Abstract
Background
A large number of elderly patients with acute myeloid leukemia (AML) are not offered curative intent treatments such as allogeneic stem cell transplantation (SCT) due to fears of toxicity and perceived futility of intensive treatment. Therefore, the outcomes of SCT in elderly AML patients remain poorly defined.
Methods
We performed a meta-analysis of all previous articles up until September 22, 2015 of SCT in AML patients >60 years. The primary endpoints were relapse-free survival (RFS) and overall survival (OS) at 6 months and 1, 2, and 3 years.
Results
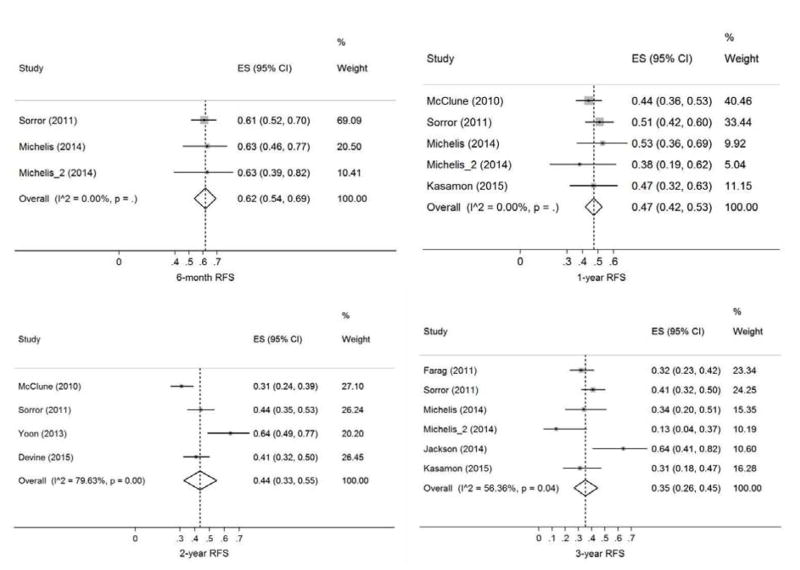
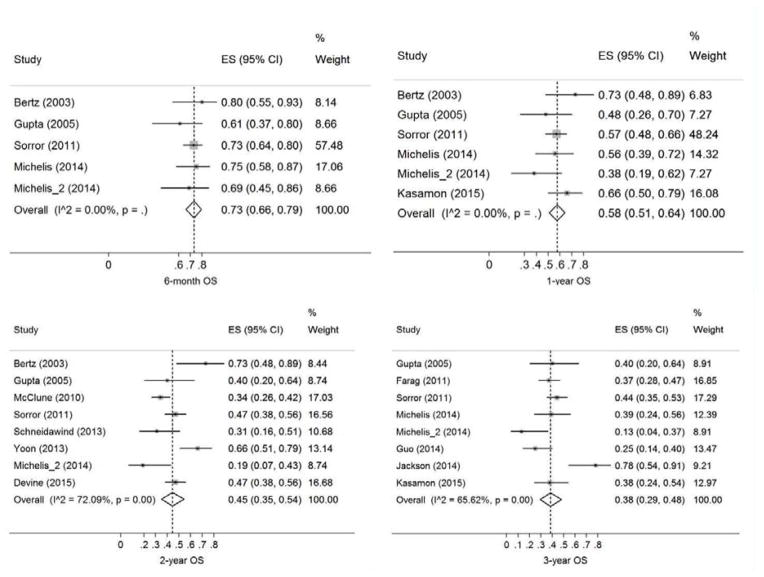
A total of 13 studies (749 patients) were included. The pooled estimates (95%CI) for RFS at 6 months, 1 year, 2 years, and 3 years were 62 (54–69)%, 47 (42–53)%, 44 (33–55)%, and 35 (26–45)%, respectively. The corresponding numbers for OS were 73 (66–79)%, 58 (50–65)%, 45 (35–54)%, and 38 (29–48)%, respectively. We found no evidence for publication bias in our primary endpoints with the exception of relapse where there appeared to be a relative lack of small studies with high relapse rates. Sensitivity analysis did not identify an overtly influential study for our primary endpoints with one exception in 2-year RFS analysis.
Conclusions
The present analysis argues against significant publication bias and demonstrates consistency among reports despite differences in patient-, disease-, center-, and transplant-related characteristics. Our results suggest that reduced-intensity SCT is a viable treatment option for elderly AML patients with a 3-year RFS of 35% for those over the age of 60. These results argue against using age per se as the sole criterion against SCT and would help remove some of the barriers that often preclude curative intent treatment. Correct identification of patients who would benefit from SCT can improve outcomes in this frequently undertreated population.
Keywords: allogeneic, elderly, leukemia, outcome, reduced-intensity, transplantation
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly, with a rapidly increasing incidence by age from approximately 50 years, median age at diagnosis of 72 years, and the peak incidence at approximately 80 years of age.1, 2 Outcomes of treatment in AML decline with age with 2-year overall survival (OS) rates less than 20% in those over the age of 60.3–5 Comorbidities and intrinsic biologic factors underlying disease resistance are among causes of poor outcomes in the elderly with AML.4, 6 Nonetheless, 40–60% of these patients achieve a complete remission (CR) with standard intensive chemotherapy.7 Even some of the less intensive therapies such as hypomethylating agents can result in CR rates up to 20%.8–10 Although most elderly AML patients still succumb to their disease, a recent analysis of the Surveillance, Epidemiology and End Results (SEER) database demonstrated improved outcomes of older adults (65–74 years) with AML over the past three decades, with 1-year OS rates of 20% between 1977–1986 and 30% between 1997–2006.11 Reasons for this improvement include better supportive care, infection control, and patient selection.
Allogeneic stem cell transplantation (SCT) is a potentially curative consolidative treatment for patients with AML. While myeloablative (MA) conditioning regimens are associated with unacceptably high toxicity and non-relapse mortality (NRM) in the elderly,12 reduced-intensity (RI) regimens are both effective and better tolerated, and are increasingly used in this population. In patients older than 50 years of age, RI conditioning is associated with less NRM and similar relapse-free survival (RFS) compared to MA conditioning.13
A major challenge in the treatment of older adults with AML is our limited ability to identify those who would likely tolerate induction (intensive or less intensive) chemotherapy and/or consolidative SCT. A prevailing perception is that intensive therapy results in unacceptable rates of toxicity in the elderly. As a result, a large number of elderly patients with AML are not offered curative intent treatment due to fears of toxicity, high rates of relapse, and high treatment-related mortality. Between 2000 and 2007, fewer than 40% of AML patients >65 years in the US received anti-leukemia treatment within three months of diagnosis.14 Similarly, according to recent estimates, only about 6% of AML patients older than 60 in the US undergo SCT.15 Publication bias and inconsistency between the results of the available studies are two of the usually stated limitations that, although based on little systematically derived evidence, tend to prevent clinicians from applying the available results to more widespread clinical practice.
Since older patients are often excluded from clinical trials, transplant outcome data in this population are limited, making retrospective reviews and meta-analyses potentially valuable. The purpose of the present study was to determine the outcomes of SCT in elderly AML patients using a systematic review and meta-analysis.
Materials and Methods
Data sources and searches
We performed this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 PRISMA is an evidence-based minimum set of items for reporting in meta-analyses including a 27-item checklist (pertaining to the title, abstract, methods, results, discussion and funding) and a flow diagram (the flow of information through the different phases of a systematic review). We searched Medline (PubMed) and Embase since their inception for articles written in English published up until September 22, 2015. The Appendix lists the keywords used to find studies that included AML patients older than 60 years who underwent SCT. Considering that studies with a focus on patients with myelodysplastic syndrome (MDS) may have included AML patients and reported their outcomes separately, we used MDS-related keywords in our search as well.
Selection criteria and data extraction
Duplicates were first removed from the search results. The remaining reports were then screened by scanning titles and abstracts for the following exclusion criteria: reviews or meta-analyses, commentaries, editorials, conference abstracts, reports in languages other than English, no primary endpoints reported, and studies of patients <60 years only. The remaining studies were reviewed in detail. Studies that used both RI and MA conditioning but did not report the outcomes separately were excluded. The corresponding authors of eligible studies with partially missing information were contacted for additional data. Studies were included in data extraction if they reported at least one of the two primary endpoints. AR and ME independently reviewed the studies, extracted the data, and resolved discrepancies by consensus.
Quality assessment
All studies were evaluated for quality using a 2-item scoring system. The categories were (i) specific conditioning regimen(s) and (ii) median age. For each item, studies received a score of 1 if the information was provided in the report and zero otherwise. The total quality score (range 0–2) was calculated by adding the scores for individual items. A higher total score indicated a higher-quality study. These scores were not a basis for inclusion or exclusion of studies.
Statistical analysis
The primary endpoints were OS and RFS at 6 months, 1 year, 2 years, and 3 years, measured from the time of SCT. OS was defined as time to death or last follow up if alive. Secondary endpoints were the cumulative incidence of relapse (CIR) and NRM (death unrelated to relapse). Study heterogeneity was assessed using the Cochran Q test and quantified using the I2 statistic. A random effects model was first used to calculate pooled proportions with 95% confidence intervals (95%CI) in proportion meta-analysis.17 In analyses with no significant heterogeneity, the model was then changed to fixed effects. Publication bias was assessed using funnel plots and Egger test. Meta-regression (one covariate at a time) was used to determine the effect of potential variables (median age, maximum age, accrual initiation year, gender, cytogenetic risk, and donor type) on outcomes. Regression was performed only when the number of eligible studies was larger than 5. Two-sample independent Student’s t-test was used to evaluate the effect of study scale (single-center vs. multicenter). Finally, sensitivity analysis was performed by removing individual studies and repeating the analysis to determine the influence of each study on the pooled estimate. A study was considered overtly influential if the change in the pooled estimate for proportion after removing the study was >10%. STATA 13 (College Station, TX) was used for analysis. P values <0.05 were considered statistically significant.
Results
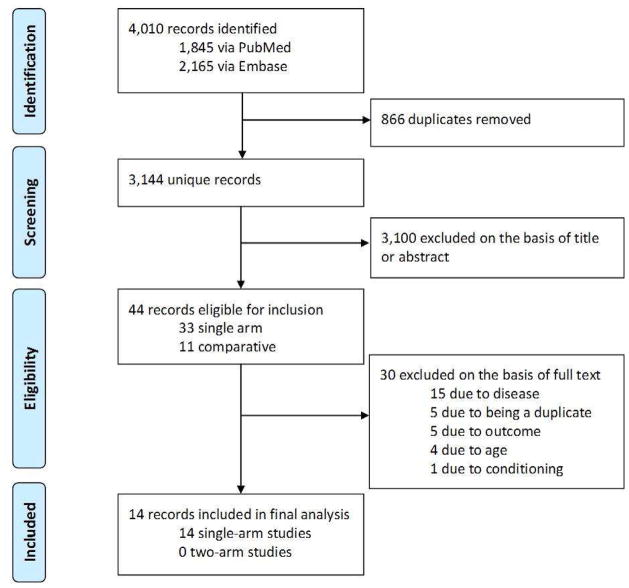
A total of 14 reports were studied in detail (Figure 1, Table 1).12, 18–29. All but two studies were retrospective and six were multicenter. All studies were single-arm, 13 using RI and one using MA conditioning. Since only one study used MA conditioning,12 this study was not analyzed. The included studies had a total of 749 eligible patients. The sample size ranged between 6 and 195. The proportion of patients with poor-risk cytogenetics ranged between 6% and 29%. 11 studies scored 2, and three scored 1.
Figure 1. Flow diagram of the studies in meta-analysis.
A total of 14 reports were studied in detail. One study was counted twice because it reported patients in first and second complete remission separately.23 Only one study used myeloablative conditioning and was not analyzed.12
Table 1.
Summary of the eligible studies
| Study | Accrual initiation year | # Centers | # Patients | Males (%) | Median/ Max age | Poor-risk cytogenetics (%) | Remission (%), CR1 |
|---|---|---|---|---|---|---|---|
| Age ≥ 60 years | |||||||
| Bertz, et al. JCO 2003† | NA | Single | 15 | NA | 65/70 | 9 | 2 (13), 1 |
| Wallen, et al. JCO 2005* | 1979 | Multiple | 6 | NA | NA | NA | 1 (17), 1 |
| Gupta, et al. BBMT 2005 | 2000 | Single | 16 | 10 (63) | 64 | 6 | 15 (94), NA |
| McClune, et al. JCO 2010 | 1995 | Multiple | 195 | 111 (57) | NA | 29 | 195 (100), 195 |
| Faraq, et al. BBMT 2011 | 1999 | Multiple | 94 | 55 (59) | 63 | 22 | 94 (100), 94 |
| Sorror, et al. JAMA 2011 | 1998 | Multiple | 109 | 70 (64) | 65 | NA | NA, NA |
| Schneidawind, et al. Ann Hematol 2013 | 2006 | Single | 24 | NA | 67/NA | NA | NA, NA |
| Yoon, et al. AJH 2013 | 2002 | Single | 41 | 19 (46) | 62/68 | 15 | NA, NA |
| Michelis, et al. BBMT 2014 | 1999 | Single | 32 | 20 (63) | 63/71 | 25 | 32 (100), 32 |
| Michelis_2, et al. BBMT 2014 | 1999 | Single | 16 | 6 (38) | 66/70 | 6 | 16 (100), 0 |
| Guo, et al. BBMT 2014 | 2005 | Single | 40 | NA | NA | NA | 40 (100), NA |
| Jackson, et al. APJCO 2014 | 1999 | Multiple | 17 | NA | 63/66 | NA | 17 (100), 17 |
| Devine, et al. JCO 2015† | 2004 | Multiple | 114 | 71 (62) | 65/74 | 28 | 114 (100), 114 |
| Kasamon, et al. JCO 2015 | 2003 | Single | 36 | NA | NA | NA | NA |
| Age > 65 years | |||||||
| Bertz, et al. JCO 2003† | NA | Single | 6 | NA | 68 | 25 | 1 (17), 1 |
| Gupta, et al. BBMT 2005 | 2000 | Single | 7 | 6 | 67 | 20 | 6 (86), 4 |
| McClune, et al. JCO 2010 | 1995 | Multiple | 63 | 34 | 67 | 26 | 63 (100), 63 |
| Michelis, et al. BMT 2015 | 1999 | Single | 40 | NA | NA | NA | 40 (100), 40 |
CR1: first complete remission; NA: not available
Myeloablative conditioning. This study was not included in analysis.
Prospective
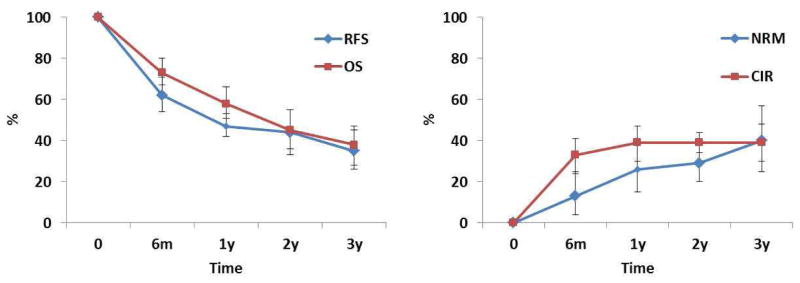
The pooled estimates (95%CI) for RFS at 6 months, 1 year, 2 years, and 3 years were 62 (54–69)%, 47 (42–53)%, 44 (33–55)%, and 35 (26–45)%, respectively (Figure 2). The corresponding numbers for OS were 73 (66–79)%, 58 (50–65)%, 45 (35–54)%, and 38 (29–48)%, respectively (Figure 3). The pooled estimates (95%CI) for CIR at 6 months, 1 year, 2 years, and 3 years were 33 (25–42)%, 39 (31–48)%, 39 (34–44)%, and 39 (30–48)%, respectively (Figure S1). The corresponding numbers for NRM were 13 (4–25)%, 26 (15–39)%, 29 (20–40)%, and 40 (25–57)%, respectively (Figure S2). Figure 4 shows reconstructed curves for outcomes at different time points.
Figure 2. Relapse-free survival (RFS) at 6 months, 1 year, 2 years, and 3 years.
Effect size (ES) is odds ratio or relative risk depending on the study.
Figure 3. Overall survival (OS) at 6 months, 1 year, 2 years, and 3 years.
Effect size (ES) is odds ratio or relative risk depending on the study.
Figure 4. Reconstructed Kaplan-Meyer curves for relapse-free survival (RFS), overall survival (OS), cumulative incidence of relapse (CIR), and non-relapse mortality (NRM).
Pooled estimates demonstrate that allogeneic stem cell transplantation is a viable treatment option for elderly patients with acute myeloid leukemia.
There was no evidence of statistical heterogeneity among studies in 2-year CIR (χ2 = 2.6, I2 = 0), 6-month RFS (χ2 = 0.1, I2 = 0), 1-year RFS (χ2 = 2.1, I2 = 0), 6-month OS (χ2 = 1.6, I2 = 0), and 1y OS (χ2 = 5.4, I2 = 6.9%). In contrast, a significant proportion of inter-study variation in 3-year CIR (χ2 = 8.9, I2 = 54.9%), 6-month NRM (χ2 = 6.5, I2 = 53.5%), 1-year NRM (χ2 = 15.5, I2 = 74.1%), 2-year NRM (χ2 = 32.1, I2 = 78.2%), 3-year NRM (χ2 = 24.3, I2 = 83.5%), 2-year RFS (χ2 = 14.7, I2 = 79.6%), 3-year RFS (χ2 = 11.5, I2 = 56.4%), 2-year OS (χ2 = 25.1, I2 = 72.1%), and 3-year OS (χ2 = 20.4, I2 = 65.6%) was attributable to study heterogeneity. There were too few studies for heterogeneity analysis of other outcomes. The plots and Egger’s bias coefficients argued against significant publication bias in our primary or secondary endpoints with the exception of relapse rates where there appeared to be a relative lack of small studies with high 2- and 3-year CIR rates (Figures S3-S6).
In sensitivity analysis of 6-month NRM, the studies by Gupta et al.20 and Sorror et al.24 were influential (change in pooled estimate after study removal: −40% and +54%, respectively). With regards to 1-year NRM, the same two studies were again influential (change in pooled estimate: −12% and +23%, respectively). For 2-year and 3-year NRM, the studies by Devine et al.29 and Sorror et al.24 were influential (change in pooled estimate: +14% and +18%, respectively). The study by Yoon et al. was influential (change in pooled estimate: −14%) in 2-year RFS analysis.27 Meta-regression of outcomes on our pre-planned set of predictors was non-significant in all analyses. There was no significant difference in outcomes between single-center and multicenter studies.
Four studies (116 patients) had outcome information regarding patients older than 65 years. The pooled estimates for 6-month, 1-year, and 2-year OS were 0.89 (0.63–1.00), 0.77 (0.48–0.97), and 0.52 (0.24–0.79), respectively. The corresponding estimates for NRM were 0.05 (0–0.28), 0.26 (0.16–0.38), and 0.32 (0.21–0.44), respectively. The sample was too small for a meaningful analysis of other outcomes.
Discussion
Outcomes of SCT using RI conditioning in elderly patients with AML have been only sporadically reported and not systematically analyzed. We conducted this meta-analysis to fill the evidence gap by analyzing the existing literature on transplant outcomes in elderly AML patients. We analyzed 13 eligible studies with a total sample size of 749 patients. On our pre-planned scoring system, 11 studies scored 2, and three scored 1, suggesting a satisfactory quality for the available studies. Sample sizes varied substantially, ranging from 6 to 195 patients, with 10 studies having fewer than 50 patients. Our results demonstrate 3-year RFS and CIR rates of 35% and 39%, respectively. There is a plateau in relapse after 1 year. We did not observe significant study heterogeneity for early survival outcomes at 6 months and 1 year.
A number of registry-based and single-center studies have demonstrated that anti-leukemia treatment improves survival in the elderly.3, 30, 31 However, only a small proportion of elderly patients receive induction therapy, and even a smaller minority are considered for SCT. There is no consensus on the ideal method to select patients who would benefit from SCT. Our results from this meta-analysis suggest that a sizable proportion of older adults with AML may achieve durable remissions with SCT. This observation argues against the utility of age as the only factor in patient selection. Consistent with these results, in a large retrospective analysis by McClune et al. age was not associated with NRM or RFS.22 The optimal approach to patient selection would take into account both patient characteristics (e.g. age and comorbidities) and disease characteristics (e.g. cytogenetic and molecular features). Less intensive induction therapies such as hypomethylating agents have been used with success as a “bridge” to transplant in frail or older patients with AML.32, 33 It should be noted that although the present meta-analysis was largely negative for significant publication bias and overtly influential studies, it only pertains to patients who underwent SCT and does not address the potential variability of the criteria used in different studies to select transplant-eligible patients.
In conclusion, the results of our meta-analysis demonstrate that reduced-intensity SCT is a viable treatment option for elderly AML patients. Age should not be the sole factor in selecting patients for intensive therapy and/or SCT. Interestingly, although the number of patients >65 years in the available studies is small, and therefore, any comparison would be limited, our results do not suggest worse NRM or OS for this population when compared to patients >60 years. Lack of significant publication bias and the consistency of results among different studies question the prevailing perception that most elderly AML patients cannot be treated with curative intent. An unresolved challenge, unaddressed by the present analysis, is how to select the subgroup of patients who benefit from SCT. More accurate prognostication using disease- and patient-related characteristics is critical in this regard. One limitation of the present study is related to the lack of data on patients with active disease at the time of SCT. Therefore, our results may not be generalizable to active disease patients. Also, the limited numbers of patients over the age of 70 years precluded a meaningful analysis of outcomes in this cohort. Finally, although we tried to eliminate studies which overlapped in their patient population with others, some degree of patient overlap might have inevitably occurred among publications. This may have falsely narrowed the confidence interval for some of the pooled estimates.
Supplementary Material
Effect size (ES) is odds ratio or relative risk depending on the study.
Effect size (ES) is odds ratio or relative risk depending on the study.
There is potential publication bias with a relative lack of small studies with high relapse rates. See text for details.
There is no evidence for publication bias.
There is no evidence for publication bias.
There is no evidence for publication bias.
Highlights.
Elderly AML patients are often undertreated.
Transplantation outcomes in elderly AML patients are poorly defined.
A meta-analysis shows efficacy of allografting in elderly AML and little publication bias.
35% of elderly AML patients are alive and in remission 3 years post-transplant.
Age should not be the sole factor in making treatment decisions in elderly AML.
Acknowledgments
Funding: AR was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences and JFD by the Specialized Program of Research Excellence (SPORE) in Leukemia NIH 1 P50 CA171963-01 from the National Cancer Institute. The funding sources had no role in study design, data collection, analysis, or interpretation of results. The authors thank Drs. Kanamori, Michelis, and Sorror for providing additional data.
Appendix
The following were used for PubMed search:
(“transplantation”[Title] OR “SCT”[Title] OR “HSCT”[Title]) AND (“stem cell”[Title] OR “stem-cell”[Title] OR “marrow”[Title] OR “marrow”[Title] OR “hematopoietic”[Title] OR “haematopoietic”[Title] OR “cord”[Title]) AND (“elderly” OR “older” OR “age over” OR “aged” OR “old”) AND (“leukemia” OR “leukaemia” OR “AML” OR “myeloid” OR “MDS” OR “myelodysplastic” OR “myelodysplasia”) AND (“conditioning” OR “preparation” OR “preparatory” OR “busulfan” OR “fludarabine” OR “cyclophosphamide” OR “total body” OR “treosulfan” OR “melphalan” OR “reduced-intensity” OR “reduced intensity” OR “ablative” OR “myeloablative” OR “clofarabine”) AND (“outcome” or “survival”)
The following were used for Embase search:
‘transplantation’:ti OR ‘sct’:ti OR ‘hsct’:ti AND (‘stem cell’:ti OR ‘stem-cell’:ti OR ‘marrow’:ti OR ‘hematopoietic’:ti OR ‘haematopoietic’:ti OR ‘cord’:ti) AND (‘elderly’ OR ‘older’ OR ‘age over’ OR ‘aged’ OR ‘old’) AND (‘leukemia’ OR ‘leukaemia’ OR ‘aml’ OR ‘myeloid’ OR ‘mds’ OR ‘myelodysplastic’ OR ‘myelodysplasia’) AND (‘conditioning’ OR ‘preparation’ OR ‘preparatory’ OR ‘busulfan’ OR ‘fludarabine’ OR ‘cyclophosphamide’ OR ‘total body’ OR ‘treosulfan’ OR ‘melphalan’ OR ‘reduced-intensity’ OR ‘reduced intensity’ OR ‘ablative’ OR ‘myeloablative’ OR ‘clofarabine’) AND (‘outcome’ OR ‘survival’)
Footnotes
Conflicts of interest
None
Authors’ contributions
AR and ME reviewed the studies and extracted the data. AR and GAC analyzed the data. AR, ME, GAC, and JFD interpreted the results and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juliusson G, Lazarevic V, Horstedt AS, Hagberg O, Hoglund M Swedish Acute Leukemia Registry G. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhayat F, Das-Gupta E, Smith C, McKeever T, Hubbard R. The incidence of and mortality from leukaemias in the UK: a general population-based study. BMC cancer. 2009;9:252. doi: 10.1186/1471-2407-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. The New England journal of medicine. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 6.Motyckova G, Stone RM. Treatment of elderly acute myeloid leukemia patients. Current treatment options in oncology. 2011;12:341–353. doi: 10.1007/s11864-011-0162-4. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AK, Milligan D, Goldstone A, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. British journal of haematology. 2009;145:318–332. doi: 10.1111/j.1365-2141.2009.07604.x. [DOI] [PubMed] [Google Scholar]
- 8.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or lowdose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubbert M, Ruter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97:393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119:2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallen H, Gooley TA, Deeg HJ, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:3439–3446. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 14.Lang K, Earle CC, Foster T, Dixon D, Van Gool R, Menzin J. Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs & aging. 2005;22:943–955. doi: 10.2165/00002512-200522110-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone marrow transplantation. 2013;48:1497–1505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo RJ, Atenafu EG, Craddock K, Chang H. Allogeneic hematopoietic cell transplantation may alleviate the negative prognostic impact of monosomal and complex karyotypes on patients with acute myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:690–695. doi: 10.1016/j.bbmt.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, Daly A, Lipton JH, et al. Nonmyeloablative stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia in patients 60 years or older. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:764–772. doi: 10.1016/j.bbmt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Jackson K, Kennedy G, Mollee P, Marlton P, Morris K. Intensive chemotherapy and reducedintensity allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia in elderly patients. Asia Pac J Clin Oncol. 2014;10:246–254. doi: 10.1111/ajco.12188. [DOI] [PubMed] [Google Scholar]
- 22.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelis FV, Messner HA, Atenafu EG, et al. The benefit of allogeneic hematopoietic cell transplantation in older patients with acute myeloid leukemia is restricted to those in first complete remission at the time of transplantation. Blood. 2012;120:21. [Google Scholar]
- 24.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA - Journal of the American Medical Association. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:1480–1484. doi: 10.1200/JCO.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 26.Schneidawind D, Federmann B, Faul C, Vogel W, Kanz L, Bethge WA. Allogeneic hematopoietic cell transplantation with reduced-intensity conditioning following FLAMSA for primary refractory or relapsed acute myeloid leukemia. Ann Hematol. 2013;92:1389–1395. doi: 10.1007/s00277-013-1774-5. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Cho BS, Kim HJ, et al. Outcomes of elderly de novo acute myeloid leukemia treated by a risk-adapted approach based on age, comorbidity, and performance status. American journal of hematology. 2013;88:1074–1081. doi: 10.1002/ajh.23576. [DOI] [PubMed] [Google Scholar]
- 28.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of Nonmyeloablative HLAHaploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devine SM, Owzar K, Blum W, et al. A phase II study of allogeneic transplantation for older patients with AML in first complete remission using a reduced intensity conditioning regimen: Results from CALGB 100103 (Alliance)/BMT CTN 0502. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.62.7273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a populationbased study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baz R, Rodriguez C, Fu AZ, et al. Impact of remission induction chemotherapy on survival in older adults with acute myeloid leukemia. Cancer. 2007;110:1752–1759. doi: 10.1002/cncr.22976. [DOI] [PubMed] [Google Scholar]
- 32.Lubbert M, Bertz H, Ruter B, et al. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone marrow transplantation. 2009;44:585–588. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]
- 33.Ustun C, Kalla A, Farrow S, DeRemer DL, Jillella A. Decitabine as “bridge therapy” to a MUD transplant in relapsed AML postautologous stem cell transplantation. American journal of hematology. 2008;83:825–827. doi: 10.1002/ajh.21267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect size (ES) is odds ratio or relative risk depending on the study.
Effect size (ES) is odds ratio or relative risk depending on the study.
There is potential publication bias with a relative lack of small studies with high relapse rates. See text for details.
There is no evidence for publication bias.
There is no evidence for publication bias.
There is no evidence for publication bias.