Abstract
Because testing of nerve agents is limited to only authorized facilities, our laboratory developed several surrogates that resemble nerve agents because they phosphylate the acetylcholinesterase (AChE) with the same moiety as the actual nerve agents. The inhibition kinetic parameters were determined for AChE by surrogates of cyclosarin (NCMP), sarin (NIMP, PIMP and TIMP) and VX (NEMP and TEMP) and other organophosphorus compounds derived from insecticides. All compounds were tested with rat brain and a subset was tested with mouse brain and purified human erythrocyte AChE. Within the compounds tested on all AChE sources, chlorpyrifos-oxon had the highest molecular rate constant followed by NCMP and NEMP. This was followed by NIMP then paraoxon and DFP with rat and mouse brain AChE but DFP was a more potent inhibitor than NIMP and paraoxon with human AChE. With the additional compounds tested only in rat brain, TEMP was slightly less potent than NEMP but more potent than PIMP which was more potent than NIMP. Methyl paraoxon was slightly less potent than paraoxon but more potent than TIMP which was more potent than DFP. Overall, this study validates that the pattern of inhibitory potencies of our surrogates is comparable to the pattern of inhibitory potencies of actual nerve agents (i.e., cyclosarin>VX>sarin), and that these are more potent than insecticidal organophosphates.
Keywords: organophosphate, anticholinesterase, inhibition kinetics
1.1 Introduction
Following the introduction of organophosphates (OPs) as insecticides in the early 1940s, several OP compounds were developed into chemical weaponry and ultimately designated as nerve agents due to their neurotoxic mechanism of action (Johnson et al., 2009; Tucker, 2006). The use of nerve agents against civilian populations has been reported in several incidences including against the Kurdish minorities in Halabja, Iraq in 1988 and in the Tokyo subway attack by Aum Shinrikyo in 1995 (Gupta, 2009). More recently, suspected use of nerve agents against Syrian civilians in 2013 has been reported (Pita and Domingo, 2014; Eisenkraft et al., 2014). There is currently a concern with the threat of terrorist organizations utilizing nerve agents, and possibly other OPs, as weapons in their attacks.
OPs exert toxicity by inhibiting the enzyme acetylcholinesterase (AChE; EC 3.1.1.7), a serine hydrolase that converts the neurotransmitter acetylcholine (ACh) into choline and acetate. Inhibition of AChE causes ACh to accumulate in synaptic and neuromuscular junctions resulting in hyperactivation within the cholinergic nervous system (Ecobichon, 2001). Spasm of respiratory muscle, bronchoconstriction, increased secretion of mucus and shutdown of the brain’s respiratory control center lead to death in mammals from respiratory failure in lethal dose exposures to OP anticholinesterases.
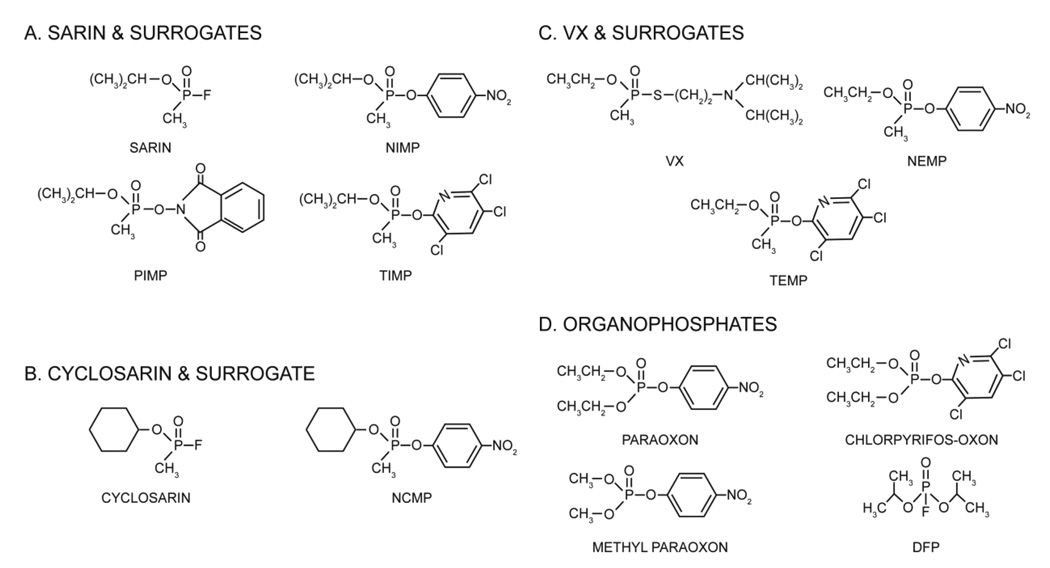
OP nerve agents are highly toxic and research on the actual nerve agents has been limited to only authorized facilities by the Chemical Weapons Convention. It would be beneficial to have chemicals that behave similarly to the nerve agents but are less toxic in order to allow laboratories that are not authorized to use the actual nerve agents to conduct research relevant to nerve agents. Therefore our laboratories developed surrogates for sarin and VX (Meek et al., 2012) and more recently to cyclosarin which substitute the nerve agent leaving group with another moiety (i.e., 4-nitrophenyl, 3,5,6-trichloro-2-pyridinyl, or phthalimidyl) (Fig. 1). These surrogates are highly relevant for AChE inhibition studies because they phosphylate the serine residue of the AChE active site with the same moiety as the actual nerve agents.
Figure 1.
Structures of (A) sarin and its surrogates NIMP (nitrophenyl isopropyl methylphosphonate), TIMP (3,5,6-trichloro-2-pyridinyl isopropyl methylphosphonate) and PIMP (phthalimidyl isopropyl methylphosphonate); (B) cyclosarin and its surrogate NCMP (nitrophenyl cyclohexyl methylphosphonate); C) VX and its surrogates NEMP (nitrophenyl ethyl methylphosphonate) and TEMP (3,5,6-trichloro-2-pyridinyl ethyl methylphosphonate); and; (D) Organophosphates: paraoxon, chlorpyrifos-oxon, methyl paraoxon, and DFP (diisopropylfluorophosphate).
Other OPs are not directly toxic as nerve agents. The phosphorothioate insecticides (e.g., parathion) must undergo bioactivation via cytochrome P450-mediated desulfuration to create their corresponding oxon metabolites (e.g., paraoxon) to be toxic. These oxons are substantially more potent anticholinesterases than the parent insecticide (Hodgson et al., 1991). These chemicals are also considered a potential terrorist tool and could be utilized to poison civilian populations (Ballantyn, 2009). For this reason, the oxons of three OP insecticides (parathion, methyl parathion and chlorpyrifos) were included in this study.
The present study was designed to assess the anticholinesterase potency through steady state kinetics investigating several OP molecules, including seven nerve agent surrogates and three insecticidal oxons. In addition to the surrogates synthesized in our laboratories, diisopropylfluorophosphate (DFP), which is commonly used as a surrogate of nerve agents by a number of investigators, was also studied. Three different enzyme sources were investigated: preparations of rat and mouse whole brain, and commercially available purified AChE isolated from human erythrocytes. The need to perform basic research on nerve agent effects as well as research to identify potential therapies for nerve agents must necessarily involve the use of laboratory animals. Thus information on the similarities of inhibition of AChE of two common laboratory rodents to that of humans will be valuable in extrapolating the animal data to humans.
1.2 Materials and Methods
1.2.1 Chemicals
AChE from human erythrocytes, DFP and all reagent grade chemicals were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). The insecticidal oxons paraoxon and methyl paraoxon were synthesized as described previously (Chambers and Chambers, 1989) from commercially available intermediates from Sigma-Aldrich Chemical Co. and chlorpyrifos-oxon was synthesized by similar methods from 3,5,6-trichloro-2-pyridinol generously donated by Dow Chemical Co. (Midland, MI). The nerve agent surrogates were synthesized as described below.
1.2.2 Synthesis of Nerve Agent Surrogates
1.2.2.1 Nitrophenyl Ester Homologs
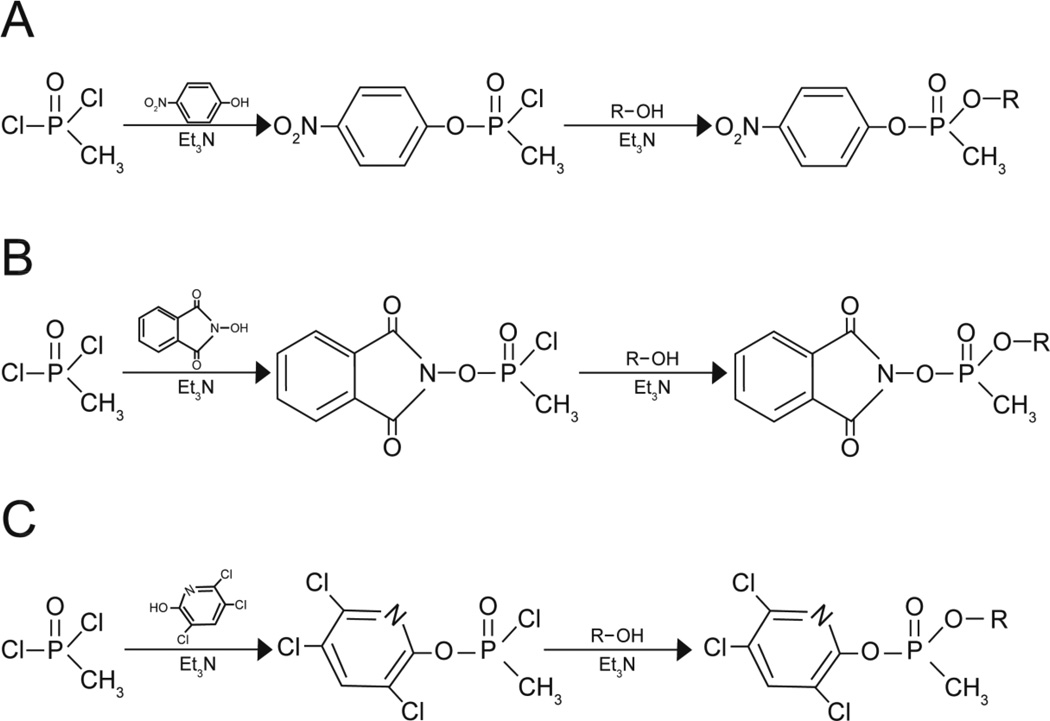
The synthesis reaction was as described by Meek et al. (2012). Briefly, triethylamine in benzene was slowly added to a mixture of methylphosphonic dichloride and 4-nitrophenol in benzene (80 ml) at room temperature. Then triethylamine in benzene and 2-propanol, ethanol, or cyclohexanol for NIMP (sarin surrogate), NEMP (VX surrogate), and NCMP (cyclosarin surrogate), respectively, were added slowly (Fig. 2A).
Figure 2.
Synthesis reactions for (A) the nitrophenyl surrogates for VX (NEMP), sarin (NIMP), and cyclosarin (NCMP); (B) the phthalimidyl surrogate for sarin (PIMP); and (C) the trichloropyridinyl surrogates for VX (TEMP) and sarin (TIMP). Et3N = triethyl amine.
1.2.2.2 Phthalimidyl Ester Homolog
The synthesis of PIMP (sarin surrogate) was the same as for the nitrophenyl ester homologs above except that N-hydroxyphthalimide was used instead of 4-nitrophenol (Fig. 2B).
1.2.2.3 Trichloropyridinyl Ester Homologs
The synthesis of 3,5,6-trichloro-2-pyridinyl ethyl methylphosphonate (TEMP, VX surrogate) and 3,5,6-trichloro-2-pyridinyl isopropyl methylphosphonate (TIMP, sarin surrogate) were the same as for the nitrophenyl ester homologs above except that 3,5,6-trichloro-2-pyridinol was used instead of 4-nitrophenol (Fig. 2C).
1.2.3 Animals
Adult male Sprague Dawley-derived rat and male C57BL/6 mice were used for this experiment. The animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility at a constant temperature (22 ± 2C) and on a 12-h light/dark cycle. LabDiet rodent chow and tap water were freely available. Euthanasia of the rats and of the mice was by carbon dioxide asphyxiation. All procedures were approved by the Mississippi State University Institutional Animal Care and Use Committee.
1.2.4 Determination of bimolecular rate constants
A continuous spectrophotometric assay was used to determine AChE activities (modification of Ellman et al., 1961) with acetylthiocholine (ATCh) as the substrate and 5,5’-dithiobis(nitrobenzoic acid) (DTNB) as the chromogen (Chambers et al., 1988).
The bimolecular rate constant (ki), phosphorylation constant (kp), and association constant (KA) for each inhibitor in brain homogenates and human erythrocyte AChE were determined for each inhibitor according to the method of Johnson and Wallace (1987) and Carr and Chambers (1996) based on the work of Kitz and Wilson (1962) and Segel (1975). The values were determined using a SpectraMax M5 microplate reader with SoftMax Pro Software (Molecular Devices Corporation, Sunnyvale, CA). The software measured each well's absorbance and calculated the velocity of each reaction by determining the slope of the line from a plot of product formed as a function of time.
Frozen whole brains from rats and mice were homogenized in 0.05M Tris-HCl (pH 7.4, 25C) buffer at 40 mg/ml and diluted with the same buffer to the final assay concentration of 6 mg wet weight of tissue/ml. Commercial erythrocyte AChE was diluted in 0.05M Tris-HCl (pH 7.4, 25C) to the final assay concentration of 10−4 mg/ml of purified enzyme. For both brain and human erythrocyte AChE, 155µl of each sample was added into each well of a 96-well plate. No additional proteins was added to the assays for enzyme stabilization to avoid any additional potential binding sites for the test compounds. To correct for non-enzymatic hydrolysis 60µM eserine sulfate was incubated with parallel wells of control samples. Non-enzymatic hydrolysis of ATCh was typically <10% and <5% for brain and human AChE, respectively. Each plate was warmed to 37C before starting the assay.
The inhibition reaction was initiated by the pre-incubation of brain homogenates and human erythrocyte AChE with ethanol vehicle or eight concentrations of each OP which were predetermined to give 10–90% AChE inhibition. Six pre-incubation periods were used (0–5 mins at 1 min intervals). For all enzyme sources, the OPs paraoxon, chlorpyrifos-oxon, DFP, NEMP, NIMP, and NCMP were tested. In addition, methyl paraoxon, PIMP, TIMP, and TEMP were tested using rat brain only because of the greater availability of rat brain tissue in our laboratories.
Following the pre-incubation, the inhibition reaction was terminated by addition of the substrate which, at least 3 orders of magnitude greater concentration than the inhibitors, would have out-competed for binding to the active site. The AChE reaction was initiated by the addition of a 40µl mixture of 5mM substrate ATCh and 25mM DTNB. For the 0-min time point, the substrate/chromogen mixture was added at the same time with the OPs. Each reaction was monitored by recording the absorbance at 412nm for 3 min at 30 second intervals (6 readings) and the velocity of each pre-incubation time was obtained for each concentration and control.
For each pre-incubation time, the AChE velocity following inhibition ([E]t) was divided by the original uninhibited velocity ([E]o) to obtain the fraction of AChE velocity remaining ([E]t/[E]o). The apparent rate of AChE phosphorylation (slope = -kapp) for each concentration was calculated by linear regression of the natural log (ln) of the [E]t/[E]o as a function of time. Finally, a double reciprocal plot of kapp as a function of the molar inhibitor concentration of AChE was used to calculate each inhibitor’s ki (slope = 1/ki), kp (y intercept = 1/kp), and KA (x intercept= -KA). The reaction describing the inhibition process and definition of kinetics parameters were based on the following scheme:
Assuming kp ≪ k−1
where [E] was the concentration of free enzyme, [I] was the concentration of free inhibitor, [EI]R was the reversible enzyme-inhibitor complex, and [EI]I was the irreversibly phosphorylated enzyme-inhibitor complex (Johnson and Wallace, 1987).
For each species and each inhibitor, all linear regressions and calculations were performed using Microsoft Excel 2010 to obtain the AChE velocity remaining ([E]t/[E]o) and apparent rate of AChE phosphorylation (kapp) for determination of the bimolecular rate constants (ki), association constants (KA), phosphorylation constants (kp) for each OP.
1.3 Results and Conclusions
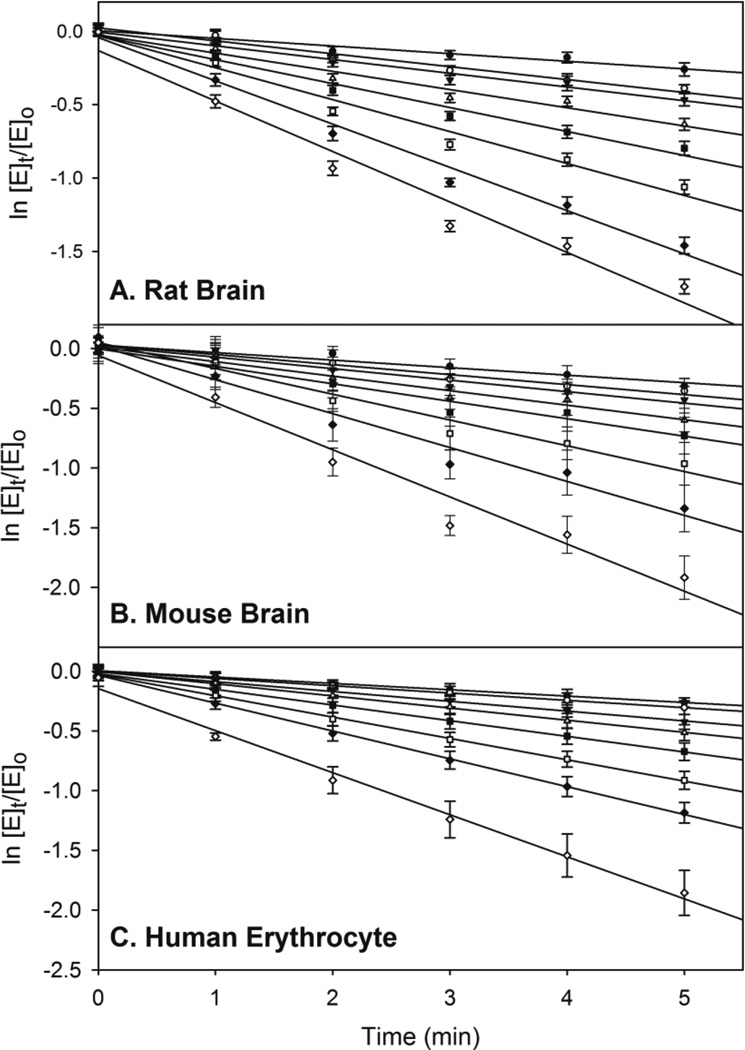
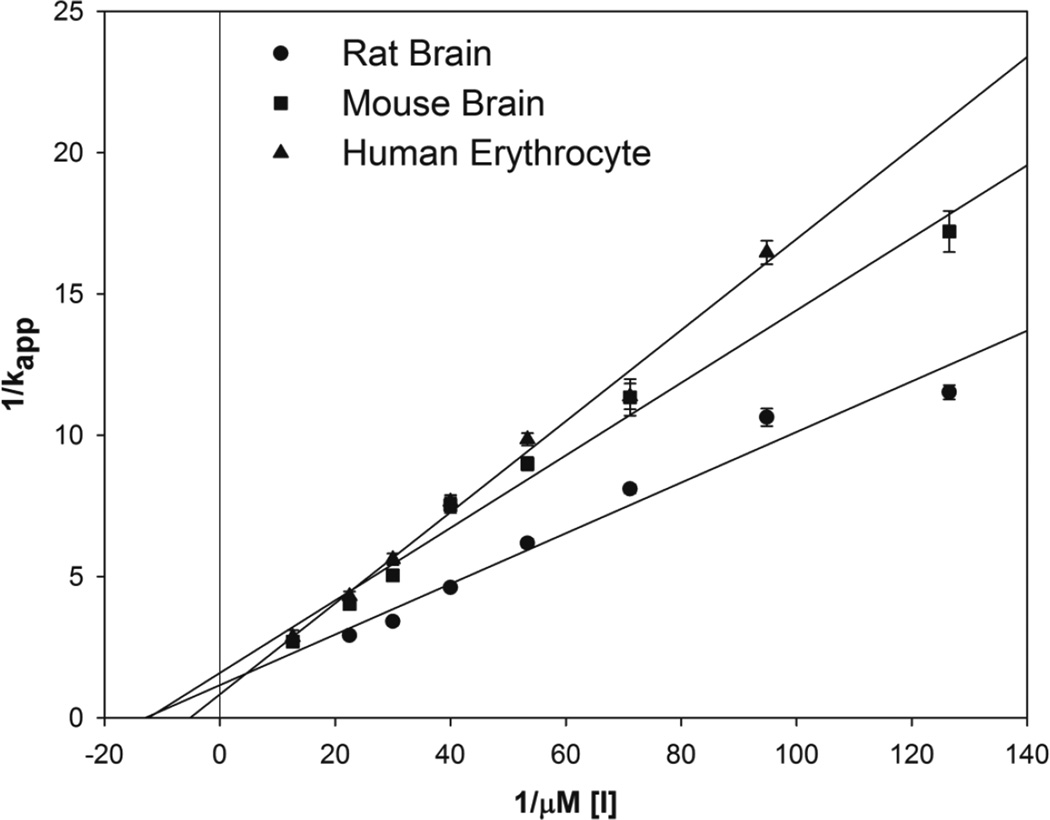
Representative data used for determining the apparent rates of inhibition (kapp) of NCMP in rat brain, mouse brain, and human erythrocyte AChE are presented in Figure 3. Representative double reciprocal plots of kapp as a function of NCMP concentration for rat brain, mouse brain, and human erythrocyte AChE are displayed in Figure 4. From these plots, the ki, kp, and KA were determined for each OP and are presented in Table 1. The oxons and surrogates are ranked from greatest potency to lowest potency based on the ki.
Figure 3.
Averages of data sets used for determining the –kapp (slope= -kapp) of NCMP (nitrophenyl cyclohexyl methylphosphonate) for (A) rat brain ChE, (B) mouse brain ChE, and (C) human erythrocyte AChE. Values represent means ± SEM (n=3–6). Linear regression coefficients of each line used for determination of inhibition kinetic values were between 0.92 and 0.99.
Figure 4.
Double reciprocal plot of apparent rate of phosphorylation (kapp) as a function of molar inhibitor concentration ([I]) of NCMP (nitrophenyl cyclohexyl methylphosphonate) for rat brain ChE, mouse brain ChE, and human erythrocyte AChE. Values represent as means ± SEM (n=3–6).
Table 1.
Bimolecular rate constant (ki), association constant (KA), and phosphorylation constant (kp) for the inhibition of rat and mouse brain and purified human erythrocyte AChE by several organophosphates, including some nerve agent surrogatesa.
|
ki (µM−1 min−1) |
KA (µM−1) |
kp (min−1) |
|
|---|---|---|---|
| Rat brain | |||
| CPOb | 15.885 ± 0.198 | 22.679 ± 0.309 | 0.704 ± 0.065 |
| NCMP | 12.045 ± 0.174 | 17.074 ± 0.274 | 0.714 ± 0.048 |
| NEMP | 3.976 ± 0.096 | 4.951 ± 0.173 | 0.813 ± 0.059 |
| TEMP | 2.967 ± 0.155 | 4.326 ± 0.273 | 0.694 ± 0.102 |
| PIMP | 2.102 ± 0.149 | 3.894 ± 0.290 | 0.597 ± 0.121 |
| NIMP | 1.670 ± 0.080 | 2.665 ± 0.125 | 0.632 ± 0.071 |
| PO | 1.162 ± 0.107 | 0.512 ± 0.082 | 2.293 ± 0.118 |
| MPO | 1.021 ± 0.105 | 1.760 ± 0.151 | 0.583 ± 0.079 |
| TIMP | 0.670 ± 0.081 | 0.517 ± 0.073 | 1.329 ± 0.151 |
| DFP | 0.382 ± 0.086 | 0.755 ± 0.167 | 0.533 ± 0.119 |
| Mouse Brain | |||
| CPO | 11.310 ± 0.217 | 15.576 ± 0.426 | 0.760 ± 0.080 |
| NCMP | 8.072 ± 0.301 | 13.126 ± 0.586 | 0.630 ± 0.109 |
| NEMP | 3.414 ± 0.347 | 3.185 ± 0.476 | 1.139 ± 0.345 |
| NIMP | 1.677 ± 0.118 | 2.848 ± 0.266 | 0.602 ± 0.103 |
| PO | 1.588 ± 0.165 | 2.667 ± 0.285 | 0.602 ± 0.169 |
| DFP | 0.118 ± 0.035 | 0.164 ± 0.075 | 0.725 ± 0.132 |
| Human Erythrocyte AChE | |||
| CPO | 9.674 ± 0.191 | 6.338 ± 0.582 | 2.488 ± 0.330 |
| NCMP | 6.027 ± 0.201 | 4.365 ± 0.170 | 1.381 ± 0.044 |
| NEMP | 1.586 ± 0.214 | 2.550 ± 0.350 | 0.627 ± 0.110 |
| DFP | 1.243 ± 0.153 | 0.901 ± 0.194 | 1.408 ± 0.291 |
| NIMP | 0.942 ± 0.211 | 1.447 ± 0.180 | 0.659 ± 0.214 |
| PO | 0.861 ± 0.112 | 1.378 ± 0.192 | 0.642 ± 0.124 |
Data are expressed as mean ± SEM, n = 3–6.
CPO (chlorpyrifos-oxon), NCMP (nitrophenyl cyclohexyl methylphosphonate, cyclosarin surrogate), NEMP (nitrophenyl ethyl methylphosphonate, VX surrogate), TEMP (3,5,6-trichloro-2-pyridinyl ethyl methylphosphonate, VX surrogate), PIMP (phthalimidyl isopropyl methylphosphonate, sarin surrogate), NIMP (nitrophenyl isopropyl methylphosphonate, sarin surrogate), PO (paraoxon), MPO (methyl paraoxon), TIMP (3,5,6-trichloro-2-pyridinyl isopropyl methylphosphonate, sarin surrogate), DFP (diisopropyl fluorophosphate)
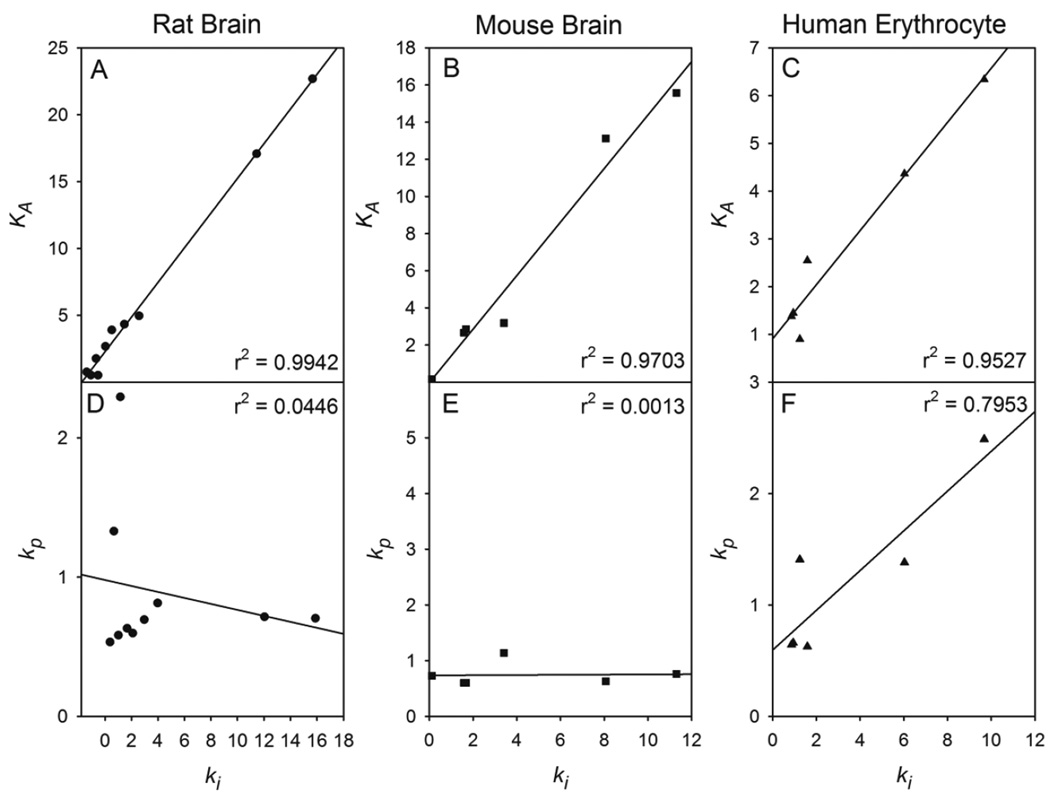
The bimolecular rate constant is a measure of the inhibitory power of an organophosphate and is comprised of both the binding affinity to the active site and rate of phosphorylation. The rate of phosphorylation of AChE is fast compared to the association of the inhibitor with the enzyme and the most important factor in determining the inhibitory power of an organophosphate is the affinity for the active site. The greater importance ascribed to the inhibitor’s affinity constant is evident for all tissue preparations demonstrated by the linear regression of ki vs KA which yielded correlation coefficients of r2 = 0.9942 for rat (Figure 5A), r2 = 0.9703 for mouse (Figure 5B), and r2 = 0.9527 for human erythrocyte AChE (Figure 5C). The poor correlation coefficients observed when linear regression of ki vs kp was performed (Figures 5D-5F) also suggests the lesser role of the rate of phosphorylation in determining the inhibitory power.
Figure 5.
Linear regression plots of the bimolecular rate constant (ki) vs the association constant (KA) of all inhibitors for AChE from (A) rat brain; (B) mouse brain; and (C) purified human erythrocytes and linear regression plots the bimolecular rate constant (ki) vs the phosphorylation constant (kp) of all inhibitors for AChE from (A) rat brain; (B) mouse brain; and (C) purified human erythrocytes.
Chlorpyrifos-oxon was the most potent inhibitor of AChE as shown by the higher ki values. In addition, it had the highest KA values in all tissue preparations. This is in agreement with our previous report using pelleted rat brain membranes (Carr and Chambers, 1996). While we could not find any studies directly comparing chlorpyrifos-oxon with nerve agents, chlorpyrifos-oxon is frequently reported to be a more potent in vitro inhibitor of AChE in studies comparing its inhibitory potency to other OPs (Atterberry et al., 1997; Sultatos et al., 1982; Mortensen et al., 1998; Amitai et al., 1998; Kropp and Richardson, 2003; Wille et al., 2011). For rat brain, the ranking of potencies for the non-surrogate organophosphates was chlorpyrifos-oxon>paraoxon>methyl paraoxon>DFP. This pattern is in agreement with other in vitro inhibition studies (Wang and Murphy, 1982; Amitai et al., 1998; Kousba et al., 2004; Worek et al., 2004).
With respect to the organophosphonate nerve agent surrogates, the most potent inhibitor was NCMP, the cyclosarin surrogate. This was followed by NEMP and TEMP, the VX surrogates, and then by PIMP, NIMP and TIMP, the sarin surrogates. This ranking of potency between the nerve agent surrogates based on their ki values agrees with the ranking of potency of the actual nerve agents based on their ki values where cyclosarin>VX>sarin (Worek et al., 2004). While not all surrogates were tested with mouse brain and human erythrocyte AChE, the pattern of inhibitory potency of the organophosphonates is similar across tissue preparations.
In general, with the exception of chlorpyrifos-oxon, the organophosphonates were more potent inhibitors than the organophosphates with respect to inhibiting AChE in the rat brain assay. For example, the NCMP rate constant was 10- and 11.8-fold higher than that of paraoxon and methyl paraoxon, respectively. However, an exception to this occurred with the sarin surrogate TIMP. Comparison of the three sarin surrogates clearly demonstrates that differences in the substituents caused notable differences in ki values. The inhibitory potency can be influenced by the leaving group attached to the isopropyl methylphosphonate base structure. PIMP was developed as a less stable sarin surrogate to be used in in vitro assays to prevent significant reinhibition of reactivated AChE (Meek et al., 2012). Although it degrades within about 15 min in aqueous solution, PIMP was a more potent inhibitor than NIMP, indicating that the optimal leaving group is a phthalimidyl substituent which is 1.25-fold more potent than when the leaving group is a nitrophenyl substituent. TIMP was developed with the same leaving group as chlorpyrifos-oxon (i.e., trichloropyridinyl leaving group) with the assumption that it would be a very potent inhibitor since chlorpyrifos-oxon is a very potent inhibitor. In contrast to this assumption, TIMP was the weakest of the three sarin surrogates being 3- and 2.5-fold less potent than PIMP and NIMP, respectively. However, if the base structure is a diethyl phosphate, chlorpyrifos-oxon, which contains a trichloropyridinyl substituent, was a 13.7-fold better inhibitor than paraoxon which contains a nitrophenyl substituent. These differences indicate that the trichloropyridinyl group does not impart high inhibitory potency in all cases. In addition, differences in the substituents comprising the phosphonate structure also caused notable differences in ki values. For example, replacement of the isopropyl group of NIMP (sarin surrogate) with a cyclohexyl substituent (NCMP, cyclosarin surrogate) resulted in a 7.2-fold increase in inhibitory potency while replacement with an ethyl group (NEMP, VX surrogate) only resulted in a 2.4-fold increase in inhibitory potency. Together, these data indicate that the ultimate inhibitory potency of the surrogates depends on both the interaction of the leaving group moiety to the anionic binding site and the orientation to the active site conferred by the substituent composing the phosphonate structure.
NEMP, NIMP, NCMP, DFP, chlorpyrifos-oxon, and paraoxon were the six compounds tested on mouse brain and human erythrocyte AChE. As stated above, the pattern of potency of the organophosphonates is similar in all tissue preparations. The pattern of potency of the organophosphates with rat brain AChE is similar to that with mouse brain AChE and, with the exception of DFP, similar to that with human erythrocyte AChE. In rat and mouse brain, DFP was a surprisingly weak AChE inhibitor considering that it is frequently used in many laboratories as a nerve agent surrogate (presumably because its fluoride leaving group is similar to that of sarin and soman) and is considered a highly toxic compound. Our kinetic analysis does not suggest that it is a highly potent inhibitor in brain preparations. In human erythrocyte ghosts, the ki for DFP was lower than all nerve agents tested and lower than paraoxon and methyl paraoxon (Worek et al., 2004). This is in contrast to our data with purified human erythrocyte AChE data where DFP had a higher ki than paraoxon and the sarin surrogate NIMP. It is possible that the purified human erythrocyte AChE, which is the amphiphilic form extracted together with its GPI anchor, may not have the same conformational tertiary structure as the membrane embedded enzyme that would be present in erythrocyte ghosts. This difference could have affected the affinity of the purified enzyme for certain inhibitors. In addition, the two isopropyl groups in DFP are bulkier substituents than the other compounds tested and may have created more steric hindrance to the membrane-bound AChE than to the purified enzyme. The fact that DFP was not the weakest inhibitor with the purified AChE indicates that the DFP was not degraded.
It was previously shown that when equal 0.1 M concentrations of different buffers (phosphate buffer, MOPS, tyrode, and TRIS) were used to control the pH of the AChE assay, significant differences in substrate affinity were observed. The use of TRIS caused a noteworthy decrease in substrate affinity (Km using ACh). It was suggested that this difference was the result of TRIS acting as either an allosteric effector or as a competitive inhibitor to AChE (Pavlic, 1967; Wille et al., 2011). Our laboratories have routinely used TRIS buffer for cholinesterase assays. As there is no one universally accepted AChE assay system, it should come as no surprise that reports from different laboratories may present significantly different literature values for AChE with respect to its kinetic parameters and its inhibition by the same OP inhibitor (Wille et al., 2011).
Studies have demonstrated that at 1pM concentration, the ki values for paraoxon and chlorpyrifos-oxon were 1,000 and 10,000-fold higher than when the ki values determined at 100nM concentration, respectively. It was suggested that, when assayed under “high” inhibition concentrations, paraoxon, methyl paraoxon and chlorpyrifos-oxon (Kaushik et al., 2007; Kousba et al., 2004) bind to a secondary peripheral site on human AChE or rat brain AChE. This is turn affects the resulting activation/inhibition and thus affects changes in the ki value as a function of oxon concentration in the presence of acetylcholine, particularly at low, environmentally relevant concentrations.
In summary, the kinetics constants that have been calculated for several nerve agent surrogate organophosphonates and several non-surrogate organophosphates showed similar patterns of potency between rat and mouse brain AChE, with generally the surrogates showing greater potency (except for chlorpyrifos-oxon, which was the most potent compound tested). Human erythrocyte AChE showed a similar pattern to the rodent brain AChE except for DFP which was reasonably potent for human AChE but considerably weaker for rodent AChE. Generally the surrogate organophosphonates reflected potencies proportional to the toxicities of their respective nerve agents. These data indicate that inhibition kinetics analysis can be useful in predicting the general potency of organophosphate/organophosphonate anticholinesterases and the values obtained in laboratory rodents are reflective of the potencies observed on humans. Such data can be of predictive value and lead to a reduced use of laboratory animals. Because nerve agent research must be conducted on animals, the similarities observed here in potencies indicate that laboratory rodent data should be useful to estimate relative potencies in humans.
Highlights.
Inhibition kinetic assays were conducted with several highly relevant surrogates for sarin, VX and cyclosarin and insecticidal organophosphates in rat brain, mouse brain and purified human erythrocyte acetylcholinesterase.
With the exception of chlorpyrifos-oxon, the nerve agent surrogates were more potent inhibitors than the other organophosphates.
The potencies of the nerve agent surrogates were proportional to the toxicities of the nerve agents, cyclosarin > VX > sarin.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number U01NS083430. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AChE
acetylcholinesterase
- ATCh
acetylthiocholine iodide
- DFP
diisopropyl fluorophosphate
- DTNB
5,5’-dithio-bisnitrobenzoic acid
- MOPS
3-[N-morpholino] propane sulfonic acid
- NCMP
nitrophenyl cyclohexyl methylphosphonate
- NEMP
nitrophenyl ethyl methylphosphonate
- NIMP
nitrophenyl isopropyl methylphosphonate
- PIMP
phthalimidyl isopropyl methylphosphonate
- TEMP
3,5,6-trichloro-2-pyridinyl ethyl methylphosphonate
- TIMP
3,5,6-trichloro-2-pyridinyl isopropyl methylphosphonate
- TRIS
tris[hydroxymethyl]-aminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alper Coban, Email: acoban@umc.edu.
Russell L. Carr, Email: russell.carr@cvm.msstate.edu.
Howard W. Chambers, Email: hwc2@msstate.edu.
Kenneth O. Willeford, Email: kwilleford@bch.msstate.edu.
Janice E. Chambers, Email: chambers@cvm.msstate.edu.
References
- Amitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyrylcholinesterase by chlorpyrifos-oxon. Biochem. Pharmacol. 1998;56:293–299. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol. Appl. Pharmacol. 1997;147:411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Ballantyn B. Chemical terrorism. In: Ballantyne B, Marrs TC, Syversen T, editors. General, Applied and Systems Toxicology. Volume 5. Military and Homeland Security Toxicology Issues. Chichester, UK: Wiley; 2009. [Google Scholar]
- Carr RL, Chambers JE. Kinetic analysis of the in vitro inhibition, aging, and reactivation of brain acetylcholinesterase from rat and channel catfish by paraoxon and chlorpyrifos-oxon. Toxicol. Appl. Pharmacol. 1996;139:365–373. doi: 10.1006/taap.1996.0177. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Chambers HW. An investigation of acetylcholinesterase inhibition and aging and choline acetyltransferase activity following a high level acute exposure to paraoxon. Pestic. Biochem. Physiol. 1989;33:125–131. [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW. Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Commun. 1988;3:85–92. [Google Scholar]
- Ecobichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett and Doull’s Toxicology. New York, NY: McGraw-Hill; 2001. pp. 763–810. [Google Scholar]
- Eisenkraft A, Gilburd D, Kassirer M, Kreiss Y. What can we learn on medical preparedness from the use of chemical agents against civilians in Syria. Am. J. Emerg. Med. 2014;32:186. doi: 10.1016/j.ajem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Introduction. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. San Diego, CA, USA: Academic Press, Elsevier; 2009. pp. 3–5. [Google Scholar]
- Hodgson E, Silver IS, Butler LE, Lawton MP, Levi PE. Metabolism. In: Hayes WJ Jr, Laws ER Jr, editors. General Principles, Handbook of Pesticide Toxicology. Vol. 1. New York: Academic Press; 1991. pp. 107–168. [Google Scholar]
- Johnson JA, Wallace KB. Species-related differences in the inhibition of brain acetylcholinesterase by paraoxon and malaoxon. Toxicol. Appl. Pharmacol. 1987;88:234–241. doi: 10.1016/0041-008x(87)90009-3. [DOI] [PubMed] [Google Scholar]
- Johnson NH, Larson JC, Meek EC. Historical perspectives of chemical warfare agents. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. San Diego, CA, USA: Academic Press, Elsevier; 2009. pp. 7–16. [Google Scholar]
- Kaushik R, Rosenfeld CA, Sultatos LG. Concentration-dependent interactions of the organophosphates chlorpyrifos oxon and methyl paraoxon with human recombinant acetylcholinesterase. Toxicol. Appl. Pharmacol. 2007;221:243–250. doi: 10.1016/j.taap.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitz R, Wilson IB. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J. Biol. Chem. 1962;237:3245–3249. [PubMed] [Google Scholar]
- Kousba AA, Sultatos LG, Poet TS, Timchalk C. Comparison of chlorpyrifosoxon and paraoxon acetylcholinesterase inhibition dynamics: potential role of a peripheral binding site. Toxicol. Sci. 2004;80:239–248. doi: 10.1093/toxsci/kfh163. [DOI] [PubMed] [Google Scholar]
- Kropp TJ, Richardson RJ. Relative inhibitory potencies of chlorpyrifos oxon, chlorpyrifos methyl oxon, and mipafox for acetylcholinesterase versus neuropathy target esterase. J. Toxicol. Environ. Health. 2003;66:1145–1157. doi: 10.1080/15287390306360. [DOI] [PubMed] [Google Scholar]
- Meek E, Chambers H, Coban A, Funck K, Pringle R, Ross M, Chambers J. Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol. Sci. 2012;126:525–533. doi: 10.1093/toxsci/kfs013. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Hooper MJ, Padilla S. Rat brain acetylcholinesterase activity: developmental profile and maturational sensitivity to carbamate and organophosphorus inhibitors. Toxicology. 1998;125:13–19. doi: 10.1016/s0300-483x(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Pavlic M. The inhibitory effect of Tris on the activity of cholinesterases. Biochim. Biophys. Acta. 1967;139:133–137. doi: 10.1016/0005-2744(67)90119-2. [DOI] [PubMed] [Google Scholar]
- Pita R, Domingo J. The use of chemical weapons in the Syrian conflict. Toxics. 2014;2:391–402. [Google Scholar]
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. New York: Wiley; 1975. [Google Scholar]
- Sultatos LG, Costa LG, Murphy SD. Factors involved in the differential acute toxicity of the insecticides chlorpyrifos and methyl chlorpyrifos in mice. Toxicol. Appl. Pharmacol. 1982;65:144–152. doi: 10.1016/0041-008x(82)90372-6. [DOI] [PubMed] [Google Scholar]
- Tucker JB. War of Nerves: Chemical Warfare from World War I to Al-Qaeda. New York, NY: Pantheon Books; 2006. [Google Scholar]
- Wang C, Murphy SD. Kinetic analysis of species difference in acetylcholinesterase sensitivity to organophosphate insecticides. Toxicol. Appl. Pharmacol. 1982;66:409–419. doi: 10.1016/0041-008x(82)90307-6. [DOI] [PubMed] [Google Scholar]
- Wille T, Thiermann H, Worek F. Effect of different buffers on kinetic properties of human acetylcholinesterase and the interaction with organophosphates and oximes. Arch. Toxicol. 2011;85:193–198. doi: 10.1007/s00204-010-0578-9. [DOI] [PubMed] [Google Scholar]
- Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]