., guilt or regret) about the role of smoking in causing their lung cancer, there is an opportunity for physicians to respond in an empathic way to these concerns. Despite the benefits of responding empathically to patients, research indicates that physicians in oncology settings frequently (70-90%) “miss” empathic opportunities.26 One study found that as many as 90% of empathic opportunities were missed within the lung cancer patient population,14 and that statements readily perceived by patients as blaming (e.g., “your smoking has done a number on your lungs”) were common.14
Prior research has demonstrated the link between lung cancer stigma and negative psychosocial outcomes, 5-13 but little work has examined ways to reduce lung cancer stigma effectively. Determining targets for lung cancer stigma-reducing interventions could improve lung cancer patients’ psychosocial and clinical care outcomes. The current analysis addresses the potentially important connection between lung cancer stigma and patient-provider communication. Given the benefits of empathic physician communication,23,24 the prevalence of missed empathic opportunities among clinical encounters with lung cancer patients, 14 and the presence of potentially stigmatizing communication, 16 there is much potential for targeting providers’ communication to reduce patients’ self-reported lung cancer stigma. As such, we hypothesized that better patient-provider communication would be associated with lower levels of patient-reported lung cancer stigma.
Materials and Methods
Analyses in the present study utilized data from 231 lung cancer patients collected as part of a multi-site, cross-sectional study of lung cancer stigma, psychosocial concerns, and behavioral outcomes. Outcomes assessed in the present analyses focus on the relationship between patient-provider communication and lung cancer stigma. Participants were recruited from two NCI-designated cancer centers located in the northeast and southern parts of the United States. This study was approved by all participating institutions’ Institutional Review Boards.
Patient Recruitment
Potentially eligible individuals included those with a confirmed lung cancer diagnosis (both non-small cell lung cancer [NSCLC] and small cell lung cancer [SCLC], any stage). Patient eligibility criteria included: being adults (18 years and older) with a lung cancer diagnosis in the last 12 months, being able to read and comprehend English, and having capacity to comprehend study information. At time of study enrollment, participants were also either: a) undergoing anti-cancer treatment (chemotherapy or radiation therapy) or, b) had undergone surgical resection, radiation treatment, or chemotherapy within the prior 12 months. Participants were excluded who did not speak English, or had a speech or cognitive impairment that rendered them incapable of informed consent and/or participation.
Study Materials and Procedures
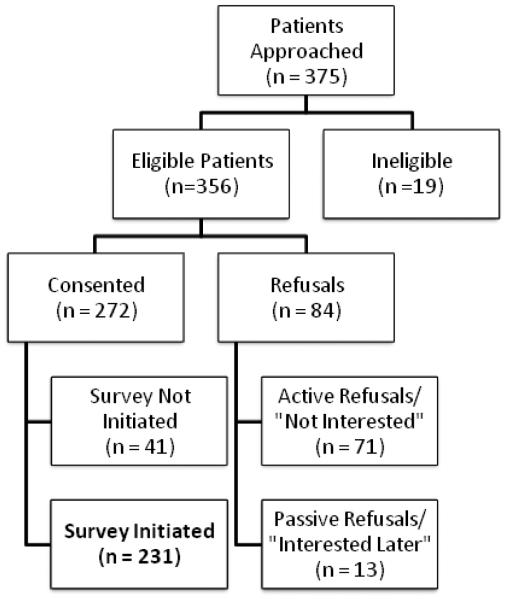
Once participants were identified as potentially eligible, they were approached inperson by a member of study staff during scheduled clinic appointments. Once eligibility was confirmed, patients were informed about the study and asked about their interest. As indicated in Figure 1, 76.4% of eligible patients consented to study procedures. Consented study participants were asked to complete a 30-40 minute survey battery which contained questionnaires carefully chosen to reflect potentially important concepts related to lung cancer stigma. Participants completed the questionnaire in one of the following ways: a) on a tablet computer provided by the trained research team member, b) through a secure electronic (web-based) portal from their own computer, or c) a paper-based version of the survey.
Figure 1.
Consort diagram of patient recruitment and enrollment.
Web-based study materials utilized REDCap (Research Electronic Data Capture),1 a data management software system. REDCap is a tool for the creation and collection of customized, secure data management systems including web-based data entry forms, reporting tools, and a full array of security features including user- and group-based privileges and an audit trail of data manipulation and export procedures. Data from participants who completed paper surveys were entered into RedCap by study staff.
Measures
Demographic and clinical information
The majority of the demographic and clinical information was assessed via patient self-report (e.g., age, gender, race, ethnicity, education level, marital status, time since diagnosis, treatment history, smoking status). Disease type and stage were determined both through self-report and the electronic medical record (EMR), with preference given to EMR status when information was inconsistent.
Smoking status
Smoking status was evaluated with the following two questions regarding smoking history and status: a) “Have you smoked at least 100 cigarettes in your lifetime?” and b) “Do you smoke cigarettes every day, some days, or not at all?” Those participants who reported smoking at least 100 cigarettes and that they are currently smoking (e.g., “Every day” or “Some days”) were categorized as “current” smokers; those who reported they are not currently smoking (e.g., “Not at all”) were categorized as “former” smokers. Finally, those who reported < 100 cigarettes/lifetime were considered “never” smokers.
Patient-provider communication
Patient-provider communication was assessed using six items from the Provider Communication Subscale of the widely used, Consumer Assessment of Health Care Providers and Systems Program (CAHPS). 27 Participants were asked to specifically rate the quality of interactions with their health care providers since their lung cancer diagnosis. Examples of items include: “How often did your [health care] providers explain things in a way that was easy to understand?” and “How often did your providers seem to know important information about your medical history?” (1 = never, 2 = sometimes, 3 = usually, 4 = always). Prior studies indicate the communication subscale of CAHPS is an internally reliable scale, with Cronbach’s α = .86.27 This measure of provider communication has been widely used to assess patients’ perceptions of provider communication across several studies and is noted for having strong psychometric properties.28,29
Lung cancer stigma
To measure lung cancer stigma, patients completed the Cataldo Lung Cancer Stigma Scale (CLCSS),13 a 31-item validated instrument that assesses stigma and shame, social isolation, discrimination, and smoking related to lung cancer. Examples of items include: “I feel guilty because I have lung cancer” and “Some people have told me lung cancer is what I deserved for smoking” (1 = strongly disagree, 4 = strongly agree). Prior studies indicate the CLCSS is an internally reliable scale, with Cronbach’s α = .96. 13 This measure of lung cancer stigma has been used across several recent studies assessing lung cancer stigma.5,30,31
Analytic Plan
Descriptive statistics were calculated for demographic and clinical variables, patient-provider communication, and lung cancer stigma. Bivariate correlations were run in order to examine the degree of association between demographic variables, patient-provider communication, and lung cancer stigma. Finally, a multivariate linear regression was run in order to examine patient-provider communication as a predictor of lung cancer stigma. To rule out potential confounding variables, relevant demographic and medical characteristics were included in this regression model that showed an association with the outcome variable―lung cancer stigma―that trended towards significance (p≤.10).
Results
Demographic and Clinical Characteristics
Participants included 231 lung cancer patients. The mean age of participants was 62.8 years old (SD= 11.0). The majority of participants identified as White (78.8%), married (62.8%), and female (63.6%). There was a fairly even split on patients’ educational level, with approximately half (49.0%) reporting having a college degree or higher. Patients were represented across all four disease stages: 11.7% Stage I, 8.2% Stage II, 18.6% Stage III, and 55.4% Stage IV (with 6.1% of patients not having staging information). Finally, the majority of participants were former smokers (65.1%), while 26.0% were never smokers and 8.7% were current smokers. For more detailed demographic and clinical characteristics, see Table 1.
Table 1.
Demographic and clinical characteristics of the sample (N=231).
| Characteristic | Number | Percentage |
|---|---|---|
| Age (in years) | M=62.80 | SD=10.96 |
| Gender | ||
| Female | 147 | 63.6 |
| Male | 84 | 36.4 |
| Race* | ||
| White | 182 | 78.8 |
| Black/African-American | 33 | 14.3 |
| Asian/Pacific-Islander | 8 | 3.5 |
| Other | 6 | 2.6 |
| Ethnicity* | ||
| Hispanic | 7 | 3.0 |
| Non-Hispanic | 220 | 95.2 |
| Education | ||
| Less than college degree | 118 | 51.0 |
| College degree or higher | 113 | 49.0 |
| Marital Status* | ||
| Married/Partnered | 145 | 62.8 |
| Single, Never Married | 26 | 9.1 |
| Divorced | 27 | 11.3 |
| Widowed | 27 | 11.7 |
| Type of Lung Cancer | ||
| Non-small cell lung cancer (NSCLC) | 183 | 79.2 |
| Small cell lung cancer (SCLC) | 22 | 9.5 |
| Missing | 26 | 11.3 |
| Disease Stage | ||
| Stage I | 27 | 11.7 |
| Stage II | 19 | 8.2 |
| Stage III | 43 | 18.6 |
| Stage IV | 128 | 55.4 |
| Missing | 14 | 6.1 |
| Smoking Status* | ||
| Never smoker (<100 packs in lifetime) | 60 | 26.0 |
| Former smoker (>100 packs in lifetime; not smoking some days or everyday at time of study) |
149 | 65.1 |
| Current smoker (>100 packs in lifetime; smoking some days or everyday at time of study) |
20 | 8.7 |
Not all percentages = 100% due to missing data.
Associations between Main Study Variables
Bivariate correlations were run between demographic and clinical characteristics, patient-provider communication, and lung cancer stigma. For demographic and clinical characteristics, the following dummy variables were created from categorical variables in order to more clearly interpret results: gender (0 = female, 1 = male); education (0 = less than college degree, 1 = college degree or higher), race (0 = non-White, 1 = White), marital status (0 = other, 1 = married/partnered), Hispanic (0 = non-Hispanic, 1 = Hispanic). Additional demographic and clinical variables included in analyses were the following: age, lung cancer type, lung cancer stage, and smoking status. Results indicated that age (r=-.16, p <.05) and marital status (r=-.17, p< .01) were the only demographic and clinical characteristics significantly associated with lung cancer stigma, such that older age and being married/partnered were associated with less stigma (see Table 2). There was no association between smoking status and lung cancer stigma. As hypothesized, results indicated that higher levels of good patient-provider communication were associated with lower levels of patient-reported lung cancer stigma (r=-.18, p<.05).
Table 2.
Descriptive statics and inter-correlations of main study variables (N=231).
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | M | SD | Range | α |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CAHPS | --- | 22.03 | 2.87 | 6-24 | .89 | ||||||||||
| 2. Stigma | −.18* | --- | 47.93 | 12.74 | 31-83 | .93 | |||||||||
| 3. Age | .08 | −.16* | --- | 62.94 | 11.02 | 22-89 | -- | ||||||||
| 4. Gender | −.11 | .04 | −.05 | --- | .36 | .48 | 0-1 | -- | |||||||
| 5. Education | .01 | −.08 | −.02 | .05 | --- | .49 | .50 | 0-1 | -- | ||||||
| 6. Marital status |
.14* | −.17* | −.09 | .21** | .28** | --- | .63 | .48 | 0-1 | -- | |||||
| 7. White race | −.11 | −.11 | .18** | .08 | .21** | .19** | --- | .79 | .41 | 0-1 | -- | ||||
| 8. Hispanic/ Latino |
.06 | −.08 | −.19** | −.03 | −.03 | −.07 | −.04 | --- | .03 | .17 | 0-1 | -- | |||
| 9. Lung cancer type |
.07 | .08 | .11 | .11 | −.22** | −.26** | −.21** | .11 | --- | -- | -- | 1-3 | -- | ||
| 10. Lung cancer stage |
.05 | .11 | −.09 | .06 | −.03 | .00 | −.06 | .01 | .10 | --- | 3.36 | 1.11 | 1-5 | -- | |
| 11. Smoking status |
.17* | −.02 | .16* | −.07 | .17** | .11 | −.07 | .23** | −.07 | .12 | --- | -- | -- | 0-2 | -- |
Note: CAHPS = Consumer Assessment of Health Care Providers and Systems Program; Stigma = Cataldo lung cancer stigma survey; gender (0 = female, 1 = male); education (0 = less than college degree, 1 = college degree or higher); marital status (0 = “other,” 1= married/partnered); White race (0 – non-White, 1 = White); Hispanic/Latino (0 = not Hispanic/Latino, 1 = Hispanic/Latino); cancer type (1= non-small cell lung cancer, 2= small cell lung cancer, 3 = I don’t know); smoking status (0 = current, 1 = former, 2 = never).
p < .05
p < .01
Patient-Provider Communication’s Association with Lung Cancer Stigma
To examine if patient-provider communication was associated with lung cancer stigma, even after controlling for relevant demographic and clinical characteristics, a multivariate regression analysis was run. Age and marital status were the only variables included in the model as control variables because they were the only demographic and clinical characteristics with an association with lung cancer stigma of p ≤ .10. Results indicated that age, marital status, and patient-provider communication were all significant predictors of lung cancer stigma in the final model (see Table 3). Thus, even when controlling for relevant demographic variables, better patient-provider communication was associated with lower levels of patient-reported lung cancer stigma.
Table 3.
Regression equation predicting lung cancer stigma (N=231).
| Predictors | Beta (SE) | Stand. β | t-value | p value |
|---|---|---|---|---|
| Age | −.17 (.08) | −.15 | −2.05 | .041 |
| Marital status | −4.24 (1.92) | −.16 | −2.21 | .028 |
| Communication (CAHPS) | −.63 (.31) | −.15 | −2.02 | .045 |
|
| ||||
| Adjusted R2 | .06 | |||
| F-value | 4.90** | .003 | ||
*Note: Only demographic and medical variables associated with the outcome variable (stigma) at the p≥.10 level in initial bivariate analyses were included in the final model reported here.
Marital status (0 = other, 1 = married/partnered); CAHPS = Consumer Assessment Health Care Providers and Systems Program;
*p <.05;
p < .01;
*** p < .001
Discussion
The present study examined the relationship between patient-provider communication and lung cancer stigma. Supporting our hypothesis, good patient-provider communication (e.g., explaining things in a way that’s easier to understand, listening carefully, showing respect for the patient) was associated with lower levels of patient-reported lung cancer stigma. This negative relationship between patient-provider communication and lung cancer stigma remained, even when controlling for relevant demographic and clinical variables. Of note, the present study also found that there was no significant association between lung cancer stigma and smoking status, lending support to prior findings which found no significant differences in reported levels of lung cancer stigma among ever and never smokers.5 These findings suggest that lung cancer patients report experiencing stigma, regardless of their smoking status. Thus, it is critical to develop interventions designed to reduce lung cancer stigma among all lung cancer patients, regardless of smoking status.
The novel results from this study suggest that improving providers’ communication may be a fruitful intervention target for reducing lung cancer stigma among lung cancer patients. Although prior research indicates that lung cancer stigma is associated with a variety of adverse outcomes―such as depression and other negative psychological outcomes, 5-13 delaying20 and underreporting21,22 of symptoms, and misreporting of smoking behaviors6―no research has highlighted a potential intervention target for improving clinical encounters surrounding lung cancer. Given the high prevalence rates (90%) of missed empathic opportunities among this patient population14 and high levels of perceiving stigma from medical providers (48%),7 improving communication is likely to lead to reduced stigma among a large number of lung cancer patients.
Guided by results from the present study, developing a communication intervention to improve physicians’ communication with lung cancer patients could improve patients’ psychological and clinical outcomes through a reduction in stigma. In support of the concept of targeting patient-provider communication as a means to reduce patients’ lung cancer stigma, prior research has shown that an empathic, caring approach (e.g., degree of empathy) to a physician-delivered smoking cessation program was more effective at promoting cessation than an information-driven approach.32 Similarly, improving patient-physician communication may reduce lung cancer stigma, and thus improve outcomes associated with stigma such as psychosocial outcomes like depression. 5-13 Although some empathic communication training interventions for oncologists exist,33,34 none have been tested with oncologists treating lung cancer patients who may be especially prone to experience stigmatizing interactions related to smoking history and current smoking status. This may be a fruitful next step in developing targets for lung cancer stigma-reducing interventions.
Study Strengths and Limitations
This study had a number of strengths, including a relatively large, two-site heterogeneous sample of patients with varied stage of disease and smoking status and the use of validated measures of stigma13 and patient-provider communication.27 These results highlight a potentially fruitful intervention target for reducing perceived stigma within lung cancer clinical encounters. Despite these strengths, the present study does have limitations that should be considered. First, the study was cross-sectional, precluding directional analyses of the causal effects of patient-provider communication on lung cancer stigma. For example, it is possible that patients who report lower levels of stigma have improved communication with their providers rather than communication leading to reduced levels of stigma. As such, future longitudinal research could examine patients’ level of lung cancer stigma pre- and post-clinical encounters to determine if better patient-physician communication is associated with reductions in perceived stigma. Second, the present study utilized a patient-reported measure of patient-provider communication rather than an observational measure. Future research could examine how actual patient-provider communication may be associated with patients’ perceptions of lung cancer stigma in order to see how communication processes occur in the context of clinical care for lung cancer patients. Finally, another limitation of the present study is that the measure of communication utilized (CAHPS) asks patients to report on communication of providers after being diagnosed with lung cancer broadly rather than thoracic oncologists per se. As such, the results of this study could refer to satisfaction with communication with lung cancer patients’ oncologists, surgeons, radiation oncologists, nurses, or other oncology providers. Nevertheless, each individual member of the oncology team (oncologists, surgeons, nurses, etc.) plays a critical role in the context of cancer care. Thus, the present study highlights the importance of good communication within the context of cancer care.
Clinical Implications
Although the interpretation of directionality is limited in the present study due to the cross sectional data used, the present study suggests that good communication may help reduce the perception of stigma among lung cancer patients. A prior review of the literature in patient-physician communication in oncology has highlighted the following areas as learnable communication skills associated with specific outcomes:35 1) warmth, respect, empathy, and being encouraging (increases patient satisfaction); 2) listening, clarifying, and summarizing (enhances information exchange); 3) explaining and using humor (increases treatment compliance; e.g., potentially smoking cessation); and 4) checking understanding and endorsing question-asking (facilitates shared decision making). It is possible that engaging in these “best practices” of patient-provider communication may also reduce the deleterious effects of lung cancer stigma. As such, providers working with lung cancer patients may benefit from being trained in good communication practices, especially as it relates to discussing sensitive topics such as taking a smoking history or assessing current smoking status. To develop a communication-based intervention targeting providers working with lung cancer patients, future work should focus on best practices for developing communication-based provider trainings, which includes identifying the communication difficulties and determining communication practices associated with better outcomes specific to this patient population.36
Conclusions
By focusing on patient-provider communication, these data illuminate a potentially fruitful area for intervention that could reduce patients’ perception of lung cancer stigma. Namely, good patient-provider communication was found to be associated with lower levels of lung cancer stigma, suggesting that targeting the patient-provider relationship by improving communication may lead to a reduction in lung cancer stigma. Understanding the link between communication and stigma is critical, given that this patient population consistently reports stigmatizing interactions. Future research could develop communication-based interventions designed to improve thoracic physicians’ communication with their patients and determine these interventions effectiveness at reducing lung cancer stigma.
Acknowledgments
Sources of funding: This work was supported by the following grants: National Cancer Institute (T32-CA009461), National Cancer Institute (1R03CA154016), the North Carolina Chapter (Young Investigator Award), and the Lung Cancer Research Foundation.
Footnotes
References
- 1.Bell K, Salmon A, Bowers M, Bell J, McCullough L. Smoking, stigma and tobacco ‘denormalization’: Further reflections on the use of stigma as a public health tool. A commentary on Social Science & Medicine's Stigma, Prejudice, Discrimination and Health Special Issue. Social Science & Medicine. 2010;70(6):795–799. doi: 10.1016/j.socscimed.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 2.Bayer R, Stuber J. Tobacco control, stigma, and public health: rethinking the relations. American Journal of Public Health. 2006;96(1):47–50. doi: 10.2105/AJPH.2005.071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gritz ER, Sarna L, Dresler C, Healton CG. Building a united front: aligning the agendas for tobacco control, lung cancer research, and policy. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(5):859–863. doi: 10.1158/1055-9965.EPI-07-0342. [DOI] [PubMed] [Google Scholar]
- 4.Hamann HA, Howell LA, McDonald JL. Causal attributions and attitudes toward lung cancer. Journal of Applied Social Psychology. 2013 [Google Scholar]
- 5.Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2012;16(3):264–269. doi: 10.1016/j.ejon.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ (Clinical research ed.) 2004;328(7454):1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, Lee SJC. Stigma among patients with lung cancer: a patient reported measurement model. Psycho-Oncology. 2013 doi: 10.1002/pon.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LoConte NK, Else-Quest NM, Eickhoff J, Hyde J, Schiller JH. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clinical lung cancer. 2008;9(3):171–178. doi: 10.3816/CLC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 9.Weiss T, Weinberger M, Schwerd AM, Holland J. A 30-Year perspective on psychosocial issues in lung cancer: How lung cancer “came out of the closet”. Thoracic surgery clinics. 2012;22(4):449–456. doi: 10.1016/j.thorsurg.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Holland JC, Kelly BJ, Weinberger MI. Why psychosocial care is difficult to integrate into routine cancer care: stigma is the elephant in the room. Journal of the National. Comprehensive Cancer Network. 2010;8(4):362–366. doi: 10.6004/jnccn.2010.0028. [DOI] [PubMed] [Google Scholar]
- 11.Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychology & health. 2009;24(8):949–964. doi: 10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez BD, Jacobsen PB. Depression in lung cancer patients: the role of perceived stigma. Psycho-oncology. 2012;21(3):239–246. doi: 10.1002/pon.1882. [DOI] [PubMed] [Google Scholar]
- 13.Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ. Measuring stigma in people with lung cancer: Psychometric testing of the Cataldo Lung Cancer Stigma Scale. Oncology nursing forum. 2011;38(1):E46–E54. doi: 10.1188/11.ONF.E46-E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse DS, Edwardsen EA, Gordon HS. Missed opportunities for interval empathy in lung cancer communication. Archives of internal medicine. 2008;168(17):1853–1858. doi: 10.1001/archinte.168.17.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijnders M, Van Der Meij S. The fight against stigma: an overview of stigma-reduction strategies and interventions. Psychology, health & medicine. 2006;11(3):353–363. doi: 10.1080/13548500600595327. [DOI] [PubMed] [Google Scholar]
- 16.Hamann HA, Lee J-W, Schiller JH, et al. Clinician perceptions of care difficulty, quality of life, and symptom reports for lung cancer patients: an analysis from the Symptom Outcomes and Practice patterns (SOAPP) study. Journal of Thoracic Oncology. 2013;8(12):1474–1483. doi: 10.1097/01.JTO.0000437501.83763.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball DL, Irving LB. Are patients with lung cancer the poor relations in oncology? The Medical journal of Australia. 2000;172(7):310–311. doi: 10.5694/j.1326-5377.2000.tb123974.x. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA. Perceptions of prognosis, treatment, and treatment impact on prognosis in non-small cell lung cancer. Chest. 1998;114(2):593–604. doi: 10.1378/chest.114.2.593. [DOI] [PubMed] [Google Scholar]
- 19.Wassenaar TR, Eickhoff JC, Jarzemsky DR, Smith SS, Larson ML, Schiller JH. Differences in primary care clinicians' approach to non-small cell lung cancer patients compared with breast cancer. Journal of Thoracic Oncology. 2007;2(8):722–728. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]
- 20.Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: a qualitative study. Journal of advanced nursing. 2008;61(3):336–343. doi: 10.1111/j.1365-2648.2007.04542.x. [DOI] [PubMed] [Google Scholar]
- 21.Koller M, Kussman J, Lorenz W, et al. Symptom reporting in cancer patients: the role of negative affect and experienced social stigma. Cancer. 1996;77(5):983–995. doi: 10.1002/(sici)1097-0142(19960301)77:5<983::aid-cncr27>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Westerman MJ, Sprangers MA, Groen HJ, van der Wal G, Hak T. Small-cell lung cancer patients are just ‘a little bit' tired: response shift and self-presentation in the measurement of fatigue. Quality of Life Research. 2007;16(5):853–861. doi: 10.1007/s11136-007-9178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Evaluation & the health professions. 2004;27(3):237–251. doi: 10.1177/0163278704267037. [DOI] [PubMed] [Google Scholar]
- 24.Lelorain S, Brédart A, Dolbeault S, Sultan S. A systematic review of the associations between empathy measures and patient outcomes in cancer care. Psycho-oncology. 2012;21(12):1255–1264. doi: 10.1002/pon.2115. [DOI] [PubMed] [Google Scholar]
- 25.Pollak KI, Arnold RM, Jeffreys AS, et al. Oncologist communication about emotion during visits with patients with advanced cancer. Journal of Clinical Oncology. 2007;25(36):5748–5752. doi: 10.1200/JCO.2007.12.4180. [DOI] [PubMed] [Google Scholar]
- 26.Hsu I, Saha S, Korthuis PT, et al. Providing support to patients in emotional encounters: A new perspective on missed empathic opportunities. Patient education and counseling. 2012;88(3):436–442. doi: 10.1016/j.pec.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays RD, Shaul JA, Williams VS, et al. Psychometric properties of the CAHPS™ 1.0 survey measures. Medical care. 1999;37(3):MS22–MS31. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- 28.Lee Hargraves J, Hays RD, Cleary PD. Psychometric properties of the consumer assessment of health plans study (CAHPS®) 2.0 adult core survey. Health services research. 2003;38(6):1509–1528. doi: 10.1111/j.1475-6773.2003.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browne K, Roseman D, Shaller D, Edgman-Levitan S. Analysis & commentary measuring patient experience as a strategy for improving primary care. Health Affairs. 2010;29(5):921–925. doi: 10.1377/hlthaff.2010.0238. [DOI] [PubMed] [Google Scholar]
- 30.Cataldo JK, Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncology. 2013;85(1):33–40. doi: 10.1159/000350834. [DOI] [PubMed] [Google Scholar]
- 31.Lee JL, Kim KS. The relationships between stigma, distress, and quality of life in patients with lung cancer. Journal of Korean Oncology Nursing. 2011;11(3):237–246. [Google Scholar]
- 32.Willms DG, Best J, Wilson D, et al. Patients' perspectives of a physician-delivered smoking cessation intervention. American Journal of Preventive Medicine. 1990;7(2):95–100. [PubMed] [Google Scholar]
- 33.Kissane DW, Bylund CL, Banerjee SC, et al. Communication skills training for oncology professionals. Journal of Clinical Oncology. 2012;30(11):1242–1247. doi: 10.1200/JCO.2011.39.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Medical care. 2007;45(4):340–349. doi: 10.1097/01.mlr.0000254516.04961.d5. [DOI] [PubMed] [Google Scholar]
- 35.Baile WF, Aaron J. Patient-physician communication in oncology: past, present, and future. Current opinion in oncology. 2005;17(4):331–335. doi: 10.1097/01.cco.0000167738.49325.2c. [DOI] [PubMed] [Google Scholar]
- 36.Schofield PE, Butow PN. Towards better communication in cancer care: a framework for developing evidence-based interventions. Patient education and counseling. 2004;55(1):32–39. doi: 10.1016/j.pec.2003.07.001. [DOI] [PubMed] [Google Scholar]