Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of Metabolic Syndrome (MetS). As the prevalence of obesity and MetS increases, the number of NAFLD patients will increase. The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) which is characterized by fat accumulation, inflammation, and hepatocyte ballooning. While simple steatosis is more likely considered as a non-progressive entity, NASH is a progressive disease which can result in significant hepatic fibrosis, cirrhosis; and, poses significant risk for hepatocellular carcinoma (HCC) development. Recent published data suggest that nearly one-fourth of HCCs result as a consequence of NAFLD (1). Finally, mortality and morbidity of NASH are significantly higher than simple steatosis. Importantly, patients with the diagnosis of NAFLD have a higher risk for cardiovascular mortality than patients who do not carry the diagnosis (2). Although investigators have learned a significant amount on the platform of the ‘two-hit hypothesis’, important molecular mechanisms to account for NAFLD progression remain elusive.
In this issue of HEPATOLOGY, Kwon, et al, report a novel molecular mechanism with therapeutic potential targeting hedgehog (HH) signaling for NAFLD (Kwon et al). HH proteins were initially identified in association with embryonic development. In Drosphilia, mutated HH gene develop “hedgehog”-like spiky process and denticles on their dorsal surface, which is how the HH molecular pathway got its name (3). Mammalian cells express three HH ligands, Sonic HH (Shh), Indian HH, and Desert HH. The HH ligands bind to Patched receptors (Ptch). In the absence of ligand, Ptch represses the signal transduction of its co-receptor Smoothened (Smo). Upon Shh binding to Ptch, the inhibitory effect on Smo is abolished which leads to the activation of Smo. Activated Smo signaling induces nuclear-translocation of transcription factor Glioma-associated oncogene (Gli1, Gli2, Gli3) proteins, resulting in the induction of HH-target genes, such as Ptch and Gli, to control cell proliferation, migration, differentiation, and survival.
In adult healthy liver, HH signaling is barely active, but is reactivated to induce a regenerative response when the liver is injured and inflamed. Hepatic HH signaling has been implicated in hepatic progenitor cell fate, liver development, liver regeneration, and liver fibrosis. In liver fibrosis, HH signaling is activated in hepatic stellate cells (HSCs) and induces trans-differentiation of quiescent HSCs into activated myofibroblasts (4). In NAFLD patients, the hepatic expression of Shh is known to be increased, and the ballooned hepatocytes, a histological hallmark of human NASH, are the major source of Shh (5). Increased expression of Shh is associated with severity of hepatocyte ballooning and liver fibrosis stage in NASH patients (5). It is conceivable the activation of the Shh pathway may be a mechanism of triggering NASH progression. Moreover, NASH patients treated with vitamin E in the PIVENS trial showed improved NASH severity accompanied by reduced numbers of ballooned hepatocytes as well as Shh-expressing cells (6). Taken together previous studies suggest Shh expression could be used as a biomarker to evaluate effectiveness of NASH therapy (7). The molecular mechanisms how Shh signaling pathway contributes to NAFLD progression and whether targeting Shh pathway can be effective therapies for NAFLD remain elusive.
The present study highlights a crucial role of HH signaling in hepatocytes in NAFLD pathogenesis. High fat diet (HFD)-induced NAFLD elevated hepatic expression of HH target genes Gli1, Ptch1, and Smo, were attenuated when hepatocyte Smo was ablated (Kwon et al) (Fig. 1). Further the investigators demonstrate hepatocyte HH signaling is required for osteopontin expression through Smo and Gli1 activation; that osteopontin induces liver macrophage infiltration (Kwon et al) (Fig. 1). However, Smo ablation in hepatocytes did not attenuate hepatic lipid accumulation, elevated liver triglyceride levels, or serum transaminase levels, or the overall NAFLD activity score (NAS). These data reveal only a minor contribution of hepatocyte HH signaling in lipid metabolism and hepatocyte injury. Rather, cell-cell crosstalk between hepatocytes and other liver non-parenchymal cells is suggested. This hypothesis is supported by reduced hepatic and serum lipid levels when HH signaling was pharmacologically inhibited by systemic administration of two Smo inhibitors LDE225 (erismodegib) or GDC-0449 (vismodegib). In turn Smo inhibition also suppressed liver osteopontin expression and macrophage infiltration. Although the present study did not account for exactly how the number of liver macrophages is increased, hepatocyte-derived osteopontin may induce the recruitment of bone-marrow-derived monocytes into the liver rather than proliferation of resident Kupffer cells.
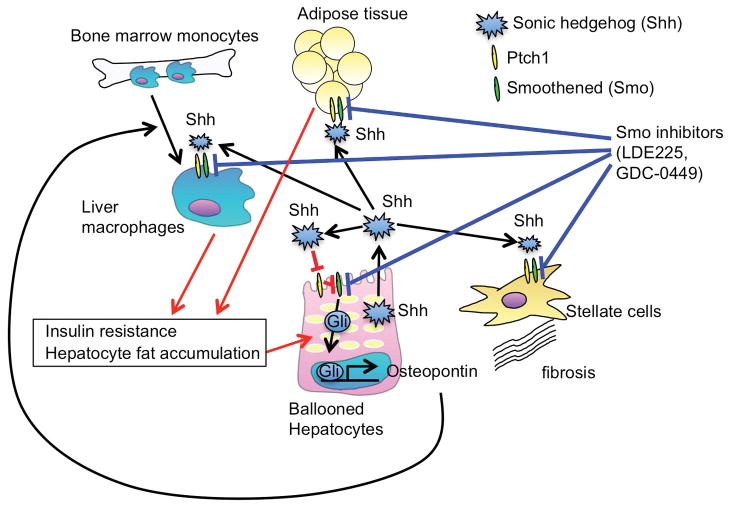
Figure 1. Activation of hedgehog signaling in hepatocytes promotes NAFLD development.
Sonic hedgehog (Shh) binds to Ptch1, which abolishes the inhibitory effect of Smoothened (Smo) on hedgehog signaling, resulting in activation of hedgehog signaling and transcription factor Gli. In NAFLD, ballooned hepatocyte-derived Shh stimulates hedgehog signaling in hepatocytes, which produce osteopontin to induce liver macrophage accumulation and NAFLD development. Hedgehog signaling is also activated in hepatic stellate cells, macrophages, and adipose tissues, which promotes NAFLD development and insulin resistance. Blocking hedgehog signaling systemically by the administration of Smo inhibitors suppress the progression of NAFLD and insulin resistance.
In addition to the reduction of hepatic and serum lipid levels, Smo inhibitors also improved systemic insulin resistance assessed by glucose tolerance test, but the improvement of insulin resistance was not seen in hepatocyte specific Smo knockout mice. This suggests the HH signaling in adipose tissue may play a pivotal role in the development of insulin resistance and it is also possible that HH signaling in liver non-parenchymal cells affect hepatocyte insulin resistance through intercellular crosstalk (Fig. 1). Additional studies are needed to dissect the role of HH signaling in adipose tissues and hepatic non-parenchymal cells in systemic and hepatic insulin resistance, respectively.
Unfortunately, the effect of inhibition of HH signaling on liver fibrosis, a cardinal feature of advanced NASH, was not tested in the present study because of the limitation of the currently available animal models of NAFLD that cannot entirely recapitulate the pathophysiology of human NASH. For example, HFD induces fatty liver, weight gain, and insulin resistance, but liver inflammation is very mild and fibrosis is not developed. Methionine-choline deficient diet, commonly used for NASH study, does show NASH histology but no insulin resistance. Choline-deficient amino acid defined diet feeding induces fibrosis along with weight gain and mild insulin resistance. Future studies may require to use several animal model approaches to fully understand mechanisms of NAFLD pathogenesis.
The present study demonstrates to link HH hepatocyte signal transduction with NAFLD by production of osteopontin and subsequent recruitment of liver macrophages. Unfortunately, the present study did not provide sufficient evidence that treatment with Smo inhibitors, as well as the modification of HH signaling in hepatocytes could attenuate NASH as indicated by no reduction in liver enzyme levels and NAS. However, The treatment strategy using HH inhibition is still very important since it may serve as an effective way to treat simple steatosis and prevent NAFLD-related cardiovascular risk and other MetS. Additional work to more clearly define the role of HH ligands and signals in NAFLD disease progression is in order.
Acknowledgments
Financial support:
This study was supported by NIH grant R01AA02172, R01DK085252, P42 ES010337.
List of Abbreviations
- Gli
Glioma-associated oncogene
- HCC
hepatocellular carcinoma
- HFD
High fat diet
- HH
hedgehog
- HSCs
hepatic stellate cells
- MetS
metabolic Syndrome
- NAFLD
non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
non-alcoholic steatohepatitis
- Ptch
Patched receptors
- Shh
Sonic HH
- Smo
Smoothened
Footnotes
Conflicts of interest: There is no conflict of interest to disclose for all authors.
References
- 1.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015 doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandan A, Ang B, Bettencourt R, Hooker J, Behling C, Lin GY, Valasek MA, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:1513–1520. e1511. doi: 10.1016/j.cgh.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294:G595–598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. e1311–1311. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy CD, Suzuki A, Abdelmalek MF, Burchette JL, Diehl AM, Nash CRN. Treatment response in the PIVENS trial is associated with decreased Hedgehog pathway activity. Hepatology. 2015;61:98–107. doi: 10.1002/hep.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]