Abstract
Adipocytes are widely distributed in the dermis, in a unique fat depot referred to as dermal white adipose tissue (dWAT). In rodents, dWAT is present as widespread thin layers, whereas in pigs and humans it is present in clusters referred to as “dermal cones” around the pilosebaceous units. This distinct layer of fat cells located above the subcutaneous white adipose tissue is important for proper hair follicle (HF) cycling in rodents. Murine HFs produce spatially restricted synchronous patches after their second postnatal cycle which correlates with a spatial heterogeneity of murine dWAT. Similarly, the cycling of HFs in humans may also be related to the spatial distribution of dWAT, making the difference between murine and human HF cycling of more quantitative than of qualitative nature. This should allow the production of small spatially correlated HF patches in human skin and we propose that this process can be regulated by paracrine signaling and involving a number of signaling modules, including the hedgehog pathway. This pathway is an established player in HF cycling, but is also involved in the regulation of adipogenesis and may therefore be a key regulator of the process across species. We also suggest that the spatial heterogeneity of dWAT is connected not only to HF cycling, but may also be related to other physiological and pathological processes in the skin.
Keywords: dermal adipocytes, hair follicle, cycling, murine, human, synchronous, mosaic
Introduction
Whereas the views on different morphological and physiological characteristics of adipose tissue were revised during the last years, one feature was never discussed in depth: it is generally implicitly assumed that the content and metabolic properties of adipocytes from the same fat depot are spatially homogeneous. This implies that there are no macroscopic structural and signaling gradients within a fat pad. Such assumptions should however not be taken for granted and seem to be incorrect at least for the dermal white adipose tissue whose behavior is tightly connected with the cycling of the hair follicles (HFs). Cyclic development is a remarkable feature of HFs, a phenomenon intensively investigated over decades. Despite tremendous efforts, our knowledge in this field is however still far from satisfactory (1). One of the most intriguing questions remains the remarkable difference between rodent and human HF cycling: whereas in rodents, HFs cycle proceeds spatially quasi-synchronously, human HFs demonstrate fully asynchronous cycling.
Effectively, the fully synchronous HFs cycling in rodents can be observed in the postnatal period only up to the second HF cycle. During the following cycles, synchronous HF growth occurs only in relatively small patches (2), thus demonstrating a spatially restricted correlation radius. This phenomenological observation was supported by time-course experiments looking at gene expression profile data (3,4). Such specific spatiotemporal HF behavior leads to production of a mosaic skin pattern with HFs belonging to a single patch being quasi-synchronized in the same phase of the cycle. On the other side, the idea of fully stochastic cycling of HFs in humans goes back to the classical publication of Chase (5) which was not significantly revised until present day. Whereas HFs cycling in humans is also often described as occurring in a “spatially mosaic structure”, the correlation radius of HF patches in humans is assumed to be restricted to the single hairs, making neighboring human HFs fully asynchronous. Such a model amongst a number of issues strongly restricts the search area for signals initiating the HF growth and transitions between the phases to proximity of a single HF, thus excluding any long-range or spatially correlated effects.
More recent insights in this field connected the murine HF cycling with activity of dermal adipocytes (6). Observations like that make the idea of spatially homogeneous structures of dWAT and the idea of fully stochastic HF cycling also in humans questionable.
Dermal adipocytes in HF cycling
Adipocytes are an abundant cell type present in dermis, giving rise to a fat depot referred to as dermal white adipose tissue (dWAT). This depot can spatially produce widespread thin layers (as in rodents) or form clusters (sometimes also named “dermal cones”) around the pilosebaceous units (as in pigs and humans) (7-10). This is a distinct layer located above the subcutaneous white adipose tissue (sWAT) (Fig. 1). Whereas the adipocytes in the dermis for a long time were considered to be physiologically irrelevant, this opinion has dramatically changed during the last years after they were implicated in processes such as HF cycling (6), wound healing (11), homeostatic temperature regulation (12), cutaneous fibrosis (13), protection against skin infections (14) and many additional phenomena. Recently, dermal adipocytes have even been implicated in such processes as long-term skin reactions to the soft tissue fillers (15) and some other processes (16).
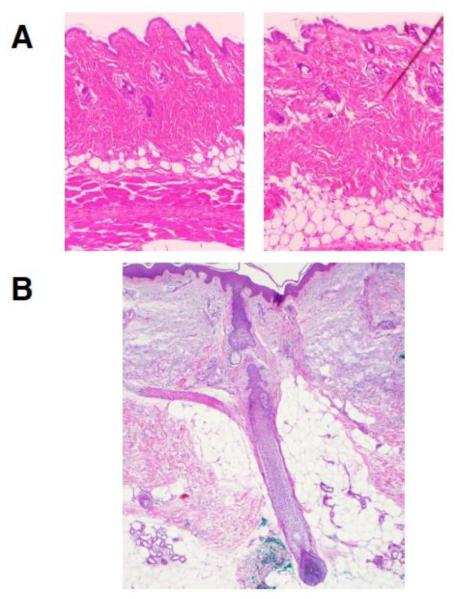
Figure 1.
Typical dWAT structures in murine and human skin: a – two samples from the same back area of a wildtype mouse at room temperature demonstrating spatially heterogeneous dWAT structure, presumably connected with the phase of HF cycle; b – “dermal cone” around the pilosebaceous unit in humans. Pictures courtesy of Drs. Annabel Wang and Travis Vandergriff, UT Southwestern Medical Center.
According to (6), a temporal re-distribution of immature dermal adipocytes in the vicinity of HF is not only necessary, but also sufficient for proper HF cycling; at the same time, the mature dermal adipocytes do not appear to be essential for this process, since the lipodystrophic A-ZIP/F1 mouse that lacks mature white adipocytes system-wide and only has immature adipocytes demonstrates almost normal HF cycling. This idea can be supported by the fact that co-culturing of adipocytes with HFs enhances the differentiation of HFs (17). In parallel, it was shown that epidermal Wnt signaling can activate adipocyte differentiation in dWAT during the hair cycle (18) which indicates that HFs-dWAT interactions may be reciprocal and not a one-way communication. In any case, the number of mature dermal adipocytes reaches its maximum during late anagen phase (6,19), thus demarcating the transition from the downward growth of a HF accompanied by a strong mesenchymal remodeling processes at the growth zone of the hair shaft. In contrast to these dynamics of mature dermal adipocytes, the number of adipocyte precursors increases more than four-fold during anagen induction, and remains high in middle of the anagen phase and then returns back to baseline in late anagen (6). Although dermal adipocytes are involved in different physiological and pathological processes in the skin, their primary function seems to be connected with HF morphogenesis and cycling, which is supported by the fact that morphogenetic development of murine dWAT occurs independently of sWAT, and this process temporally correlates with the appearance of HFs in murine fetuses (20).
Given that dermal adipocytes are essential for proper murine HF cycling (6) and given that murine HFs produce spatially restricted synchronous patches after their second postnatal cycle (2), this would suggest that murine dWAT should be spatially heterogeneous (Fig. 1). Every phase of the HF cycle and even different sub-phases of anagen are characterized by a special arrangement of mature and immature dermal adipocytes (6). This indicates that neighboring skin patches in different phases of HF cycle should demonstrate different patterning of dermal adipocytes, whereas the HFs from the same patch should have similar characteristics. Indeed, dWAT thickness near the anagen HFs can be twice the size relative to telogen (1). The length of such morphologic heterogeneities corresponds to the characteristic size of a given HF patch.
Such a model, if correct, postulates that the difference in the cycling of HFs in rodents and in humans is not of qualitative, but of quantitative character and must primarily be related to the HFs density as well as to the spatial distribution of dermal adipocytes. Additionally, spatial gradients of cells and paracrine signals between neighboring patches should be apparent. This highlights the spatial heterogeneity that we have to bear in mind when sampling specific skin areas.
Mesenchymal remodeling processes – short- or long-range effects?
Mesenchymal remodeling is, among others, tightly connected with local activity of Hedgehog (Hh) signaling, which is most active in the early and middle anagen sub-phases and decreases to near zero during late anagen stage (21,22). Since Hh expression predominantly takes place at the distal end of HFs, this process correlates also spatially with the areas of the maximal activity of mesenchymal remodeling and with the location of immature dermal adipocytes. Whereas Hh is not the only pathway which can be involved in these highly complex processes, we will focus our discussion here to Hh signaling in the context of peculiarities of short- and long-range HF modulation.
The hedgehog pathway is involved not only in HF cycling, but also in the regulation of adipogenesis. Indeed, differentiation of preadipocytes into the mature adipocytes can be inhibited by expression of sonic hedgehog (Shh), one of the signaling components of the hedgehog system (23). Accordingly, proliferation and differentiation of adipocyte precursor cells into mature adipocytes should be suppressed in the active mesenchymal remodeling phase of HF cycle during which Shh levels are high. At the same time, suppression of Hh signaling is a necessary condition for adipocyte differentiation (24). As a result, the appearance of mature dermal adipocytes in the vicinity of HF can only be seen during or after the mesenchymal remodeling phase. This corresponds to the temporal sequence of immature and mature adipocytes surrounding murine HFs reported by Festa and colleagues (6). Additionally, adipocytes co-cultured with HFs embedded in a collagen gel exert a suppressive effect on the proliferation of peri-follicular fibroblasts (17). This interaction between adipocytes and HFs can provide a reduction of mesenchymal remodelling at the distal end of HF at late anagen phase. Moreover, during late anagen, the mature adipocytes can regulate the differentiation processes of HFs (25,26). Whether the appearance of dermal adipocytes is the primary driving force suppressing mesenchymal remodeling or whether mesenchymal remodeling prompts the appearance of dermal adipocytes, is hard to say. Either way, mesenchymal remodeling temporally and spatially correlates with proliferation of preadipocytes and differentiation of dermal adipocytes around cycling HFs.
Shh expression is normally spatially restricted to the cells of the pilosebaceous unit. The activity of Shh can be detected at the highest levels several cell diameters away from Shh expressing cells (27), which could mediate the stochastic nature of HFs cycling in humans in which the inter-follicular distance is much bigger than the characteristic Shh paracrine activity. At the same time, lipidation of hedgehog can significantly modulate its transport properties, increasing the effectively targeted area of activity (28). Long-range transduction of Shh signaling involves megalin (29), as the megalin null mice demonstrate a loss of hedgehog signaling (30). At the same time, megalin levels vary temporally during the HF-cycle, present at the highest levels in early anagen and the lowest levels in catagen (31). Megalin levels are regulated by PPARγ, the key pro-adipogenic transcription factor, and exposure to the PPARγ agonist rosiglitazone significantly increases megalin expression in vivo (32). PPARγ is indeed highly expressed in dermal preadipocytes (6), which are particularly active at early and middle anagen, and this effect can substantially enhance the physical range of Shh signaling.
The existence of a long-range effect of Shh could be involved into the development of follicular tumors. The mutated inter-follicular cells induce Shh targets promoting the development of trichoblastomas or basal cell carcinomas (33). This is further supported by the fact that 25-hydroxyvitamin D in pharmacological doses can significantly inhibit Shh signaling (34). Vitamin D receptor (VDR) and PPAR signaling pathways belong to the same nuclear receptor family and are interconnected (35), whereas VDR signaling inhibits both PPARγ activity and adipogenesis (36). This correlates with influence of 1,25-dihydroxyvitamin D on the structure of murine dWAT: this fat depot is depleted by high levels of this vitamin and significantly expanded in its absence (37).
Paracrine signaling of dermal adipocytes
Aggregates of human dermal adipocytes around single pilosebaceous units can communicate with each other through paracrine signaling realized through diffusion of paracrine molecules (Fig. 2). This mechanism can mediate long-range effects amongst the “dermal cones”. In turn, this communication can effectively produce the spatial clusters of characteristic size containing several “dermal cones”.
Figure 2.
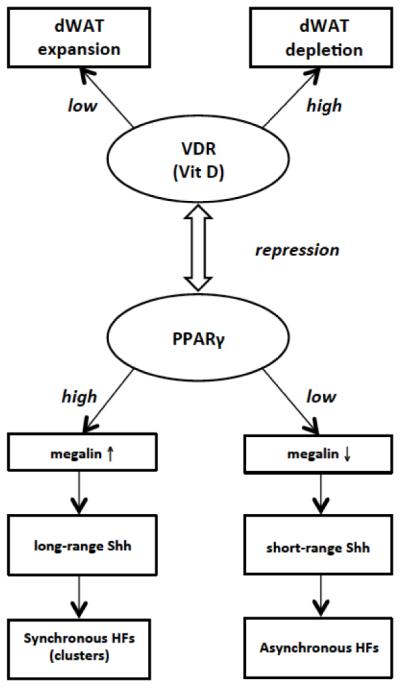
Involvement of dWAT in Hair Follicle (HF) Synchronization.
Synchronous cycling of HFs is connected with long-range paracrine signaling. High PPARγ activity is associated with high megalin expression, which is an endocytic sonic hedgehog receptor (Shh). High megalin reflects high Shh sensitivity, translating into a long-range signaling activity of the Shh pathway. As a result, the mesenchymal remodeling processes in neighboring HFs synchronized, which is typical for the first two postnatal hair cycles in rodents. In contrast, low PPARγ activity can reduce the effective range of Shh paracrine signaling, thereby resulting in seemingly asynchronous cycling of HFs, which is typical for HF cycling in humans and beyond the third postnatal HF cycling in rodents. PPARγ activity is connected with local morphology and the physiological state of dWAT, which in turn can be regulated by different factors, among them by Vitamin D Receptor (VDR) signaling. On the other hand, PPARγ activity correlates through dWAT with the phase of HF cycle.
Such signaling can be achieved through factors such as TGF-β, a well-known paracrine mediator significantly determining the expression levels of hyaluronan and collagen (38), which can produce long-range effects. TGF-β is involved in many cellular differentiation processes (39). A recent report demonstrated that adipocytes undergo reprogramming into myofibroblast-like cells upon treatment with TGF-β (13), and it was inferred that this is the main pathophysiological mechanism for cutaneous fibrosis. This transition is not only of importance in wound healing and fibrosis but can also connect the dermal adipocytes with HF cycling, since different isoforms of TGF-β are potently involved in this process (40,41). Moreover, blocking TGF-β signaling leads to delayed skin wound healing (42) once more emphasizing a possible connection between the processes of HF cycling and wound healing.
To serve as a paracrine signaling molecule in HFs cycling, TGF-β should effectively be regulated by dermal adipocytes. While there is no direct evidence at this point that human dermal adipocytes indeed secrete TGF-β and that the secretion varies according to the physiological state of these cells, it is very likely to be the case, since TGF-β is widely expressed in other adipocytes. TGF-β expression is found in the dermis and in the fat lobules around HFs in fetal and early postnatal pigs (43). Pigs display similar structures as seen for human dermal adipose tissue (8,9). Such expression should be mainly tied to the activity of immature adipocytes. Adipose-derived stem cells can indeed secrete TGF-β1 (44). Moreover, a significantly increased release of TGF-β1 was reported for adipose tissue explants from obese subjects. More than 90% of this secretory pool was provided by the stromal-vascular cell fraction which contains adipose-derived stem cells (45). In the context of HF signaling discussed here, this infers that maximum TGF-β1 signaling should be observed during the early and middle anagen phase when the immature adipocytes are most prominently present in the vicinity of HFs.
Long-range effects of dermal adipocytes
Dermal adipocytes were shown to be of substantial importance at least in murine wound healing (11). In contrast to HF cycling, where the appearance of immature adipocytes is of primary importance, the main effects in wound healing are related with mature adipocytes. These cells repopulate the skin wound concurrently with fibroblasts, and this process is thought to be mainly driven via adipogenesis (11). The relevance of mature adipocytes in wound healing can be seen from experiments with A-ZIP/F1 mice which demonstrate a high degree of instability of the healed wounds. Such interplay between the processes of HF cycling and wound healing explains why the anagen phase of the HF cycle promotes wound healing, an observation reported many times in the literature (46).
For an effective repopulation of the wound, dermal adipocytes should demonstrate two characteristics: 1) a very high dynamic range with characteristic half-lives comparable to wound healing times (which are much shorter than the renewal times of 10 years generally accepted for sWAT (47)); 2) the ability of at least immature dermal adipocytes to migrate over the distances relevant to wound size. Both of these properties mean that dermal adipocytes significantly differ from their subcutaneous counterparts and can really provide spatially long-ranging effects. At this point, there is no direct evidence demonstrating that dermal adipocytes can migrate over such long distances in a short time, but such properties should be postulated for these cells. Indeed, migration of preadipocytes is considered to be an essential process for adipogenesis in vivo (48,49). Additionally, the recently discovered adipocyte-myofibroblast transition, which connected the appearance of myofibroblasts with the local disappearance of dermal adipocytes, clearly demonstrates the possibility of the phenotypic transition from mature (adiponectin positive) dermal adipocytes (13). That means, that the presence of mature dermal adipocytes in the wound and their consequent transition into the myofibroblasts can cause quick wound repair, making the preadipocyte migration also physiologically reasonable. At the same time, it is not known whether migration of dermal adipocytes from one follicle to another takes place and whether the appearance of such “exogenous” adipocytes can induce the cycling. This important item can be directly proven with recently developed “adipochaser” mouse model (50,51) giving the possibility to label and to trace the adipocytes’ fate.
Possible consequence for human HFs
If the long-range communication between the murine HFs is realized through paracrine activity of dermal adipocytes, such effects can be also inferred for humans. In this case, it is not, however, the single HFs, but rather HF patches (of smaller characteristic size than in rodents) which should exist in humans. This could make the difference between rodent and human HF cycling not of qualitative but of quantitative nature. The signals initiating the HF cycle should in such a case have the cooperative nature provided by the local modulation of the dermal adipose tissue in the area related to a group of HFs belonging to a spatial area of characteristic radius determined by paracrine diffusion processes. Cooperative behavior of dWAT inside a patch of characteristic radius will lead to mosaic properties of the cutis which can impact other structures than HFs as well (16).
Conclusion
Cooperation between single HFs producing the spatial patches of follicles in sync with the HF cycle must exist. Dermal adipocytes are likely players in this process. Such a model postulates that murine dermal adipose tissue is spatially heterogeneous with characteristic length of heterogeneity corresponding to the size of a single patch. How many HFs are contained in one patch depends on the intensity of signaling pathway and the distance between single follicles, which can make the correlation radius of this process dependent on the a specific area of the body. As a consequence, it can be assumed that the difference in HFs cycling between rodents and humans should not be of qualitative but of quantitative character. If correct, this significantly shifts our focus to identify signals initiating transitions between different phases of the HF cycle from short-range to long-range signaling. Further progress can be achieved by examining correlations between the HFs patches in progressive murine HF cycles and spatially adjusted dermal adipose tissue structures.
The spatial heterogeneity of dWAT is connected not only with HF cycling, but also with other physiological and pathological processes in the skin, among them the development of follicular tumors. Last but not least, we cannot exclude that spatial heterogeneity of adipose tissue can play a role in other fat depots as well.
Acknowledgements
Funding: P.E.S. is funded by US National Institutes of Health Grants R01-DK55758, R01-DK099110 and P01-DK088761 as well as a grant from the Cancer Prevention and Research Institute of Texas (CPRIT RP140412).
Footnotes
Authorship: ILK and PES have jointly drafted and edited all versions of the manuscript and both approved the final version.
Conflict of Interest: ILK is the managing partner of Wellcomet GmbH. No methods or devices of Wellcomet GmbH were used in this publication. PES does not declare and conflicts of interest.
Ethics Approval: N/A.
References
- 1.Chen CC, Plikus MV, Tang PC, et al. J Mol Biol. 2015 doi:10.1016/j.jmb.2015.07.009. [Google Scholar]
- 2.Stenn KS, Paus R. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 3.Lin KK, Chudova D, Hatfield GW, et al. Proc Nat Acad Sci USA. 2004;101:15955–15960. doi: 10.1073/pnas.0407114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathur SK, Doke AM, Sadana A. Int J Bioinf Res Appl. 2006;2:249–258. doi: 10.1504/IJBRA.2006.010603. [DOI] [PubMed] [Google Scholar]
- 5.Chase HB. Physiol Rev. 1954;34:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- 6.Festa E, Fretz J, Berry R, et al. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausman GJ, Martin RJ. J Anim Sci. 1982;54:1286–1296. doi: 10.2527/jas1982.5461286x. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura H, Engrav LH, Gibran NS, et al. Wound Repair Regen. 2001;9:269–277. doi: 10.1046/j.1524-475x.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 9.Engrav LH, Tuggle CK, Kerr KF, et al. PLoS One. 2011;6:e19024. doi: 10.1371/journal.pone.0019024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driskell RR, Jahoda CAB, Chuong CM, et al. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt BA, Horsley V. Development. 2013;140:1517–27. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasza I, Suh Y, Wollny D, et al. PLoS Genet. 2014;10:e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marangoni RG, Korman BD, Wei J, et al. Arthritis Rheumatol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang LJ, Guerrero-Juarez CF, Hata T, et al. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruglikov IL, Wollina U. Exp Dermatol. 2015;24:912–915. doi: 10.1111/exd.12852. [DOI] [PubMed] [Google Scholar]
- 16.Kruglikov IL, Scherer PE. Trend Endocrin Metabol. 2016;27:1–10. doi: 10.1016/j.tem.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misago N, Toda S, Sugihara H, et al. Br J Dermatol. 1998;139:40–48. doi: 10.1046/j.1365-2133.1998.02312.x. [DOI] [PubMed] [Google Scholar]
- 18.Donati G, Proserpio V, Lichtenberger BM, et al. PNAS. 2014;111:E1501–E1509. doi: 10.1073/pnas.1312880111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plikus MV, Chuong CM. J Invest Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojciechowicz K, Gledhill K, Ambler CA, et al. PLoS One. 2013;8:e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato N, Leopold PL, Crystal RG. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oro AE, Higgins KM. Develop Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 23.Tang QQ, Lane MD. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine C, Cousin W, Plaisant M, et al. Stem Cells. 2012;26:1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- 25.Plikus MV, Mayer JA, de la Cruz D, et al. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P, Kling RE, Ravuri SK, et al. J Tissue Eng. 2014;5:1–10. doi: 10.1177/2041731414556850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingham PW, Nakano Y, Seger C. Nature Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 28.Briscoe J, Thérond PP. Nature Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, McMahon AP, Allen BL. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Willnow TE, Hilpert J, Armstrong SA, et al. Proc Natl Acad Sci USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adly MA. Histochem Cell Biol. 2010;134:591–602. doi: 10.1007/s00418-010-0763-1. [DOI] [PubMed] [Google Scholar]
- 32.Cabezas F, Lagos J, Céspedes C, et al. PloS One. 2011;6:e16794. doi: 10.1371/journal.pone.0016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson SC, Eberl M, Vagnozzi AN, et al. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang JY, Xiao TZ, Oda Y, et al. Cancer Preven Res. 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda S, Kitagishi Y. Cancers. 2013;5:1261–1270. doi: 10.3390/cancers5041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narvaez CJ, Simmons KM, Brunton J, et al. J Cell Physiol. 2013;228:2024–2036. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- 37.Sakai Y, Kishimoto J, Demay MB. J Clin Invest. 2001;107:961–966. doi: 10.1172/JCI11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung H, Kim HH, Lee DH, et al. Cytotechnol. 2011;63:57–66. doi: 10.1007/s10616-010-9327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derynck R, Akhurst RJ. Nature Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 40.Foitzik K, Lindner G, Mueller-Roever S, et al. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 41.Hibino T, Nishiyama T. J Dermatol Sci. 2004;35:9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Tan CK, McFarlane C, et al. Am J Physiol Cell Physiol. 2012;302:C1213–C1225. doi: 10.1152/ajpcell.00179.2011. [DOI] [PubMed] [Google Scholar]
- 43.Richardson RL, Wright JT, Kim JW, et al. Growth Dev Aging. 1992;56:149–157. [PubMed] [Google Scholar]
- 44.Kim WS, Park SH, Ahn SJ, et al. Biol Pharm Bull. 2008;31:606–610. doi: 10.1248/bpb.31.606. [DOI] [PubMed] [Google Scholar]
- 45.Fain JN, Tichansky DS, Madan AK. Metabolism. 2005;54:1546–1551. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Ansell DM, Kloepper JE, Thomason HA, et al. J Invest Dermatol. 2011;131:518–528. doi: 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- 47.Spalding KL, Arner E, Westermark PO, et al. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 48.Crandall DL, Busler DE, McHendry-Rinde B, et al. J Clin Endocrinol Metab. 2000;85:2609–2614. doi: 10.1210/jcem.85.7.6678. [DOI] [PubMed] [Google Scholar]
- 49.Omatsu-Kanbe M, Inoue K, Fujii Y, et al. Biochem J. 2006;393:171–180. doi: 10.1042/BJ20051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang QA, Tao C, Gupta RK, Scherer PE. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang QA, Scherer PE. Adipocyte. 2014;3:146–150. doi: 10.4161/adip.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]