Abstract
Recent advances have uncovered a previously unknown function of vitamin C in epigenetic regulation. Vitamin C exists predominantly as an ascorbate anion under physiological pH conditions. Ascorbate was discovered as a cofactor for methylcytosine dioxygenases that are responsible for DNA demethylation, and also as a likely cofactor for some JmjC domain-containing histone demethylases that catalyze histone demethylation. Variation in ascorbate bioavailability thus can influence the demethylation of both DNA and histone, further leading to different phenotypic presentations. Ascorbate deficiency can be presented systematically, spatially and temporally in different tissues at the different stages of development and aging. Here, we review how ascorbate deficiency could potentially be involved in embryonic and postnatal development, and plays a role in various diseases such as neurodegeneration and cancer through epigenetic dysregulation.
Keywords: Epigenetics, Vitamin C, Methylcytosine dioxygenase, DNA methylation, JmjC domain-containing histone demethylases, Histone methylation, Scurvy
Introduction
The focal function of vitamin C (l-ascorbic acid) is the essential role that it plays in collagen crosslinking. Severe vitamin C deficiency can cause scurvy due to incomplete collagen crosslinking [41, 152, 159]. Besides other known functions, recent discoveries of vitamin C in epigenetic regulations, specifically promoting the demethylation of DNA and histone, are poised to revolutionize our understanding of this often overlooked vitamin in health and diseases. One recent review has provided insights with respect to the regulation of the epigenome by vitamin C [166]. This review particularly focuses on the potential roles of vitamin C in health and diseases from the perspectives of epigenetic regulation.
Vitamin C exists predominantly as ascorbate anion under physiological pH conditions. From here on we will be discussing the role of vitamin C in terms of ascorbate, not the disassociated proton. All mammals, except high primates, guinea pigs and fruit bats, synthesize ascorbate de novo in the liver. The loss of de novo ascorbate synthesis is due to a mutant and nonfunctional l-gulonolactone oxidase (Gulo), the enzyme catalyzing the last step of ascorbate biosynthesis. For high primates, guinea pigs and fruit bats, ascorbate is a vitamin that needs to be supplied via diet and/or dietary supplements.
Ascorbate, derived from either dietary sources or the liver, enters cells primarily through sodium-dependent vitamin C transporters (SVCTs). The high-capacity, low-affinity SVCT1 is primarily responsible for ascorbate absorption and reabsorption in intestinal and renal epithelial cells. In contrast, the low-capacity, high-affinity SVCT2 distributes ascorbate to most tissues and is expressed more ubiquitously [158]. As a general reducer, ascorbate can be oxidized to dehydroascorbic acid (DHA) by two rounds of single electron donation to other oxidants such as oxygen free radicals, peroxides, and superoxide [96]. DHA is no longer able to pass through SVCTs, but rather, enters cells through glucose transporters (GLUTs). Once inside the cell, DHA can be rapidly reduced back to ascorbate. In a similar manner, ascorbate must leave the cells in the form of DHA through GLUTs. In the plasma of healthy humans, the reduced form of ascorbate is dominant, and DHA is at a very low level [76, 87], suggesting that most cells take up and accumulate ascorbate primarily through SVCTs. However, it is also known that DHA, not ascorbate, can pass through the blood–brain barrier and blood–retina barrier to enter into the brain and the retina, respectively [54, 105].
In addition to being a general antioxidant, ascorbate serves as a cofactor for a list of monooxygenases and dioxygenases. Ascorbate-dependent monooxygenases contain dopamine β-hydroxylase and peptidyl-glycine α-amidating monooxygenase, which require Cu2+ as a cofactor and ascorbate as another cofactor (electron donor) [111]. On the other hand, ascorbate-dependent dioxygenases include collagen prolyl 4-hydroxylase (P4H), collagen lysyl hydroxylases, transmembrane P4H, asparaginyl hydroxylase and other enzymes, which utilize Fe2+ as a cofactor, 2-oxoglutarate (2OG, also known as α-ketoglutarate) as a co-substrate, and require ascorbate as another cofactor (electron donor) for full catalytic activity [95].
The mechanistic role of ascorbate in these enzymes is exemplified in collagen P4H and its involvement in scurvy. In the absence of ascorbate, the initial hydroxylation catalyzed by collagen P4H can proceed at a maximal rate. However, during this process the conversion of reduced iron (Fe2+) to catalytically inactive oxidized iron species (mainly Fe3+) soon results in the inactivation of collagen P4H, leading to an incomplete hydroxylation of residues in collagen, which in turn causes incomplete crosslinking and eventually the characteristic signs of scurvy [40]. When available, ascorbate has the capacity to reduce Fe3+ to catalytically active Fe2+. Thus, ascorbate repletion assists collagen P4H to complete the collagen hydroxylation, effectively curing and preventing scurvy.
By serving as a cofactor for these enzymes, the availability of ascorbate influences some important biological functions such as catecholamine synthesis, collagen crosslinking and hypoxia-induced factor-α degradation. Recent progress in the epigenetics field identified a number of Fe2+ and 2OG-dependent dioxygenases, which catalyze the epigenetic modifications of DNA and histone. Some of them may also require ascorbate to maintain their catalytic activities [102]. Therefore, the availability of ascorbate also has an influence on the epigenome, which in turn has an impact on health and diseases.
Demethylation of DNA and histone
The epigenome constitutes the interface of a dynamic environment and the genome. Identifying enzymes and essential cofactors that catalyze epigenetic modifications is the key to understanding molecular connections between the epigenome and the environment. Methylation at the C5 position of cytosine (5-methylcytosine, 5mC) is the major covalent modification of mammalian DNA and plays essential roles in regulating transcription and maintaining genome stability and cellular identity [125]. Although 5mC is relatively stable, it can be lost by dilution via a lack of maintenance during DNA replication, which would result in passive demethylation [9]. It remained largely unclear whether and how the methyl group in 5mC could be actively removed, i.e., active demethylation, until only a few years ago.
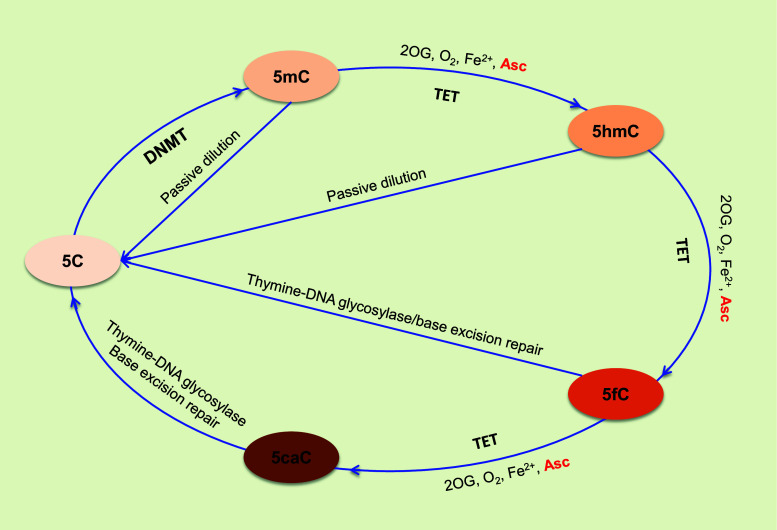
A group of enzymes termed methylcytosine dioxygenase ten-eleven translocation (TET, including TET1, TET2 and TET3) was identified to catalyze the hydroxylation of 5mC to 5-hydroxymethylcytosine (5hmC) [63, 80, 138]. TETs can further oxidize 5hmC to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) [64]. In addition to the passive dilution of 5hmC during DNA replication, both 5fC and 5caC could be excised by the DNA repair enzyme thymine DNA glycosylase to produce an abasic position, which is eventually replaced by an unmodified C, thus completing the process of DNA active demethylation (Fig. 1) [51, 92]. Although it involves multiple steps, the TET-mediated cascade oxidation in combination with base excision repair constitutes one of the most important and consistent pathways responsible for the active demethylation of DNA.
Fig. 1.
Ascorbate and DNA demethylation. The methylation of an unmodified 5C could be established by DNMT1. 5mC can also be passively diluted by the failed maintenance during DNA synthesis. Ascorbate promotes the TET catalyzed cascade oxidation of 5mC, to 5hmC, to 5fC and to 5caC. Unable to maintain 5hmC in the newly synthesized DNA leads to the passive dilution of 5hmC. Both 5fC and 5caC could be excised by the DNA repair enzyme thymine DNA glycosylase to produce an abasic position, which is eventually replaced by an unmodified C, thus completing the process of DNA active demethylation and a cycle of DNA methylation-demethylation
It has been shown that 5hmC is relatively stable with a unique distribution pattern in the genome, while 5fC and 5caC are rare in the genome [129, 133]. 5hmC recruits very different sets of binding proteins compared to 5mC [99, 134, 164]. Therefore, in addition to being a DNA demethylation intermediate, 5hmC also serves as an epigenetic mark with unique regulatory functions.
TETs belong to the Fe2+ and 2OG-dependent dioxygenase superfamily. Several lines of evidence have demonstrated that, like collagen P4H, the catalytic activity of TETs is dependent on Fe2+ and 2OG. For instance, introducing mutations at the iron-binding sites in TETs, as well as supplementation of 2-hydroxyglutarate (2HG), a competitive inhibitor of 2-oxoglutarate, suppresses their catalytic activity in converting 5mC to 5hmC [63, 138, 162, 163]. Elevated levels of 2HG and reduced levels of 5hmC have been found in multiple cancers with gain of function mutations in the isocitrate dehydrogenase (IDH) [162]. These mutant IDHs aberrantly produce 2HG instead of 2OG [78, 136]. Further, recombinant TETs require both Fe2+ and 2OG to hydroxylate 5mC to 5hmC in test tubes to initiate the conversion of 5mC to 5hmC [63, 138], which strongly supports the idea that 2OG and Fe2+ act as co-substrate and cofactor, respectively. The requirement for ascorbate as an additional cofactor for P4H and other dioxygenases suggests a potential role for this reducing cofactor in TET-mediated DNA demethylation. However, initial in vitro enzymatic analysis suggested that ascorbate was not essential for TET-mediated hydroxylation of 5mC [138].
Initial observations in the Wang lab showed that ascorbate enhanced 5hmC generation in cultured cells, most likely by acting as a cofactor for TET to hydroxylate 5mC [26, 101]. This new function of ascorbate was initially discovered in mouse embryonic fibroblasts (MEF) that expressed TETs at low, but detectable levels [27, 73]. Thus, MEFs constituted a convenient and useful tool to analyze TET enzymatic requirements in a cell-based experimental setting. Interestingly, standard cell culture media usually lack ascorbate in their formula. However, when ascorbate is available, it can enter into cells mainly via SVCT2 transporters. The content of 5hmC was extremely low in MEFs cultured in ascorbate-free medium. Addition of ascorbate dose- and time-dependently enhanced the generation of 5hmC [101]. Treatment with another reducer, glutathione, did not change the level of 5hmC. Blocking the entry of ascorbate into cells and knocking down TETs expression by short interference RNAs significantly inhibited the effect of ascorbate on 5hmC. The effect of ascorbate did not involve an increased expression of TET or IDH, the enzymes responsible for producing 2OG. Furthermore, the effect of ascorbate on 5hmC was also independent of the cellular uptake of iron [26]. It is known that ascorbate has the capacity to reduce Fe3+ to catalytically active Fe2+ for TETs, as it does for collagen P4H. Taken together, these results indicate that ascorbate promotes TETs to catalyze the hydroxylation of 5mC to 5hmC, most likely as a cofactor of TETs. The promotion of 5hmC by ascorbate is not limited to MEF, but ubiquitous to all cell types being tested.
Subsequently, the effect of ascorbate on DNA demethylation was also reported by multiple groups in other experimental settings such as embryonic stem cells, induced pluripotent stem cells (iPSC) and Gulo knockout (Gulo −/−) mice [10, 20, 165]. Blaschke et al. showed that ascorbate (l-ascorbic acid 2-phosphate), but not other antioxidants, enhanced the activity of recombinant TET1 in an in vitro assay. The ascorbate-induced conversion of 5mC to 5hmC was further demonstrated on certain chromosomal regions in cultured embryonic stem (ES) cells by genome-wide high-throughput sequencing. The ascorbate-induced changes in 5hmC and 5mC were entirely suppressed in TET1 and TET2 double knockout ES cells [10]. Liu et al. found that ascorbate (ascorbic acid) could directly enhance the catalytic activity of TET dioxygenases to oxidize 5mC by uniquely interacting with the C-terminal catalytic domain of TETs. Ascorbate was shown to significantly increase the levels of all 5mC oxidation products, particularly 5fC and 5caC. The effect of ascorbate on 5mC oxidation was further validated in vivo in Gulo −/− mice [165]. Chen et al. reported that TET1 promoted somatic cell reprogramming independent of mesenchymal-to-epithelial transition (MET) in the absence of ascorbate. When ascorbate was available, TET1 regulated 5hmC formation at loci critical for MET in an ascorbate-dependent fashion [20]. With the evidence provided by each of these four groups, a previously unknown function of ascorbate in modulating the epigenetic control of genome activity has thus been uncovered.
Besides cytosine methylation in DNA, lysine and arginine residues in histones can also be methylated in the chromatin. Histone methylation is another key component of the epigenome, which is closely associated with either the activation or silencing of transcription [67]. There are two groups of histone demethylases: (1) lysine-specific histone demethylases (LSD1 and LSD2) that can demethylate mono- and di-methylated lysine residues in histones; (2) JmjC domain-containing histone demethylases that can demethylate mono-, di-, and trimethylated histone lysine/arginine residues [71, 147]. So far, about 20 proteins that belong to the JmjC domain-containing histone demethylase family have been discovered to have the catalytic capacity to demethylate histones [102]. JmjC domain-containing histone demethylases, like TETs, belong to the Fe2+ and 2OG-dependent dioxygenase superfamily. For the first time, the Zhang group reported that ascorbate is required for optimal catalytic activity of JHDM1; additionally, the demethylation mediated by JHDM3A was halted when ascorbate was withdrawn from an in vitro assay [71, 147]. The promoting effect of ascorbate on histone demethylation was further verified in the transition from pre-iPSC phase to fully reprogrammed iPSC [21, 156]. These studies suggest that ascorbate could be a cofactor for some JmjC domain-containing histone demethylase family, thus modulating histone demethylation in a similar way as it does in the case of DNA demethylation.
To date, our understanding is that ascorbate acts as a cofactor role in DNA demethylation catalyzed by TETs and in histone demethylation mediated by some JmjC domain-containing histone demethylases by regenerating the catalytically active Fe2+. Deficiency of ascorbate, especially in the nucleus, may not be able to meet the requirement of TETs or some JmjC domain-containing histone demethylases. This will disrupt the methylation-demethylation dynamics of DNA and histone, which can subsequently contribute to phenotypic alterations or even diseases. Furthermore, the expression of these Fe2+ and 2OG-dependent dioxygenases such as TETs shows spatial–temporal dynamics in the body throughout development and aging. How to adjust diet and dietary supplementation of ascorbate to meet the needs of these enzymes and to avoid unnecessary epigenomic alterations to stay healthy remains a question yet unanswered.
Variation in ascorbate availability
The availability of ascorbate to TETs and some JmjC domain-containing histone demethylases in the nucleus is now considered critical in maintaining the epigenome. Because of the abundance of ascorbate in certain fruits and vegetables, and the comparably low price of ascorbate supplementation, there has been little attention paid to the implications of ascorbate deficiency, which is often defined as the critical level at which the signs of scurvy begin to appear. Although the current incidence of scurvy is relatively low, instances of scurvy are still reported in the industrialized countries [106]. In the USA, more than 7 % of the population (>20 million individuals) is estimated to be deficient in ascorbate (concentrations <11.4 μM in the plasma) [123]. Marginal ascorbate deficiency was defined as a plasma concentration below 23 μM, which has been estimated to affect about 10 % of adults in the industrialized world [150]. Furthermore, the turnover rate of ascorbate appears to be quite rapid [131]. In Gulo −/− mice, which like humans cannot synthesize endogenous ascorbate, the concentration of ascorbate in most tissues, except for the brain, steadily decreases to near zero within approximately 1 week after the withdrawal of dietary ascorbate [153]. If humans have a similar turnover rate, it may help explain the relatively high prevalence of ascorbate deficiency in humans.
Certain life styles, such as smoking and alcohol consumption, have significant impacts on ascorbate availability. Cigarette smoking has been shown to strongly reduce ascorbate levels in the plasma [88, 120]. Chronic alcoholic individuals also have lower levels of ascorbate, possibly due to the alcohol-enhanced excretion of ascorbate or its metabolites in the urine and low dietary ascorbate [34]. Furthermore, economically low-income populations have a much higher risk of ascorbate deficiency, estimated at 25 % incidence for men and 16 % for women [103]. People with clinical conditions that affect the absorption and reabsorption of ascorbate are also at risk of deficiency. These include digestive diseases such as Crohn’s disease, ulcerative colitis, and chronic kidney diseases [13, 25]. Patients undergoing dialysis, due to a kidney failure, have an even higher prevalence of ascorbate deficiency.
Genetic variations in ascorbate transporters also influence the availability of ascorbate. It is known that SVCT1 is responsible for the absorption and reabsorption of ascorbate. In the dbSNP database, there are about 60 non-synonymous single nucleotide polymorphisms (SNP) in the SLC23A1 gene (encoding SVCT1), all of which are rare in the population [minor allele frequency (MAF) <0.5 %]. Emerging evidence indicates that some of these variants influence the level of circulating ascorbate. For example, one study suggests that SNP rs35817838 (amino acid change M258V) causes approximately 75 % decline in human plasma ascorbate regardless of ascorbate intake (up to 2.5 g/day), while SNPs rs33972313 (V264M), rs34521685 (I218V), and synonymous rs6886922 (I60I) decrease 40–50 % of plasma ascorbate [24]. A meta-analysis of five studies with over 15,000 participants shows that SNP rs33972313 is associated with reduced circulating ascorbate [144]. Furthermore, genotype GG at intronic SNP rs4257763 is correlated with a reduced serum ascorbate level [15]. Genotype CC at another intronic SNP rs6596473 is associated with a lower level of ascorbate in aqueous humor, but not plasma [127].
SVCT2 is known to distribute ascorbate to most types of tissues. There are 40 non-synonymous SNPs in the SLC23A2 gene (encoding SVCT2) in the dbSNP database. The impact of these variants on SVCT2 function remains largely unclear. However, one study shows that genotype TT at an intronic SNP rs12479919 is associated with a reduced level of ascorbate in the lens of the eye [127]. Although the functional analysis of the genetic variation in SLC23A1 and SLC23A2 is not complete, individuals carrying certain genetic variants could have a higher risk of ascorbate deficiency [47].
Ascorbate deficiency can be presented systematically, spatially and temporally in different tissues or cells. Intracellular ascorbate, or more accurately nuclear ascorbate, refers to the ascorbate available to Fe2+ and 2OG-dependent dioxygenases in the nucleus. However, the ascorbate level in the cell nucleus remains largely unknown because a majority of published studies have conveniently measured ascorbate levels in serum or plasma. Currently, the recommended dietary allowances by the Institute of Medicine of the USA are 90 mg for adult males and 75 mg for adult females [62]. It remains unclear whether these amounts of ascorbate can meet the needs of TETs and some JmjC domain-containing histone demethylase in different tissues along different developmental and aging stages. Moreover, it is expected that once ascorbate fulfills the cofactor epigenetic enzymatic requirements, any further increase would not exert additional enhancement on these enzymatic activities.
Epigenetic regulation of ascorbate in embryonic development
Epigenetic reprogramming is critical to embryonic development. Two rounds of DNA demethylation–remethylation happen at early stages of mammalian embryonic development. It is now known that both TET-mediated oxidation and passive dilution could participate in these demethylation processes. Immediately after fertilization of an oocyte, 5mC in the paternal chromatin is rapidly replaced by 5hmC via TET3-mediated oxidation as shown initially. 5hmC cannot be maintained during the rapid DNA replication in pre-implanted embryos, leading to passive demethylation and the erase of most of the paternal 5mC patterns [61]. The demethylation of the maternal chromatin, though occurring slightly later, appears to be also mediated by TET3 from zygote to four-cell embryos [155]. Later reports suggest that other pathways such as TET3-independent passive demethylation during DNA replication might play an even bigger role in DNA demethylation of pre-implanted embryos [45, 108, 128]. A second round of epigenetic reprogramming happens in primordial germ cells (PGC), which is also involved in TET2/3-mediated active demethylation [74]. At these stages, TETs are expressed at a much higher level [139], suggesting that a significant amount of ascorbate is required to meet the need of TETs. If the available ascorbate cannot satisfy the needs of TETs, the programmed embryonic development could be disrupted by an incomplete DNA demethylation, which could potentially lead to birth defects. The function of ascorbate as a cofactor is expected during these developmental steps. In fact, ascorbate is needed for demethylation in the DNA of embryonic stem cells and is needed in the induction of TET1 and TET2 dependent DNA demethylation in stem cells [10, 22]. Further, ascorbate has been shown to maintain the methylation pattern and expression of the Dlk1–Dio3 imprinting region in embryonic stem cells [37, 135]. Overall, the current data indicate that ascorbate is essential for the global DNA demethylation in early embryonic stages. The importance of the correct DNA methylation patterns during development has been highlighted by recent publications showing that maternal or paternal nutrition has an impact on the methylation status of the offspring [28, 66, 82, 145]. Furthermore, histone demethylation mediated by JmjC domain-containing histone demethylases is also critical to embryonic development [68, 84, 130, 154, 157]. Thus, it is necessary to take consideration of dietary ascorbate consumption and supplementation during the peri-pregnancy period.
Variations in ascorbate availability, due to genetic factors or an insufficient intake during pregnancy, could affect the embryonic development by changing the catalytic activity of TETs. One genetic study showed that certain variations in SVCT1 and SVCT2 conferred the risk of spontaneous preterm birth [31]. Ascorbate deficiency has been linked to certain types of developmental defects. For example, women at a high risk for neural tube defect (NTD) recurrence tend to have lower leucocyte ascorbate levels compared with low-risk women [12, 124]. Lower intake of ascorbate also increases the risk of gastroschisis in infants, which is a congenital defect of the abdominal wall [146].
Plasma ascorbate levels, however, often decrease throughout the pregnancy [16]. The prevalence of ascorbate deficiency (<22.7 μM) can be as high as 30.8 % in pregnant women during the later pregnancy terms [90]. The maternal deficiency in ascorbate leads to fetal ascorbate deficiency, which could disrupt prenatal development [121], suggesting that ascorbate supplementation might be necessary for pregnant women. It is worth noting that ascorbate supplementation in pregnant women should be aimed at satisfying the needs of TETs and other Fe2+ and 2OG-dependent dioxygenases in both the mother and the fetus. In the case of sufficient dietary ascorbate intake, higher doses of ascorbate may not necessarily exert additional benefits [110]. Currently, it is not clear how much ascorbate is really needed from diet and/or supplements to completely satisfy the requirement of TETs in the prevention of potential embryonic defects caused by inappropriate DNA demethylation. Due to the fact that ascorbate consumption from dietary sources is almost impossible to control quantitatively in human subjects, a recent review of seven studies concluded that ascorbate supplementation does not exert obvious benefits to prevent preterm birth [137]. No significant difference was found in neonatal outcomes from the women supplemented with ascorbate in other studies [29].
Contrary to the inconsistent studies of human subject, results from animal studies suggest that ascorbate is essential to embryonic development. Maternal ascorbate deficiency in guinea pigs (which have no endogenous production of ascorbate) during pregnancy resulted in lower fetal levels of plasma ascorbate, and reduced fetal body weight and brain weight [122]. Insufficient ascorbic acid intake during the gestation of Gulo −/− mice (also have no endogenous ascorbate production) resulted in neonatal lethality [70]. These mice had abnormal cardiac dilation, congestion of the liver and lungs and incompletely expanded pulmonary alveoli, suggesting that the epigenetic regulation of ascorbate could be one of the pathways critical to normal tissue development [70]. Ascorbate improves the development of embryos produced by somatic cell nuclear transfer [58, 59, 69, 93]. Additionally, ascorbate has been shown to improve the development of the blastocyst in in vitro fertilization [55, 65, 116, 140]. The exact mechanism by which ascorbate improves embryo development in vitro was assumed to be due to its antioxidant properties [43, 86]. However, it is possible that an epigenetic role of ascorbate in the in vitro embryo development has been missed.
Overall, ascorbate could be essential to embryonic development by maintaining the catalytic activity of TETs and some of the JmjC domain-containing histone demethylases, especially during the epigenetic reprograming at early embryonic stages. However, the amount of daily ascorbate intake that is required prenatally to aid in the prevention of possible birth defects remains unknown.
Epigenetic regulation of ascorbate in postnatal development
Ascorbate deficiency may affect the postnatal development of various organs and tissue types through the aforementioned epigenetic pathways. Here we will discuss the impact of ascorbate deficiency on neurogenesis and neuronal myelination based on published results. Breast milk or formula milk is the major source of ascorbate for newborn babies. The level of ascorbate in breast milk is reduced if breastfeeding women consume insufficient ascorbate, and also declines as lactation progresses [8]. Ascorbate content in formula is often higher than breast milk, however, no ascorbate exists in cow’s milk unless artificially added [30]. Therefore, there is a risk of ascorbate deficiency in newborns if the primary food source is cow’s milk or breast milk produced by an individual with deficient ascorbate levels.
Animal studies suggest that deficient ascorbate may affect biogenesis of certain cell types during development, a process largely controlled by epigenetic events. For the newborn guinea pigs (6–7 days old), insufficient supply of ascorbate (though adequate to prevent scurvy) for 2 months caused a reduced number of neurons in the hippocampus and spatial memory deficits [149]. Consistent with this finding, maternal ascorbate deficiency during pregnancy also persistently impaired hippocampal neurogenesis in the offspring of guinea pigs [151]. It is likely that disrupted demethylation of DNA and histone underlie the impaired neurogenesis in the hippocampus.
Myelination of certain peripheral nerves by Schwann cells is critical for proper neural functioning. The myelin sheaths encircle axons to provide metabolic support and allow rapid nerve conduction. At embryonic stages, neural crest progenitor cells differentiate to Schwann cell precursors to immature Schwann cells. Then, in the postnatal stages, pre-myelinating Schwann cells appear and further differentiate to myelinating Schwann cells, which wrap individual nerve fibers to form unique myelin sheath, or differentiate to non-myelin forming Remak Schwann cells [35].
A potential role of ascorbate in myelination has long been proposed, primarily because of its function in collagen crosslinking and the synthesis of other basement membrane components [98]. In the 1980s, ascorbate (50 μg/ml) was identified to fully restore the myelination of axons in vitro by Schwann cells due to serum withdrawal [14]. Schwann cells would be arrested in a basal-lamina-free pre-myelination stage if serum and ascorbate were deprived from the medium [160]. In the defined medium used for co-culture of neurons and Schwann cells, myelin is not formed if ascorbate is absent from the medium [107]. In contrast, myelin formation by olfactory ensheathing cells is not dependent on ascorbate [6], suggesting the specificity of the requirement of ascorbate for the myelin formation by Schwann cells. However, how ascorbate mechanistically regulates the Schwann cell-mediated myelination remained largely unclear until only a few of years ago. An animal model study with reduced levels of an ascorbate transporter provided a breakthrough in the field.
Ascorbate enters and accumulates in Schwann cells primarily via SVCT2 [38]. Haploinsufficiency of the SLC23A2 gene (encoding the SVCT2 protein) caused a lower protein level of SVCT2, which resulted in deficient ascorbate within Schwann cells and other cells, but not in extracellular milieu. In SLC23A2 +/− mice, the myelin layer of sciatic nerve fibers was thinner and the nerve conduction velocity was also reduced compared to the wild type mice [39]. This suggests that the intracellular ascorbate deficiency, rather than the extracellular ascorbate deficiency, affects the process of myelination by Schwann cells.
The transformation of cell identity from precursors, to pre-myelinating Schwann cells, and to myelinating Schwann cells is largely controlled by the epigenome [109, 117]. Although intracellular ascorbate may exert multiple functions, from the available knowledge, it is likely that the demethylation of DNA and histone, which is regulated by the intracellular ascorbate, plays a key role in Schwann cell-mediated myelination. Future studies may provide experimental evidence on how ascorbate impacts the epigenome and regulates proliferation, differentiation, and myelin formation of Schwann cells.
Epigenetic regulation of ascorbate in aging
Both human and animal studies have shown a correlation between the declining ascorbate levels in tissues and the process of aging [100]. Multiple mechanisms could be involved in the age-related ascorbate decline including increased usage, accelerated turnover, decreased absorption/reabsorption, and reduced cellular uptake. For example, ascorbate level declines ~50 % in leukocytes in individuals at age 85 and older compared to those at age 60 [5]. Distinct from peripheral tissues, ascorbate crosses the blood–brain barrier in the form of DHA through GLUTs, which are expressed in endothelial cells [1]. Upon uptake by the neurons and glial cells in the brain, DHA can be converted to ascorbate. Although there are little available data on ascorbate in human brains, one early study shows that ascorbate level in the cerebral cortex is decreased 77 % from individuals at age 80 and older, compared to individuals at age 50 and younger [119]. Thus, if no additional ascorbate is provided or its uptake is not improved, there would be persistent ascorbate decline in aged brains, which may cause phenotypic changes such as neurodegeneration. Studies have examined the potential role of ascorbate in neurodegenerative diseases from the oxidative stress angle [7]. Emerging evidence indicates that dysregulation in 5hmC, which can be caused by ascorbate shortage in the brain, could contribute to the age-related neurodegenerative diseases [3].
Increased age is one primary risk factor for Parkinson’s disease (PD), which is characterized by the progressive loss of dopaminergic neurons in the substantia nigra [114]. It is not plasma ascorbate, but cellular ascorbate that may be associated with PD. One study shows that lymphocyte ascorbate levels in patients with severe PD are significantly lower compared with those at less severe stages [60]. Although the status of ascorbate in the midbrain affected by PD remains unclear, it is possible that the change could be similar to the change that occurs in lymphocytes. In the midbrain of SLC23A2 +/− mouse embryos, the number of dopaminergic neurons is decreased. Ascorbate supplementation greatly enhances the differentiation of midbrain derived neural stem cell toward dopaminergic neurons, which is correlated with TET-mediated 5hmC generation and Jmjd3 catalyzed loss of H3K27m3 [52]. Thus, it appears that ascorbate plays a role in dopaminergic neuron differentiation. It is reasonable to deduce that the failure to maintain the epigenetic signature of dopaminergic neurons (higher 5hmC and lower H3K27m3) due to the age-related ascorbate decline in the midbrain, could lead to a shift in cell identity and eventually contribute to the development of PD.
Alzheimer’s disease (AD) is another common neurodegenerative disorder, which has an increased incidence with aging [36]. Like PD, there is a lack of data on the status of ascorbate in human brain regions that are critical to AD pathogenesis. However, many studies suggest that maintaining healthy ascorbate levels can have a protective function against AD [49]. Findings on the relationship between 5hmC and AD are not consistent in published studies, most of which use immunohistochemistry, a crude method for measuring global 5hmC content. For example, levels of TET1, 5mC, and 5hmC are increased whereas levels of 5fC and 5caC are decreased in the hippocampus gyrus of AD subjects [11]. Levels of 5mC and 5hmC are low in astrocytes and microglia, but high in the neurons of the hippocampus gyrus in human AD brains [23]. A significant decrease in global 5hmC is reported in entorhinal cortex and cerebellum of AD patients [23], which, however, has not been replicated by another group [83]. Overall, the available data of DNA demethylation from human brain studies are inconsistent. Future studies may uncover the potential impact of the age-related ascorbate decline on the epigenome and AD pathogenesis.
Epigenetic regulation of ascorbate in cancer
The TET-mediated DNA active demethylation appears to be downregulated in most, if not all, types of human cancer [81]. A low level, or even loss, of 5hmC has been recognized as a novel epigenetic hallmark of cancer [85]. The major known mechanisms for the loss of 5hmC in cancer are the following. (1) Mutations in TETs; for example TET2 mutations, which are likely loss of function, impair 5hmC generation in myeloid cancers [72]. (2) Mutations in IDHs; instead of producing 2OG, the mutant IDH produces 2HG, which competes with 2OG for TETs and results in the reduction of 5hmC and DNA hypermethylation in cancers [118, 148, 162]. (3) Deficient expression of TETs or IDHs, which can reduce 5hmC generation in cancers [85, 104]. Ascorbate, if deficient, may also affect the enzymatic activity of TETs and further lead to 5hmC reduction.
Observational studies have correlated the occurrence of scurvy and cancer. One group found a higher incidence of scurvy in cancer patients in a clinic [33]. Subclinical scurvy is also observed in patients with different malignant diseases [79]. However, some reports show a role of ascorbate in enhancing the risk for certain cancers [4]. The reasons for the mixed results of ascorbate in the risk of cancer could be the following: (1) it is difficult, if not impossible, to control dietary ascorbate quantitatively in human subjects. Ascorbate supplements in treatment groups can easily be confounded by the consumption of ascorbate-rich fruits and vegetables in control/placebo groups; (2) most studies examined ascorbate supplements by questionnaire or other indirect self-report methods, but did not verify the effects of ascorbate levels in related tissues or serum directly; (3) the effect of ascorbate can be complicated by other antioxidants or micronutrients. Recent meta-analyses confirmed the benefits of ascorbate in breast cancer treatment. One meta-analysis of published studies (n = 40) indicates that higher plasma level of ascorbate is associated with reduced breast cancer risk [57]. Thus, an inverse association between ascorbate and the risk of breast cancer seems to exist.
Genetic studies indicate an association between ascorbate transporters and cancer. Variations in SVCT1 and SVCT2 have been associated with the risk of certain types of cancers including advanced colorectal adenoma [32], muscle-invasive bladder cancer [44], gastric cancer [161] and non-Hodgkin lymphoma [132]. Furthermore, recurrent mutations in the splicing factor SF3B1 have been identified in chronic lymphocytic leukemia, uveal melanoma, as well as other cancers [113]. The mutant SF3B1 causes a truncated, most likely nonfunctional, SVCT2 that can result in intracellular deficiency of ascorbate in cancer cells [113]. These studies suggest that variation in ascorbate availability due to altered transporters could contribute to the pathogenesis of cancer.
There is a long controversial history of ascorbate as a treatment for cancer. Possibly due to the fact that it is nearly impossible to quantitatively control dietary ascorbate consumption in human subjects, results from clinical trials have been inconsistent [94]. However, a recent meta-analysis of published studies clearly demonstrated benefits of vitamin C supplement in reducing the mortality caused by breast cancer in over 17,000 patients studied [48]. Furthermore, animal studies show an obvious benefit of ascorbate in cancer treatment. Depletion of ascorbate increased the growth and metastasis of murine melanoma xenografts in Gulo −/− mice, while supplementation of ascorbate inhibited the growth and metastasis of murine melanoma xenografts [17, 18]. These preliminary studies suggest that an ascorbate supplement could be included as adjuvant therapy for melanoma.
It has been shown that the overexpression of TET1 in breast cancer and the overexpression TET2 in melanoma could partially reestablish a normal 5hmC profile in these cancer cells and decrease malignancy, especially invasiveness [56, 85]. These findings suggest that a means of rebuilding the 5hmC content could offer a potential treatment for these cancers. However, it might not be feasible to clinically overexpress TET or IDH in patients. As a cofactor for TETs, ascorbate enhances, and possibly maximizes, the catalytic activity of the existing TETs in cancer cells. It is likely that some of the beneficial effects of ascorbate in treating melanoma xenografts in Gulo −/− mice are mediated by the induction of 5hmC generation in cancer cells. One recent study shows that ascorbate treatment increases 5hmC content in cultured melanoma cells, while decreasing their malignancy [46]. Future studies should assess whether ascorbate treatment can aid in the reprogramming of cancer cells by promoting the activity of TETs and some JmjC domain-containing histone demethylases.
Epigenetic regulation of ascorbate in Scurvy
Although scurvy is a forgotten disease, due to its rare occurrence in modern times, case reports of scurvy do appear periodically [2]. The pathogenic mechanism of scurvy involves mainly these aspects: (1) the hydroxylation and crosslinking of procollagen catalyzed by P4H and lysyl hydroxylase, and (2) the dramatically decreased transcription of procollagen, which has been considered to be the major effect of ascorbate deficiency [97]. The level of type IV collagen mRNA was decreased to ~50 % of the normal level in blood vessels of ascorbate deficient guinea pigs [91]. Further, in vitro culture experiments clearly supported that ascorbate was one key factor in promoting de novo collagen synthesis [19, 50, 53, 89, 112]. The role of ascorbate as a cofactor for P4H and lysyl hydroxylase to complete the procollagen hydroxylation and crosslinking is well elucidated. However, the mechanism by which ascorbate enhances the transcription of collagen genes remains largely unclear.
Overwhelming evidence has demonstrated that DNA hypermethylation, specifically the ones at the promoter regions, inhibits the transcription of various types of collagen in different cells [42, 75, 115, 126, 143]. The transcription of collagens is activated after CpG sites at its promoters have been demethylated [167]. Furthermore, inhibition of H3K27 trimethylation increases the expression of collagen [77]. It is reasonable to hypothesize that ascorbate deficiency causes the hypermethylation in the promoter regions due to the failure of promoter demethylation of DNA and histone catalyzed by TETs and some JmjC domain-containing histone demethylase, which inhibits the transcription of collagens and further leads to scurvy. We can predict that any animal model with a loss of either one or a combination of TET enzymes and JmjC domain-containing histone demethylases will have a deficiency in collagen production and will be more sensitive to ascorbate depletion. In support of this hypothesis, it has been shown there is a TET1-mediated increase in 5hmC near the transcription start site and gene body of activated collagen genes in chondrocytes [142]. In TET1 knockout mice, the expression of collagen is decreased during bone development [141]. In this regard, scurvy could also be considered as an epigenetic disease; thus, some of its clinical manifestation could be cured and prevented epigenetically by ascorbate treatment.
Conclusions
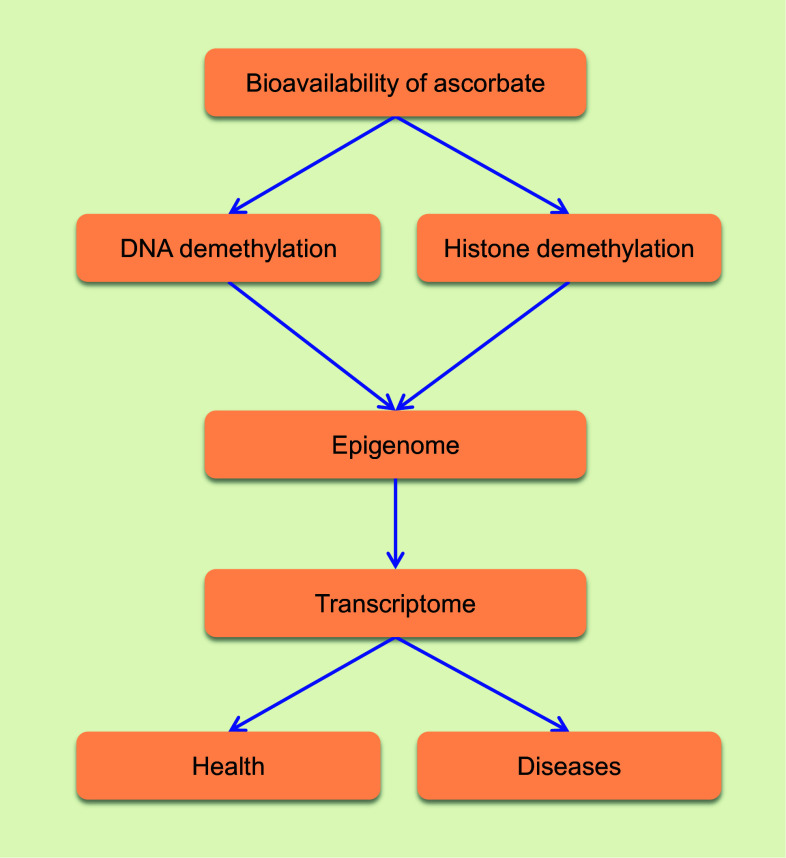
Recent progress in the epigenetics field indicates that ascorbate could play a critical role in the demethylation of DNA and histone. Although exclusive evidence is still missing, results obtained from in vitro and in vivo experiments support that ascorbate is likely serving as a cofactor for TET and some JmjC domain-containing histone demethylases by regenerating the catalytically active Fe2+. Ascorbate, if deficient, may disrupt the methylation-demethylation dynamics of DNA and histone, which can consequently contribute to phenotypic alterations. In humans, ascorbate deficiency can be presented systematically, spatially and temporally in different tissues or cells along the developmental stages and aging. By regulating the epigenome, ascorbate can be involved in embryonic development, postnatal development, aging, cancer and other diseases (Fig. 2). Future systematic studies may help uncover the epigenetic role of ascorbate in human health and diseases.
Fig. 2.
The epigenetic role of vitamin C in health and disease. The bioavailability of ascorbate influences health and diseases by regulating the demethylation of DNA and histone, further the transcriptome
Acknowledgments
We thank Lena Dennison and Sushmita Mustafi for their assistance. We apologize to our colleagues whose works were not able to cite in this review due to the space limitation. The work on the epigenetic regulation of vitamin C in the Wang lab is supported by Grants from the National Institutes of Health (R01NS089525, R21CA191668) and a James and Esther King Biomedical Research Award (3KN08).
References
- 1.Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akikusa JD, Garrick D, Nash MC. Scurvy: forgotten but not gone. J Paediatr Child Health. 2003;39:75–77. doi: 10.1046/j.1440-1754.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mahdawi S, Virmouni SA, Pook MA (2014) The emerging role of 5-hydroxymethylcytosine in neurodegenerative diseases. Front Neurosci 8:397 [DOI] [PMC free article] [PubMed]
- 4.Anthony HM, Schorah CJ. Severe hypovitaminosis C in lung-cancer patients: the utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br J Cancer. 1982;46:354–367. doi: 10.1038/bjc.1982.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attwood EC, Robey E, Kramer JJ, Ovenden N, Snape S, Ross J, Bradley F. A survey of the haematological, nutritional and biochemical state of the rural elderly with particular reference to vitamin C. Age Ageing. 1978;7:46–56. doi: 10.1093/ageing/7.1.46. [DOI] [PubMed] [Google Scholar]
- 6.Babiarz J, Kane-Goldsmith N, Basak S, Liu K, Young W, Grumet M. Juvenile and adult olfactory ensheathing cells bundle and myelinate dorsal root ganglion axons in culture. Exp Neurol. 2011;229:72–79. doi: 10.1016/j.expneurol.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 8.Bates CJ, Prentice A. Breast milk as a source of vitamins, essential minerals and trace elements. Pharmacol Ther. 1994;62:193–220. doi: 10.1016/0163-7258(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 9.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, Lorincz MC, Ramalho-Santos M. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley-Whitman MA, Lovell MA. Epigenetic changes in the progression of Alzheimer’s disease. Mech Ageing Dev. 2013;134:486–495. doi: 10.1016/j.mad.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brender JD, Werler MM, Kelley KE, Vuong AM, Shinde MU, Zheng Q, Huber JC, Jr, Sharkey JR, Griesenbeck JS, Romitti PA, Langlois PH, Suarez L, Canfield MA, The National Birth Defects Prevention, Study Nitrosatable drug exposure during early pregnancy and neural tube defects in offspring: national birth defects prevention study. Am J Epidemiol. 2011;174:1286–1295. doi: 10.1093/aje/kwr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buffinton GD, Doe WF. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic Res. 1995;22:131–143. doi: 10.3109/10715769509147535. [DOI] [PubMed] [Google Scholar]
- 14.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 15.Cahill LE, El-Sohemy A. Vitamin C transporter gene polymorphisms, dietary vitamin C and serum ascorbic acid. J Nutrigenet Nutrigenom. 2009;2:292–301. doi: 10.1159/000314597. [DOI] [PubMed] [Google Scholar]
- 16.Casanueva E, Ripoll C, Tolentino M, Morales RM, Pfeffer F, Vilchis P, Vadillo-Ortega F. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: a randomized trial. Am J Clin Nutr. 2005;81:859–863. doi: 10.1093/ajcn/81.4.859. [DOI] [PubMed] [Google Scholar]
- 17.Cha J, Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013;42:55–64. doi: 10.3892/ijo.2012.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha J, Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Ascorbate depletion increases growth and metastasis of melanoma cells in vitamin C deficient mice. Exp Oncol. 2011;33:226–230. [PubMed] [Google Scholar]
- 19.Chan D, Lamande SR, Cole WG, Bateman JF. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem J. 1990;269:175–181. doi: 10.1042/bj2690175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Guo L, Zhang L, Wu H, Yang J, Liu H, Wang X, Hu X, Gu T, Zhou Z, Liu J, Liu J, Wu H, Mao SQ, Mo K, Li Y, Lai K, Qi J, Yao H, Pan G, Xu GL, Pei D. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet. 2013;45:1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Liu H, Liu J, Qi J, Wei B, Yang J, Liang H, Chen Y, Chen J, Wu Y, Guo L, Zhu J, Zhao X, Peng T, Zhang Y, Chen S, Li X, Li D, Wang T, Pei D. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat Genet. 2013;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- 22.Chung TL, Brena RM, Kolle G, Grimmond SM, Berman BP, Laird PW, Pera MF, Wolvetang EJ. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells. 2010;28:1848–1855. doi: 10.1002/stem.493. [DOI] [PubMed] [Google Scholar]
- 23.Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging. 2014;35:1334–1344. doi: 10.1016/j.neurobiolaging.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deicher R, Horl WH. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press Res. 2003;26:100–106. doi: 10.1159/000070991. [DOI] [PubMed] [Google Scholar]
- 26.Dickson KM, Gustafson CB, Young JI, Zuchner S, Wang G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem Biophys Res Commun. 2013;439:522–527. doi: 10.1016/j.bbrc.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, Levine RL, Nik S, Chen EI, Abeliovich A. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJ, Swan GE, Zeisel SH, Innis SM, Waterland RA, Prentice AM, Hennig BJ. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dror DK, Allen LH (2012) Interventions with vitamins B6, B12 and C in pregnancy. Paediatr Perinat Epidemiol 26(Suppl 1):55–74 [DOI] [PubMed]
- 30.Elisia I, Kitts DD. Differences in vitamin E and C profile between infant formula and human milk and relative susceptibility to lipid oxidation. Int J Vitam Nutr Res. 2013;83:311–319. doi: 10.1024/0300-9831/a000173. [DOI] [PubMed] [Google Scholar]
- 31.Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, Siega-Riz AM, Olshan AF, Chanock SJ. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163:245–254. doi: 10.1093/aje/kwj035. [DOI] [PubMed] [Google Scholar]
- 32.Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, Levine M, Hayes RB, Chanock S. Genetic variation in sodium-dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer. 2008;60:652–659. doi: 10.1080/01635580802033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fain O, Mathieu E, Thomas M. Scurvy in patients with cancer. BMJ. 1998;316:1661–1662. doi: 10.1136/bmj.316.7145.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faizallah R, Morris AI, Krasner N, Walker RJ. Alcohol enhances vitamin C excretion in the urine. Alcohol Alcohol. 1986;21:81–84. [PubMed] [Google Scholar]
- 35.Feltri ML, Poitelon Y, Previtali SC (2015) How schwann cells sort axons: new concepts. Neuroscientist. pii:1073858415572361 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 36.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen project. Stockholm Neurol. 1997;48:132–138. doi: 10.1212/WNL.48.1.132. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Han Z, Li Q, Wu Y, Shi X, Ai Z, Du J, Li W, Guo Z, Zhang Y. Vitamin C induces a pluripotent state in mouse embryonic stem cells by modulating microRNA expression. FEBS J. 2015;282:685–699. doi: 10.1111/febs.13173. [DOI] [PubMed] [Google Scholar]
- 38.Gess B, Lohmann C, Halfter H, Young P. Sodium-dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of l-ascorbic acid into Schwann cells. Glia. 2010;58:287–299. doi: 10.1002/glia.20923. [DOI] [PubMed] [Google Scholar]
- 39.Gess B, Rohr D, Fledrich R, Sereda MW, Kleffner I, Humberg A, Nowitzki J, Strecker JK, Halfter H, Young P. Sodium-dependent vitamin C transporter 2 deficiency causes hypomyelination and extracellular matrix defects in the peripheral nervous system. J Neurosci. 2011;31:17180–17192. doi: 10.1523/JNEUROSCI.3457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould BS, Woessner JF. Biosynthesis of collagen; the influence of ascorbic acid on the proline, hydroxyproline, glycine, and collagen content of regenerating guinea pig skin. J Biol Chem. 1957;226:289–300. [PubMed] [Google Scholar]
- 42.Guenette DK, Ritzenthaler JD, Foley J, Jackson JD, Smith BD (1992) DNA methylation inhibits transcription of procollagen alpha 2(I) promoters. Biochem J 283(Pt 3):699–703 [DOI] [PMC free article] [PubMed]
- 43.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 44.Guey LT, Garcia-Closas M, Murta-Nascimento C, Lloreta J, Palencia L, Kogevinas M, Rothman N, Vellalta G, Calle ML, Marenne G, Tardon A, Carrato A, Garcia-Closas R, Serra C, Silverman DT, Chanock S, Real FX, Malats N, EPICURO/Spanish Bladder Cancer Study investigators Genetic susceptibility to distinct bladder cancer subphenotypes. Eur Urol. 2010;57:283–292. doi: 10.1016/j.eururo.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo F, Li X, Liang D, Li T, Zhu P, Guo H, Wu X, Wen L, Gu TP, Hu B, Walsh CP, Li J, Tang F, Xu GL. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell. 2014;15:447–458. doi: 10.1016/j.stem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Gustafson CB, Yang C, Dickson KM, Shao H, Van Booven D, Harbour JW, Liu ZJ, Wang G (2015) Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin Epigenet 7:51-015-0087-z. eCollection 2015 [DOI] [PMC free article] [PubMed]
- 47.Handelman GJ. Vitamin C deficiency in dialysis patients—are we perceiving the tip of an iceberg? Nephrol Dial Transpl. 2007;22:328–331. doi: 10.1093/ndt/gfl534. [DOI] [PubMed] [Google Scholar]
- 48.Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223–1231. doi: 10.1016/j.ejca.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J Alzheimers Dis. 2012;29:711–726. doi: 10.3233/JAD-2012-111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hata R, Senoo H. l-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- 51.He XJ, Chen T, Zhu JK. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011;21:442–465. doi: 10.1038/cr.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He X, Kim M, Kim S, Yi S, Rhee Y, Kim T, Lee E, Park C, Dixit S, Harrison FE, Lee S. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1- and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33:1320–1332. doi: 10.1002/stem.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hering TM, Kollar J, Huynh TD, Varelas JB, Sandell LJ. Modulation of extracellular matrix gene expression in bovine high-density chondrocyte cultures by ascorbic acid and enzymatic resuspension. Arch Biochem Biophys. 1994;314:90–98. doi: 10.1006/abbi.1994.1415. [DOI] [PubMed] [Google Scholar]
- 54.Hosoya K, Minamizono A, Katayama K, Terasaki T, Tomi M. Vitamin C transport in oxidized form across the rat blood-retinal barrier. Invest Ophthalmol Vis Sci. 2004;45:1232–1239. doi: 10.1167/iovs.03-0505. [DOI] [PubMed] [Google Scholar]
- 55.Hossein MS, Hashem MA, Jeong YW, Lee MS, Kim S, Kim JH, Koo OJ, Park SM, Lee EG, Park SW, Kang SK, Lee BC, Hwang WS. Temporal effects of α-tocopherol and l-ascorbic acid on in vitro fertilized porcine embryo development. Anim Reprod Sci. 2007;100:107–117. doi: 10.1016/j.anireprosci.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Hsu CH, Peng KL, Kang ML, Chen YR, Yang YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, Huang HD, Lu YY, Teng YC, Lin ST, Lin RK, Tang FM, Lee SB, Hsu HM, Yu JC, Hsiao PW, Juan LJ (2012) TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep 2:568–579 [DOI] [PubMed]
- 57.Hu F, Wu Z, Li G, Teng C, Liu Y, Wang F, Zhao Y, Pang D. The plasma level of retinol, vitamins A, C and alpha-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. J Cancer Res Clin Oncol. 2015;141:601–614. doi: 10.1007/s00432-014-1852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu J, Cheng D, Gao X, Bao J, Ma X, Wang H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod Domest Anim. 2012;47:873–879. doi: 10.1111/j.1439-0531.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y, Tang X, Xie W, Zhou Y, Li D, Zhou Y, Zhu J, Yuan T, Lai L, Pang D, Ouyang H. Vitamin C enhances in vitro and in vivo development of porcine somatic cell nuclear transfer embryos. Biochem Biophys Res Commun. 2011;411:397–401. doi: 10.1016/j.bbrc.2011.06.160. [DOI] [PubMed] [Google Scholar]
- 60.Ide K, Yamada H, Umegaki K, Mizuno K, Kawakami N, Hagiwara Y, Matsumoto M, Yoshida H, Kim K, Shiosaki E, Yokochi T, Harada K. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition. 2015;31:406–408. doi: 10.1016/j.nut.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science. 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academies Press (US), Washington, DC. ISBN-10: 0-309-06949-1, ISBN-10: 0-309-06935-1 [PubMed]
- 63.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong YW, Park SW, Hossein MS, Kim S, Kim JH, Lee SH, Kang SK, Lee BC, Hwang WS. Antiapoptotic and embryotrophic effects of alpha-tocopherol and l-ascorbic acid on porcine embryos derived from in vitro fertilization and somatic cell nuclear transfer. Theriogenology. 2006;66:2104–2112. doi: 10.1016/j.theriogenology.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Justin N, De Marco V, Aasland R, Gamblin SJ. Reading, writing and editing methylated lysines on histone tails: new insights from recent structural studies. Curr Opin Struct Biol. 2010;20:730–738. doi: 10.1016/j.sbi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Kamikawa YF, Donohoe ME. Histone demethylation maintains Prdm14 and Tsix expression and represses xIst in embryonic stem cells. PLoS One. 2015;10:e0125626. doi: 10.1371/journal.pone.0125626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J Reprod Dev. 2013;59:78–84. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kishimoto Y, Kanai T, Sato K, Lee J, Jeong K, Shimokado K, Maruyama N, Ishigami A. 2013) Insufficient ascorbic acid intake during gestation induces abnormal cardiac dilation in fetal and neonatal SMP30/GNL knockout mice. Pediatr Res. 2013;73:578–584. doi: 10.1038/pr.2013.22. [DOI] [PubMed] [Google Scholar]
- 71.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 72.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, Liu XS, Aravind L, Agarwal S, Maciejewski JP, Rao A. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kopp MU, Winterhalter KH, Trueb B. DNA methylation accounts for the inhibition of collagen VI expression in transformed fibroblasts. Eur J Biochem. 1997;249:489–496. doi: 10.1111/j.1432-1033.1997.00489.x. [DOI] [PubMed] [Google Scholar]
- 76.Koshiishi I, Mamura Y, Liu J, Imanari T. Evaluation of an acidic deproteinization for the measurement of ascorbate and dehydroascorbate in plasma samples. Clin Chem. 1998;44:863–868. [PubMed] [Google Scholar]
- 77.Kramer M, Dees C, Huang J, Schlottmann I, Palumbo-Zerr K, Zerr P, Gelse K, Beyer C, Distler A, Marquez VE, Distler O, Schett G, Distler JH. Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Ann Rheum Dis. 2013;72:614–620. doi: 10.1136/annrheumdis-2012-201615. [DOI] [PubMed] [Google Scholar]
- 78.Kranendijk M, Salomons GS, Gibson KM, Van Schaftingen E, Jakobs C, Struys EA. A lymphoblast model for IDH2 gain-of-function activity in d-2-hydroxyglutaric aciduria type II: novel avenues for biochemical and therapeutic studies. Biochim Biophys Acta. 2011;1812:1380–1384. doi: 10.1016/j.bbadis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Krasner N, Dymock IW. Ascorbic acid deficiency in malignant diseases: a clinical and biochemical study. Br J Cancer. 1974;30:142–145. doi: 10.1038/bjc.1974.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kroeze LI, van der Reijden BA, Jansen JH. 5-Hydroxymethylcytosine: an epigenetic mark frequently deregulated in cancer. Biochim Biophys Acta. 2015;1855:144–154. doi: 10.1016/j.bbcan.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lashley T, Gami P, Valizadeh N, Li A, Revesz T, Balazs R. Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2014;41(4):497–506. doi: 10.1111/nan.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, Wang HY, Chepelev I, Zhu Q, Wei G, Zhao K, Wang RF. Stage-dependent and locus-specific role of histone demethylase Jumonji D3 (JMJD3) in the embryonic stages of lung development. PLoS Genet. 2014;10:e1004524. doi: 10.1371/journal.pgen.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC, Jr, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol Reprod. 1995;52:262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- 87.Lykkesfeldt J. Ascorbate and dehydroascorbic acid as reliable biomarkers of oxidative stress: analytical reproducibility and long-term stability of plasma samples subjected to acidic deproteinization. Cancer Epidemiol Biomark Prev. 2007;16:2513–2516. doi: 10.1158/1055-9965.EPI-07-0639. [DOI] [PubMed] [Google Scholar]
- 88.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71:530–536. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- 89.Lyons BL, Schwarz RI. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984;12:2569–2579. doi: 10.1093/nar/12.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madruga de Oliveira A, Rondo PH, Mastroeni SS, Oliveira JM. Plasma concentrations of ascorbic acid in parturients from a hospital in Southeast Brazil. Clin Nutr. 2008;27:228–232. doi: 10.1016/j.clnu.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 91.Mahmoodian F, Peterkofsky B. Vitamin C deficiency in guinea pigs differentially affects the expression of type IV collagen, laminin, and elastin in blood vessels. J Nutr. 1999;129:83–91. doi: 10.1093/jn/129.1.83. [DOI] [PubMed] [Google Scholar]
- 92.Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem. 2011;286:35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mallol A, Santalo J, Ibanez E. Improved development of somatic cell cloned mouse embryos by vitamin C and latrunculin A. PLoS One. 2015;10:e0120033. doi: 10.1371/journal.pone.0120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer. 2011;63:479–494. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 95.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci. 1998;3:d1–d10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- 97.May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19:2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGarvey ML, Baron-Van Evercooren A, Kleinman HK, Dubois-Dalcq M. Synthesis and effects of basement membrane components in cultured rat Schwann cells. Dev Biol. 1984;105:18–28. doi: 10.1016/0012-1606(84)90257-4. [DOI] [PubMed] [Google Scholar]
- 99.Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michels AJ, Hagen TM. Vitamin C status decline with age. In: Asard H, May J, Smirnoff N, editors. Vitamin C: its function and biochemistry in animals and plants. Abingdon: Garland Science/BIOS Scientific Publishers; 2004. pp. 203–228. [Google Scholar]
- 101.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monfort A, Wutz A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013;14:337–346. doi: 10.1038/embor.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mosdol A, Erens B, Brunner EJ. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population . J Public Health (Oxf) 2008;30:456–460. doi: 10.1093/pubmed/fdn076. [DOI] [PubMed] [Google Scholar]
- 104.Muller T, Gessi M, Waha A, Isselstein LJ, Luxen D, Freihoff D, Freihoff J, Becker A, Simon M, Hammes J, Denkhaus D, zur Muhlen A, Pietsch T, Waha A (2012) Nuclear exclusion of TET1 is associated with loss of 5-hydroxymethylcytosine in IDH1 wild-type gliomas. Am J Pathol 181:675–683 [DOI] [PubMed]
- 105.Nualart F, Mack L, Garcia A, Cisternas P, Bongarzone ER, Heitzer M, Jara N, Martinez F, Ferrada L, Espinoza F, Baeza V, Salazar K (2014) Vitamin C transporters, recycling and the bystander effect in the nervous system: SVCT2 versus gluts. J Stem Cell Res Ther 4:209 [DOI] [PMC free article] [PubMed]
- 106.Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK. Scurvy: a disease almost forgotten. Int J Dermatol. 2006;45:909–913. doi: 10.1111/j.1365-4632.2006.02844.x. [DOI] [PubMed] [Google Scholar]
- 107.Olsen CL, Bunge RP. Requisites for growth and myelination of urodele sensory neurons in tissue culture. J Exp Zool. 1986;238:373–384. doi: 10.1002/jez.1402380310. [DOI] [PubMed] [Google Scholar]
- 108.Peat JR, Dean W, Clark SJ, Krueger F, Smallwood SA, Ficz G, Kim JK, Marioni JC, Hore TA, Reik W. Genome-wide bisulfite sequencing in zygotes identifies demethylation targets and maps the contribution of TET3 oxidation. Cell Rep. 2014;9:1990–2000. doi: 10.1016/j.celrep.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–134. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, Vitamins in Pre-eclampsia (VIP) Trial Consortium Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 111.Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol Life Sci. 2000;57:1236–1259. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quaglino D, Fornieri C, Botti B, Davidson JM, Pasquali-Ronchetti I. Opposing effects of ascorbate on collagen and elastin deposition in the neonatal rat aorta. Eur J Cell Biol. 1991;54:18–26. [PubMed] [Google Scholar]
- 113.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, Ramsay AJ, Bea S, Pinyol M, Martinez-Trillos A, Lopez-Guerra M, Colomer D, Navarro A, Baumann T, Aymerich M, Rozman M, Delgado J, Gine E, Hernandez JM, Gonzalez-Diaz M, Puente DA, Velasco G, Freije JM, Tubio JM, Royo R, Gelpi JL, Orozco M, Pisano DG, Zamora J, Vazquez M, Valencia A, Himmelbauer H, Bayes M, Heath S, Gut M, Gut I, Estivill X, Lopez-Guillermo A, Puente XS, Campo E, Lopez-Otin C. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2011;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 114.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rhodes K, Rippe RA, Umezawa A, Nehls M, Brenner DA, Breindl M. DNA methylation represses the murine alpha 1(I) collagen promoter by an indirect mechanism. Mol Cell Biol. 1994;14:5950–5960. doi: 10.1128/MCB.14.9.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saikhun K, Faisaikarm T, Ming Z, Lu KH, Kitiyanant Y. α-Tocopherol and l-ascorbic acid increase the in vitro development of IVM/IVF swamp buffalo (Bubalus bubalis) embryos. Animal. 2008;2:1486–1490. doi: 10.1017/S1751731108002541. [DOI] [PubMed] [Google Scholar]
- 117.Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. 2015;7:a020529. doi: 10.1101/cshperspect.a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, Harris IS, Holmes R, Wakeham A, Haight J, You-Ten A, Li WY, Schalm S, Su SM, Virtanen C, Reifenberger G, Ohashi PS, Barber DL, Figueroa ME, Melnick A, Zuniga-Pflucker JC, Mak TW. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaus R. The ascorbic acid content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am J Clin Nutr. 1957;5:39–41. doi: 10.1093/ajcn/5.1.39. [DOI] [PubMed] [Google Scholar]
- 120.Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158–162. doi: 10.2105/AJPH.79.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schjoldager JG, Tveden-Nyborg P, Lykkesfeldt J. Prolonged maternal vitamin C deficiency overrides preferential fetal ascorbate transport but does not influence perinatal survival in guinea pigs. Br J Nutr. 2013;110:1573–1579. doi: 10.1017/S0007114513000913. [DOI] [PubMed] [Google Scholar]
- 122.Schjoldager JG, Paidi MD, Lindblad MM, Birck MM, Kjærgaard AB, Dantzer V, Lykkesfeldt J, Tveden-Nyborg P. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur J Nutr. 2014;54(4):667–676. doi: 10.1007/s00394-014-0809-6. [DOI] [PubMed] [Google Scholar]
- 123.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 national health and nutrition examination survey (NHANES) Am J Clin Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 124.Schorah CJ, Wild J, Hartley R, Sheppard S, Smithells RW. The effect of periconceptional supplementation on blood vitamin concentrations in women at recurrence risk for neural tube defect. Br J Nutr. 1983;49:203–211. doi: 10.1079/BJN19830026. [DOI] [PubMed] [Google Scholar]
- 125.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 126.Sengupta PK, Smith BD. Methylation in the initiation region of the first exon suppresses collagen pro-alpha2(I) gene transcription. Biochim Biophys Acta. 1998;1443:75–89. doi: 10.1016/S0167-4781(98)00188-2. [DOI] [PubMed] [Google Scholar]
- 127.Senthilkumari S, Talwar B, Dharmalingam K, Ravindran RD, Jayanthi R, Sundaresan P, Saravanan C, Young IS, Dangour AD, Fletcher AE. Polymorphisms in sodium-dependent vitamin C transporter genes and plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp Eye Res. 2014;124:24–30. doi: 10.1016/j.exer.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 128.Shen L, Inoue A, He J, Liu Y, Lu F, Zhang Y. Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem Cell. 2014;15:459–470. doi: 10.1016/j.stem.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shen L, Wu H, Diep D, Yamaguchi S, D’Alessio AC, Fung HL, Zhang K, Zhang Y. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shpargel KB, Sengoku T, Yokoyama S, Magnuson T. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012;8:e1002964. doi: 10.1371/journal.pgen.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simpson GLW, Ortwerth BJ. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochimica et Biophysica Acta (BBA) Mol Basis Dis. 2000;1501:12–24. doi: 10.1016/S0925-4439(00)00009-0. [DOI] [PubMed] [Google Scholar]
- 132.Skibola CF, Bracci PM, Halperin E, Nieters A, Hubbard A, Paynter RA, Skibola DR, Agana L, Becker N, Tressler P, Forrest MS, Sankararaman S, Conde L, Holly EA, Smith MT. Polymorphisms in the estrogen receptor 1 and vitamin C and matrix metalloproteinase gene families are associated with susceptibility to lymphoma. PLoS One. 2008;3:e2816. doi: 10.1371/journal.pone.0002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, Street C, Li Y, Poidevin M, Wu H, Gao J, Liu P, Li L, Xu GL, Jin P, He C. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell. 2013;153:678–691. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, Munzel M, Wagner M, Muller M, Khan F, Eberl HC, Mensinga A, Brinkman AB, Lephikov K, Muller U, Walter J, Boelens R, van Ingen H, Leonhardt H, Carell T, Vermeulen M. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SS, Kim SY, Bestor TH, Shioda T, Park PJ, Hochedlinger K. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44(398–405):S1–S2. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Struys EA, Jansen EE, Verhoeven NM, Jakobs C. Measurement of urinary d- and l-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-l-tartaric anhydride. Clin Chem. 2004;50:1391–1395. doi: 10.1373/clinchem.2004.033399. [DOI] [PubMed] [Google Scholar]
- 137.Swaney P, Thorp J, Allen I. Vitamin C supplementation in pregnancy—does it decrease rates of preterm birth? A systematic review. Am J Perinatol. 2014;31:91–98. doi: 10.1055/s-0033-1338171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tao Y, Zhou B, Xia G, Wang F, Wu Z, Fu M. Exposure to l-ascorbic acid or alpha-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim. 2004;39:52–57. doi: 10.1046/j.1439-0531.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 141.Taylor S, Rath M, Bhutani N (2015) Loss Of Tet1 impairs endochondral ossification in the embryonic growth plate. In: Poster 402 presented at: the 2015 orthopaedic research society’s annual meeting in Las Vegas Nevada
- 142.Taylor SE, Li YH, Wong WH, Bhutani N. Genome-wide mapping of DNA hydroxymethylation in osteoarthritic chondrocytes. Arthritis Rheumatol. 2015;67:2129–2140. doi: 10.1002/art.39179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson JP, Simkevich CP, Holness MA, Kang AH, Raghow R. In vitro methylation of the promoter and enhancer of Pro alpha 1(I) collagen gene leads to its transcriptional inactivation. J Biol Chem. 1991;266:2549–2556. [PubMed] [Google Scholar]
- 144.Timpson NJ, Forouhi NG, Brion MJ, Harbord RM, Cook DG, Johnson P, McConnachie A, Morris RW, Rodriguez S, Luan J, Ebrahim S, Padmanabhan S, Watt G, Bruckdorfer KR, Wareham NJ, Whincup PH, Chanock S, Sattar N, Lawlor DA, Davey Smith G (2010) Genetic variation at the SLC23A1 locus is associated with circulating concentrations of l-ascorbic acid (vitamin C): evidence from 5 independent studies with >15,000 participants. Am J Clin Nutr 92:375–382 [DOI] [PMC free article] [PubMed]
- 145.Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Muller F, van Zwet EW, Bock C, Meissner A, Lumey LH, Eline Slagboom P, Heijmans BT (2014) DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun 5:5592 [DOI] [PMC free article] [PubMed]
- 146.Torfs CP, Lam PK, Schaffer DM, Brand RJ. Association between mothers’ nutrient intake and their offspring’s risk of gastroschisis. Teratology. 1998;58:241–250. doi: 10.1002/(SICI)1096-9926(199812)58:6<241::AID-TERA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 147.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 148.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tveden-Nyborg P, Johansen LK, Raida Z, Villumsen CK, Larsen JO, Lykkesfeldt J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am J Clin Nutr. 2009;90:540–546. doi: 10.3945/ajcn.2009.27954. [DOI] [PubMed] [Google Scholar]
- 150.Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid Redox Signal. 2013;19:2084–2104. doi: 10.1089/ars.2013.5382. [DOI] [PubMed] [Google Scholar]
- 151.Tveden-Nyborg P, Vogt L, Schjoldager JG, Jeannet N, Hasselholt S, Paidi MD, Christen S, Lykkesfeldt J. Maternal vitamin C deficiency during pregnancy persistently impairs hippocampal neurogenesis in offspring of guinea pigs. PLoS One. 2012;7:e48488. doi: 10.1371/journal.pone.0048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Robertson WB, Schwartz B. Ascorbic acid and the formation of collagen. J Biol Chem. 1953;201:689–696. [PubMed] [Google Scholar]
- 153.Vissers MC, Bozonet SM, Pearson JF, Braithwaite LJ. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit) Am J Clin Nutr. 2011;93:292–301. doi: 10.3945/ajcn.110.004853. [DOI] [PubMed] [Google Scholar]
- 154.Wang C, Lee JE, Cho YW, Xiao Y, Jin Q, Liu C, Ge K. UTX regulates mesoderm differentiation of embryonic stem cells independent of H3K27 demethylase activity. Proc Natl Acad Sci USA. 2012;109:15324–15329. doi: 10.1073/pnas.1204166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, Yang L, Zhang J, Li G, Ci W, Li W, Zhou Q, Aluru N, Tang F, He C, Huang X, Liu J. Programming and inheritance of parental DNA methylomes in mammals. Cell. 2014;157:979–991. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, Qin B, Zeng L, Esteban MA, Pan G, Pei D. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9:575–587. doi: 10.1016/j.stem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 157.Welstead GG, Creyghton MP, Bilodeau S, Cheng AW, Markoulaki S, Young RA, Jaenisch R. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc Natl Acad Sci USA. 2012;109:13004–13009. doi: 10.1073/pnas.1210787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 159.Wolbach SB, Howe PR. Intercellular substances in experimental scorbutus. Arch Pathol. 1926;1:1–26. [Google Scholar]
- 160.Wood PM, Schachner M, Bunge RP. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J Neurosci. 1990;10:3635–3645. doi: 10.1523/JNEUROSCI.10-11-03635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wright ME, Andreotti G, Lissowska J, Yeager M, Zatonski W, Chanock SJ, Chow WH, Hou L. Genetic variation in sodium-dependent ascorbic acid transporters and risk of gastric cancer in Poland. Eur J Cancer. 2009;45:1824–1830. doi: 10.1016/j.ejca.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yildirim O, Li R, Hung JH, Chen PB, Dong X, Ee LS, Weng Z, Rando OJ, Fazzio TG. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135:10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 166.Young JI, Züchner S, Wang G. Regulation of the epigenome by vitamin C. Annu Rev Nutr. 2015;35:545–564. doi: 10.1146/annurev-nutr-071714-034228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zimmermann P, Boeuf S, Dickhut A, Boehmer S, Olek S, Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008;58:2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]