“As we know, there are known knowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns – the ones we don't know we don't know.” -Donald Rumsfeld
Necroptosis is a form of programmed cell death that is both mechanistically and morphologically distinct from apoptosis, the canonical mechanism of cell suicide. Though early descriptions of necroptosis date back decades, the last 5 years have seen a proliferation of studies of this process. This surge in interest has included the recent publication of several excellent, in-depth reviews of the literature[1-4]. Rather than contribute another summary to this well-summarized field, in this Minireview I will briefly discuss key recent findings, then touch on some of the major outstanding questions— the known unknowns—that remain.
What’s the deal with necroptosis?
We used to think of cell death as a binary choice: cells could commit suicide via activation of the signaling pathways leading to apoptosis, or they could be killed “accidently” via the unprogrammed process of necrosis. However, in the last decade it has become clear that apoptosis is not the only means of programmed cell suicide. Other cell death programs exist, and that these programs rely on different mechanisms—and yield different outcomes in vivo— than classical apoptosis.
Necroptosis is one such non-apoptotic form of programmed cell death; it takes its name from the morphological resemblance between necroptosis and unprogrammed necrosis[5]. (The suffix “-ptosis” has, somewhat nonsensically, been adapted to denote a cell death program, e.g. “pyroptosis,” “ferroptosis,” and so on.) More specifically, necroptosis is a form of programmed cell death carried out by the receptor interacting protein kinases 1 and 3 (RIPK1 and RIPK3), and the pseudokinase mixed lineage kinases domain-like (MLKL).
Necroptosis, like unprogrammed necrosis, involves cellular swelling and the rupture of the plasma membrane, with concomitant release of cellular contents into the extracellular space. This makes it clearly distinct from apoptosis, which is mediated by the caspase proteases, and is characterized by nuclear condensation and the packaging of the dying cell into membrane-bound bodies that are readily cleared by phagocytes. The lytic nature of necroptotic cell death has also led to the hypothesis that cells dying by this process trigger inflammatory responses in vivo. This is thought to be mediated by the release of “danger-associated molecular patterns” (DAMPs)[6], a nebulous class of molecules whose release from damaged cells is read as a danger signal by responding immune cells; however, as discussed below, this idea remains controversial[7].
Consistent with an immune-activating role for necroptosis, this form of death can be triggered by several innate immune pattern-recognition receptors, and occurs in response to pathogen infection. Mice lacking RIPK3 or MLKL appear normal and viable, but display susceptibility to some viral infections; this is in contrast to mice lacking apoptotic mediators, many of which display defects in embryonic development and tissue homeostasis. These observations are consistent with the idea that apoptotic cell death is a developmentally programmed process required for organismal function, while necroptosis is an “emergency” program of defensive cell suicide that can be engaged to limit pathogen spread and promote inflammatory and immune responses.
Initiation and execution of necroptosis
If RIPK3 activation defines necroptosis, what activates RIPK3? The best-studied RIPK3 activator is RIPK1; both of these kinases contain small protein domains called rip homotypic interaction motifs (RHIMs), by which they interact[8,9]. RIPK1 is a well-described component of the NF-kB activation machinery downstream of receptors such as TNFR1, the TRAIL receptors, and Fas (also called CD95) as well as innate immune pattern recognition receptors like RIG-I. These platforms recruit and activate RIPK1 via its death domain (DD); once active, RIPK1 participates in the inflammatory transcriptional responses common to these signaling platforms. However, in certain cases (discussed below), active RIPK1 can also move into alternative protein complexes, where it can activate RIPK3 via RHIM-RHIM interactions, leading to necroptosis. Thus, TNF stimulation is widely used to study necroptotic responses. The pathway of TNF-driven necroptosis is summarized in Fig. 1.
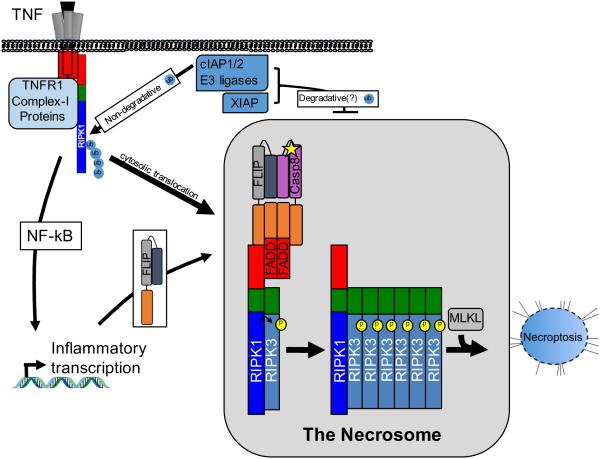
Fig. 1. TNF-driven necroptosis.
The TNFR1-driven pathway is depicted here, though the regulatory mechanisms shown generally apply to Fas, TRAIL receptor, and TLR-driven necroptosis. TNFR1 ligation triggers formation Complex I, which includes RIPK1 and cIAP1/2. Phosphorylation and ubiquitination events within this complex lead to canonical NF-kB activation. RIPK1 deubiquitination leads to its egress from complex I and formation of secondary complexes in the cytosol. The necrosome is among these. In the necrosome, RIPK1 interacts with RIPK3 via the RHIM domain (green) shared by both proteins. This interaction promotes reciprocal phosphorylation and RHIM-driven oligomerization. However, RIPK1 can also interact with a complex containing caspase-8 and FLIP, which suppresses necrosome formation. Caspase-8/FLIP are recruited via their death effector domains (DEDs, in orange) to the adapter FADD, which interacts with RIPK1 via its death domain (DD, red). The necrosome is also inhibited by the IAPs (both cIAP1/2 and XIAP) via their E3 ubiquitin ligase activity, though the molecular details of this regulation are not fully understood. Necrosome assembly promotes phosphorylation and activation of the pseudokinase MLKL, which translocates to and disrupts cellular membranes. This leads to cellular swelling and necroptosis.
The RHIM domain of RIPK3 represents the “handle” by which RIPK1 grabs and activates it; however, the adapter TRIF and the DNA sensor DAI also have RHIM domains, and these proteins are thus able to interact with RIPK3 even in the absence of RIPK1. TRIF is activated downstream of the toll-like receptors TLR3 and TLR4, while DAI senses infection by DNA viruses. These pattern-recognition receptors can recruit either RIPK1 or RIPK3 (or both) via their RHIM domains, and—unlike the RIPK1-activating receptors discussed above—they can directly activate RIPK3 independent of RIPK11. In sum, RIPK3 activation happens via its RHIM domain, and there are three other proteins in the human proteome that share this domain and can thereby activate RIPK3: RIPK1, TRIF, and DAI[8-10]. The receptors and innate immune signals that activate these proteins can, therefore, trigger necroptosis.
Morphologically recognizable necroptosis—the swelling and rupture of the cell—is driven by the RIPK3-dependent phosphorylation and activation of the pseudokinase MLKL[11]. Activated MLKL multimerizes, and this exposes a motif at the proteins N-terminus[12]; this motif interacts with phosphatidylinositol phosphates (PIPs) within the plasma membrane, leading to its disruption and thus to loss of ion homeostasis, swelling, and cell death[13-15].
How does RIPK3 activation occur?
RHIM domains stack to form amyloid-like oligomers, and RHIM-driven oligomerization of RIPK3 is both necessary and sufficient for for its activation[16]. Studies using inducible-interaction systems indicate that this may be because oligomerization allows sufficient density of RIPK3 kinase domains to promote intramolecular phosphorylation, which is required for MLKL recruitment[17-19]. However, the molecular details and structural underpinnings of these events remain obscure. How do phosphorylation events control RHIM-RHIM interactions? How does RIPK1 initiate RIPK3 activation during TNF-driven necroptosis, and how do the molecular interactions differ during RIPK1-independent activation of RIPK3 by TRIF or DAI? How does recruitment of RIPK1 to the latter complexes alter their function? As discussed below, RIPK1 may actually suppress RIPK3 activation in some cases; it’s recruitment by RIPK3 may also promote other functions, such as cytokine production. Who phosphorylates whom within the RIPK1/3 complex, and how do inter- and intramolecular phosphorylation events unfold during oligomerization and activation of the RIPKs?
How is MLKL-mediated cellular destruction controlled?
Active MLKL translocates to the cell membrane, where it interacts with PIPs to trigger membrane permeabilization, cellular swelling, and death. This mechanism of cell death execution is strikingly different to the protease-dependent effects of both apoptotic and pyroptotic caspase activation. How are the steps between MLKL activation and cell death regulated? Are these events entirely the result of MLKL-lipid interactions, or do additional regulatory mechanisms—such as membrane protein interaction partners, or anti-necroptotic MLKL phosphatases—also contribute? Furthermore, how does activated MLKL affect other cellular membranes? MLKL localization to mitochondria, ER, Golgi and lysosomes has been reported[13], though what effect MLKL might have on these organelles remains unknown; for example, while it is clear that mitochondria don’t contribute to the execution of necroptosis[20], their disruption my MLKL during necroptosis could alter the nature of the cell death by modulating immunogenicity or tissue damage[21]. Finally, we still lack a detailed understanding of the decisions points in the necroptotic program, i.e. the “point of no return” during RIPK3 and MLKL activation. One can envision a scenario in which MLKL forms a limited number of pores in the cell membrane, sufficient to alter cellular ion homeostasis or promote intake or secretion of material without triggering irreversible cell death. Can a cell survive limited MLKL activation? What cellular programs might act to limit the size or lethality of pores formed by activated MLKL? If MLKL can alter cellular membranes without killing a cell, what effect might this have on other signaling pathways such as inflammasome activation or cytokine secretion?
Regulation of necroptosis: caspase-8, FLIP, IAPs, RIPK1 and other players
While the discussion above describes TNF and TLR ligands as inducers of necroptosis, in reality in most contexts these signals drive inflammatory cytokine production rather than rapid cell death. This is because necroptosis is held in check by the action of the pro-apoptotic protease caspase-8, a role clearly demonstrated by the rescue of embryonic lethality of caspase-8−/− mice by concurrent ablation of RIPK3[22,23]. Caspase-8 interacts with its paralog cFLIP to mediate this function, and the cFLIP/caspase-8 complex is recruited to the RIPK1/RIPK3 containing necrosome via interactions mediated by the adapter protein FADD[22]. Confusingly, TNF and TLR ligation can also trigger caspase-8-mediated apoptosis, notably when NF-kB-mediated cFLIP upregulation is prevented (Fig.1). As discussed below, the molecular details of these interactions remain murky.
In addition to—or, more probably, in concert with—the anti-necroptotic function of caspase-8/cFLIP, the cellular inhibitor of apoptosis proteins (cIAPs) also exert a key check on the activation of necroptosis. The cIAPs are E3 ubiquitin ligases with complex roles in numerous cellular processes, including both canonical and non-canonical NF-kB activation. In addition to these functions, the cIAPs are able to mediate the proteasome-dependent degradation of active RIPK1/3 complexes, thereby blocking the execution of necroptosis[24-26]. The related molecule XIAP was recently shown to also participate in suppression of necroptosis, in a manner than involved its E3 ligase domain[27], though molecular details remain sketchy (Fig.1).
Recent work has added further to the complexity of these regulatory mechanisms by identifying RIPK1 as a context-specific suppressor of necroptosis. Unlike caspase-8/RIPK3 double-knockout mice, RIPK1 knockout or caspase-8/RIPK1 double-knockout animals die shortly after birth. However, additional ablation of RIPK3, to make caspase-8/RIPK1/RIPK3 triple knockouts, rescues the viability of these animals[28-30]. The implication of this finding is that caspase-8/RIPK1 double-knockout mice die due to aberrant RIPK3 engagement following birth. Thus, while RIPK1 is required for RIPK3 engagement downstream of the TNF receptor, it can also exert a suppressive effect on RIPK3 at steady state. The details of how RIPK3 is engaged in the absence of RIPK1 and TNF signaling remain poorly understood, though signaling by type-I interferons has been implicated[28-31]. Notably, while RIPK1 knockout mice die perinatally, mice expressing catalytically inactive RIPK1—which supports the NF-kB-activation function of RIPK1 but cannot activate RIPK3—are viable[32,33]. Further, these mice display notable resistance to TNF-driven septic shock and inflammatory disease models, beyond that observed with RIPK3 knockouts. Consistently, cells lacking RIPK1 display increased susceptibility to multiple inducers of both necroptotic and apoptotic cell death, while cells containing kinase-dead or inhibited RIPK1 are strongly resistant to these stimuli. This indicates that kinase-dead (or inhibitor-bound) RIPK1 exerts a dominant-negative suppressive function, possibly through recruitment of the caspase-8/FLIP complex to the RIPK3-containing necrosome[17]. These suppressive functions make RIPK1 an attractive clinical target for the mitigation of inflammatory syndromes and septic shock.
Beyond the functions caspase-8, RIPK1 and the cIAPs, additional regulators of necroptosis have recently been described. The phosphatase Ppm1b was shown to directly dephosphorylate RIPK3, thereby reducing both spontaneous and TNF-mediated necroptosis and preventing TNF-induced septic shock[34]. On the other side of the balance, a chaperone complex composed of HSP90 and CDC37 was recently shows to associate with RIPK3 and potentiate its activation[35]. Interestingly RIPK1 is a known client of the HSP90 chaperone complex, raising the possibility or broader regulatory functions of this chaperone complex. Future studies will reveal the mechanisms by which these novel regulatory events are themselves regulated, and the physiological contexts in which they are engaged.
How does caspase-8 cooperate with the IAPs to suppress necroptosis?
While it’s clear that both caspase-8 and the IAPs—including both cIAP1/2 and XIAP—suppress RIPK3 activation, it’s less clear how this suppression occurs. In particular, the specific substrates for caspase-8 within the necrosome complex are not well understood. Both RIPK1 and RIPK3 can be cleaved by caspase-8[36-38]; however, it has not been clearly demonstrated that non-cleavable forms of one or both of these enzymes mimic caspase-8 deficiency. Further, while it’s clear that the cIAPs and XIAP can mediate ubiquitin-proteosome dependent degradation of activated necrosome complexes, it’s not clear how this activity is regulated, or what substrate within that complex is required for this suppressive activity. An attractive possibility is that the caspase-8/cFLIP complex and the cIAPs cooperate in this capacity; indeed, cFLIP is itself a labile protein, and it has been proposed as a target for cIAP-mediated degradation[24,25]. However, we still lack an understanding of how RIPK oligomerization or kinase activity may potentiate these suppressive functions to negatively regulate necroptosis.
How is the activity of caspase-8 directed?
Caspase-8 has a curious dual function; it induces extrinsic apoptosis, but also suppresses necroptosis. This duality can be partially explained by its interactions with cFLIP: caspase-8 homodimers are thought to drive apoptosis, while caspase-8/cFLIP heterodimers mediate survival signaling by suppressing necroptosis. This idea is supported by cellular studies: loss of FLIP sensitizes cells to TNF-induced apoptosis and eliminates the ability of caspase-8 to block necroptosis[22]. However, this duality is not supported by the biochemical activities of the homo- vs. heterodimeric complexes, as ligand-induced formation of either complex can trigger potent apoptosis[39]. Furthermore, there is not a major alteration of substrate preferences between these complexes that could explain their divergent functions. How cFLIP is able to suppress or re-direct the activity of caspase-8 to prevent necroptosis without triggering apoptosis is therefore likely to involve additional factors. Prevention of access to pro-apoptotic substrates, altered sub-cellular localization, or degradation of active caspase-8 (perhaps in concert with RIPK degradation, as speculated above) could all play roles in this process.
How do the RIPKs promote apoptosis?
A further wrinkle in the dichotomy between apoptosis and necroptosis is added by the finding that a specific mutation in RIPK3 (D161N in the mouse) abrogates its kinase activity, but promotes caspase-8 and RIPK1-dependent apoptosis[40]. Mice expressing this mutant form of RIPK3 undergo embryonic lethality with the same kinetics and tissue involvement as caspase-8 knockouts. Thus, RIPK3D161N and caspase-8−/− mice presumably engage the same TNF-dependent pathway during development, but in the former mouse this results in caspase-8 dependent apoptosis, while in the latter it triggers RIPK3-driven necroptosis. Furthermore, a small-molecule RIPK3 inhibitor was found to confer this caspase-activating property to both wild-type and catalytically inactive (K51A) mutant RIPK3[41]. This may be due to conformational changes triggered by mutation or inhibition of RIPK3, leading to defective necroptosis but stabilization of the caspase-8/RIPK1/RIPK3 complex, caspase-8 activation within this complex, and apoptosis. More confusing still, it was found that cFLIP is required for apoptosis within this complex. CFLIP thereby plays opposite roles in controlling caspase-8 dependent apoptosis in response to TNF (where it is inhibitory) and in the chemically-stabilized RIPK1/3 complex (where it is an obligate partner of caspase-8 in the induction of apoptosis.) Oddly, in all cases caspase-8 or caspase-8/FLIP engagement requires the same adapter, FADD[41]. An understanding of how these disparate outcomes can be driven by the same proteins in different cellular contexts will require a more complete structural and biochemical understanding of these complexes.
Necroptosis in vivo
The preceding discussion of the suppressive mechanisms preventing necroptosis raises an obvious question: If caspase-8 and the IAPs effectively prevent necroptosis in most contexts, when might it occur physiologically? Indeed, the majority of studies of the necroptotic pathways have relied on either genetic ablation or chemical inhibition of caspase-8 or the IAPs; TNF in combination with the caspase inhibitor zVAD and/or IAP inhibitors such as the compound BV6[42] are commonly employed. So when does this pathway fire in normal cells in vivo?
One condition in which necroptosis is clearly engaged physiologically is during DNA virus infection. In this context, necroptotic death of infected cells benefits the host, possibly by removing the viral niche or by promoting anti-viral immune responses to infected cells (or both). The presence of viral necroptosis inhibitors is clear evidence of the importance of this pathway: the herpesviruses murine cytomegalovirus (MCMV) and herpes simplex virus (HSV) both encode RHIM domain-containing inhibitors of RIPK1/3 signaling. Ablation of these inhibitors yields robust necroptosis following infection in vitro, and RIPK3-dependent attenuation of viral replication in vivo[43-46]. At least in the context of MCMV, this is driven by viral engagement of the DNA sensor DAI, which itself contains a RHIM domain. Notably, both MCMV and HSV also encode caspase-8 inhibitors. This has led to the hypothesis that suppression of caspase-8-mediated apoptosis by these and other viral proteins may have led to evolution of the necroptotic pathway as a “trap door” to cell death[47]. In this scheme, the necroptotic pathway would act as a fail-safe, a form of death to be engaged in case the standard extrinsic apoptotic pathway is blocked. This idea has been tested, at least in vitro, using vaccinia virus (VV) infection. VV encodes a caspase inhibitor called B13R, and infection with wild-type, but not B13R-mutant, VV sensitizes cells to TNF-driven necroptosis[48]. Notably, RIPK3−/− mice are acutely susceptible to VV infection, consistent with engagement of necroptosis acting to prevent viral replication and control infection[49].
Other physiological roles for the pathways of necroptosis are less well-defined. Fascinatingly, TNF-mediated sterile septic shock appears to engage necroptosis, as RIPK3-deficient mice or animals expressing a kinase-dead version of RIPK1 are resistant to this stimulus[33,50]. How necroptosis is engaged by TNF administration, in the presence of intact caspase-8 and IAP-mediated suppression of RIPK3, is not clear. Beyond this model, detrimental roles for RIPK signaling have been defined in other inflammatory settings, such as ischemia-reperfusion in the kidney[51], myocardial infarction[52], atherosclerosis[53], non-alcoholic steatohepatitis[54] and multiple sclerosis[55]. Together, these observations point to the idea that engagement of necroptosis evolved to promote beneficial inflammation during immune responses to pathogens, but that this pathway can be aberrantly engaged to drive inflammatory pathologies.
Where does necroptosis occur?
A major question in the context of both viral infection and septic shock models is: which cells are responsible for the observed effects? Most in vitro work has used a limited subset of cell types (transformed cell lines, primary MEFs or macrophages), and it is clear that cells of different origins have distinct susceptibility to necroptosis. Indeed, a major challenge in the field has been the lack of a robust method to identify necroptotic cells in vivo. While staining for cleaved caspase-3, or TUNEL techniques, are routinely used to identify apoptotic cells in primary tissues, comparable reagents are only now becoming available to find necroptotic cells[13,56]. Applying these tools, along with recently-developed conditional expression systems[57], to models in which RIPK3-dependent effects have been observed in vivo, will yield important information on how necroptosis occurs in living tissue.
Do RIPK3 or MLKL engagement have outcomes beyond necroptosis?
RIPK1 can drive necroptosis, but it also plays a key role in the NF-kB transcriptional response. This dual functionality of RIPK1 has been thought to depend on its ubiquitination state, with K63- and linear ubiquitin conjugation at the TNFR1 complex associated with transcriptional NF-kB activation and deubiquitination required for necrosome formation. Interestingly, a recent report showed that RIPK1 can undergo non-degradative ubiquitination within the necrosome complex, implying that this complex may itself be able to drive inflammatory transcription in some cases[58]. Recent work has found a death-independent role for RIPK3 in DC-driven inflammation and tissue repair in models of DSS-induced colitis[59]. Other studies have found unexpected links between RIPK3 and activation of inflammasomes[60-62]. These effects are likely mediated by caspase-8, though reports conflict on how this prototypical apoptotic caspase interacts with the inflammatory caspases-1 and −11 (or −4 and −5 in humans) to drive IL-1β processing[63].
The definition of MLKL as an obligate downstream effector of RIPK3-driven necroptosis presents an important tool, as comparison between RIPK3 and MLKL-deficient animals may yield insight into death-independent functions of RIPK3. As discussed above, it also remains to be seen whether MLKL has additional functions beyond RIPK3-driven membrane disruption. Identification of additional regulators of MLKL function may be required to tease apart this question.
Is necroptosis tumor suppressive?
Apoptotic death of damaged or transformed cells is a key mechanism of tumor suppression, and evasion of apoptosis is a classical hallmark of cancer. It is notable, however, that nearly all transformed cell lines commonly employed in the laboratory do not express RIPK3. A recent study demonstrated that this lack of RIPK3 is mediated by promoter methylation, and can be restored by demethylating agents[64]. However, why RIPK3 is broadly lost in tumor cells remains unclear; could necroptosis be engaged during transformation, or by DNA damage, to limit tumor initiation or progression? DNA-damaging chemotherapeutics have been reported to trigger necroptosis by promoting cIAP degradation[25,64]. This work raises the possibility that re-activation of RIPK3 expression could act to retard tumor growth or improve chemotherapeutic responses. However, RIPK3-deficient mice do not display overt susceptibility to cancer, though the responses to these mice to carcinogen-mediated or genetic cancer models have not been reported.
Does it matter how cells die?
Necroptosis is an important part of the innate immune response to DNA viruses. But, in this context does necroptosis cause inflammation, and if so does this contribute to the immune response to pathogens? Or, is elimination of infected cells the key, and necroptosis is simply a means of achieving this when apoptosis is blocked? If cell death pathways could be re-wired such that apoptotic stimuli instead engaged necroptosis and vice-versa, what would be the effect during development and infection? As mentioned above, necroptosis and apoptosis are morphologically distinct processes, and the lytic nature of necroptosis has led to the hypothesis that it is an intrinsically inflammatory form of cell death. On the other hand, recent work has pointed out that inflammatory cytokine production stimulated by TLR or TNFR1 engagement in living cells is a much more potent mediator of inflammation than necroptotic cell debris generated by killing cells through these signaling pathways[7]; in essence, necroptosis may act to terminate inflammatory transcription programs.
These thought-provoking findings do not countenance the presence of pathogens within dying cells. It may be that necroptotic death of infected cells is uniquely able to drive recruitment of inflammatory immune cells or promote the presentation of antigen derived from necroptotic cells to the adaptive immune system; in effect, necroptotic cell death could act as an endogenous adjuvant. Seemingly countering this idea, mice lacking RIPK3, or even RIPK1, RIPK3 and caspase-8, demonstrate intact cross-priming of CD8 T cell responses to necroptosis- and apoptosis-inducing mutants of MCMV[30]. However, these mice also fail to control viral infection, such that viral loads are higher in these knockouts than in wild-type animals. Thus, increased viral loads may drive inflammatory signals that compensate for the lack of necroptosis-mediated inflammation and promote immunity in this context[65].
The debate over the inflammatory nature of necroptosis has focused on the release of DAMPs by this form of death; however, as discussed above there are likely non-death functions of the core necroptotic machinery, and most physiological inducers of necroptosis are themselves able to drive inflammatory transcription. It may therefore be the case that physiological necroptosis involves the coordination of transcriptional and cell death programs, and that this combination yields a unique immune signature. Lurking in the wings of this discussion is the phenomenon of pyroptosis, another pathogen-driven form of lytic cell death, associated with IL-1β and IL-18 processing in macrophages. How immune responses to necroptosis and pyroptosis differ remains an unexplored question, complicated by recently described cross-talk between these two pathways[60-62].
So…what’s the deal with necroptosis?
Despite an explosion of interest in necroptosis it is obvious that many key questions remain. A parting shot: Why does necroptosis work this way? That is, why is caspase-8, a well-described member of the apoptotic machinery, required for survival due to the suppression of another cell death program? And what are we to make of MLKL-mediated membrane permeabilization as a means of cell death execution, when it differs so completely from the protease-dependent mechanisms of apoptosis and pyroptosis? Why did evolution favor this arrangement?
An intriguing clue to the evolutionary origins of this pathway may lie in the observation that Drosophila encode RHIM-like domains within proteins involved in innate sensing of peptidoglycans, in a kinase called IMD that also contains a death domain (like RIPK1), as well as in the NF-kB component Relish[1]. Notably, Relish is cleaved and activated by a Drosophila caspase-8 homologue Dredd. Thus, the fly has a kinase and caspase-containing complex that can be activated by pathogen-associated molecules, and whose interactions are mediated by RHIM and death domains, a situation clearly evocative of the mammalian necrosome. However, this pathway is not associated with death in the fly; instead, it drives Relish-mediated inflammatory signaling in response to bacterial cell wall components. Mammalian NF-kB does not share the requirement for caspase-mediated proteolysis observed in Drosophila, but could links between the necrosome and inflammatory transcription remain in mammals? Indeed, an unexpected role for caspase-8 in LPS-mediated pro-IL-1β expression was recently described[66]. But understanding how evolution may have reconfigured the Drosophila IMD pathway into the mammalian necroptotic machinery will require understanding of these pathways in additional evolutionary intermediates.
Ultimately, while I’ve described some of the Known Unknowns within this field, many of the most fascinating aspects of necroptotic signaling likely remain Unknown Unknowns. And for a researcher, that is a tantalizing state of affairs.
References
- 1.Chan FK-M, Luz NF, Moriwaki K. Programmed Necrosis in the Cross Talk of Cell Death and Inflammation. Annu. Rev. Immunol. 2014;33 doi: 10.1146/annurev-immunol-032414-112248. 141210135520002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Mol. Cell. 2014;56:469–480. doi: 10.1016/j.molcel.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nature Immunology. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 4.Linkermann A, Green DR. Necroptosis. N. Engl. J. Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 6.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 7.Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, Martin SJ. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ. 2015;22:1313–1327. doi: 10.1038/cdd.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. The Journal of Immunology. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. Journal of Biological Chemistry. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. The Journal of Immunology. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang J-G, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RCJ, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. The Pseudokinase MLKL Mediates Necroptosis via a Molecular Switch Mechanism. Immunity. 2013 doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Sun L, Su L, Rizo J, Liu L, Wang L-F, Wang F-S, Wang X. Mixed Lineage Kinase Domain-like Protein MLKL Causes Necrotic Membrane Disruption upon Phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou J-C, Bertrand MJM, Vandenabeele P. MLKL Compromises Plasma Membrane Integrity by Binding to Phosphatidylinositol Phosphates. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Cai Z, Jitkaew S, Zhao J, Chiang H-C, Choksi S, Liu J, Ward Y, Wu L-G, Liu Z-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2013 doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao Y-S, Damko E, Moquin D, Walz T, McDermott A, Chan FK-M, Wu H. The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. Wu JLTMASJNKMY-SHEDDMTWAMF-MCH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X-N, Yang Z-H, Wang X-K, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. Diverse Sequence Determinants Control Human and Mouse Receptor Interacting Protein 3 (RIP3) and Mixed Lineage Kinase domain-Like (MLKL) Interaction in Necroptotic Signaling. J. Biol. Chem. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tait SWG, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013;5:878–885. doi: 10.1016/j.celrep.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazama H, Ricci J-E, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Moulin M, Anderton H, Voss AK, Thomas T, Wong WW-L, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL. IAPs limit activation of RIP kinases by TNF receptor 1 during development. The EMBO Journal. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yabal M, Müller N, Adler H, Knies N, Groß CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwalder M, Strasser A, Gross O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong Y-N, Janke LJ, Kelliher MA, Kanneganti T-D, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 Regulates RIPK3-MLKL-Driven Systemic Inflammation and Emergency Hematopoiesis. Cell. 2014 doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proceedings of the National Academy of Sciences. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proceedings of the National Academy of Sciences. 2013;110:E3109–18. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van T-M, Lee TH, Chan FKM, Pasparakis M, Kelliher MA. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Wu J, Li L, Zhang Z, Ren J, Liang Y, Chen F, Yang C, Zhou Z, Sean Su S, Zheng X, Zhang Z, Zhong C-Q, Wan H, Xiao M, Lin X, Feng X-H, Han J. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell Biol. 2015;17:434–444. doi: 10.1038/ncb3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proceedings of the National Academy of Sciences. 2015;112:5017–5022. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & Development. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rébé C, Cathelin S, Launay S, Filomenko R, Prévotat L, L'Ollivier C, Gyan E, Micheau O, Grant S, Dubart-Kupperschmitt A, Fontenay M, Solary E. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007;109:1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S, Yang Y, Mei Y, Ma L, Zhu D-E, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cellular Signalling. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR, Salvesen GS. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newton K, Dugger DL, Wickliffe KE, Kapoor N, Cristina de-Almagro M, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of Protein Kinase RIPK3 Determines whether Cells Die by Necroptosis or Apoptosis. Science. 2014 doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 41.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK-M, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-κB Activation, and TNFα-Dependent Apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Upton JW, Kaiser WJ, Mocarski ES. Virus Inhibition of RIP3-Dependent Necrosis. Cell Host and Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host and Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. Herpes Simplex Virus Suppresses Necroptosis in Human Cells. Cell Host and Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Z, Wu S-Q, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong C-Q, Xia W, Zhou R, Zheng C, Han J. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host and Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Current Opinion in Virology. 2013 doi: 10.1016/j.coviro.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Beg AA. Induction of Necrotic-Like Cell Death by Tumor Necrosis Factor Alpha and Caspase Inhibitors: Novel Mechanism for Killing Virus-Infected Cells. Journal of virology. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK-M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011 doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller J-O, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proceedings of the National Academy of Sciences. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe H-J, Linkermann A, Wolf MJ, Rose-John S, Lüllmann-Rauch R, Adam D, Flögel U, Heikenwalder M, Luedde T, Frey N. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc. Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 53.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Gautheron J, Vucur M, Reisinger F, Vargas Cardenas D, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, Canbay A, Reeves HL, Luedde M, Tacke F, Trautwein C, Heikenwalder M, Luedde T. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014 doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, Geng J, Py B, Zhou W, Amin P, Lima JB, Qi C, Yu Q, Trapp B, Yuan J. Activation of Necroptosis in Multiple Sclerosis. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, Chen T, Green DR. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, Kelliher MA. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proceedings of the National Academy of Sciences. 2014;111:14436–14441. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Almagro MC, Goncharov T, Newton K, Vucic D. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 2015;6:e1800. doi: 10.1038/cddis.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK-M. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vince JE, Wong WW-L, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Häcker G, Silke J, Tschopp J. Inhibitor of Apoptosis Proteins Limit RIP3 Kinase-Dependent Interleukin-1 Activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, Rashidi M, Wicks IP, Alexander WS, Mitsuuchi Y, Benetatos CA, Condon SM, Wong WW-L, Silke J, Vaux DL, Vince JE. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Comms. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, Roback L, Kaiser W, Oberst A, Sagara J, Fitzgerald KA, Green DR, Zhang J, Mocarski ES, Alnemri ES. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Comms. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang T-B, Yang S-H, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012 doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Koo G-B, Morgan MJ, Lee D-G, Kim W-J, Yoon J-H, Koo JS, Kim Il S, Kim SJ, Son MK, Hong SS, Levy JMM, Pollyea DA, Jordan CT, Yan P, Frankhouser D, Nicolet D, Maharry K, Marcucci G, Choi KS, Cho H, Thorburn A, Kim Y-S. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015 doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, Upton JW, Daley-Bauer LP. True Grit: Programmed Necrosis in Antiviral Host Defense, Inflammation, and Immunogenicity. The Journal of Immunology. 2014;192:2019–2026. doi: 10.4049/jimmunol.1302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurung P, Anand PK, Malireddi RKS, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti T-D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]