Abstract
Purpose
Fatigue is a prevalent, distressing side effect of cancer and cancer treatment which commonly co-exists with insomnia. Cognitive behavioral therapy for insomnia (CBT-I) has been shown to improve insomnia in cancer patients, but less is known about its ability to impact fatigue. This work is the analysis for a secondary aim of a 4-arm RCT study assessing the combined and comparative effect of CBT-I and a wakefulness-promoting agent, armodafinil (A), to improve sleep and daytime functioning in cancer survivors. Herein, we examine the effect of CBT-I, with and without A, on fatigue in cancer survivors.
Patients and Methods
This study was a four arm factorial study with CBTI-I (Yes/No) versus A (Yes/No). It consisted of 96 cancer survivors (Average age 56 years; 88% female; 68% breast cancer). Fatigue was assessed by the Brief Fatigue Inventory (BFI) and the FACIT-Fatigue scale. The analysis assessed the additive effects of CBT-I and A, and possible non-additive effects where the effect of CBT-I changes depending on the presence or absence of A.
Results
Analyses adjusting for baseline differences showed that CBT-I improved fatigue as measured by two separate scales (BFI: P=0.002, Std. Error=0.32, effect size (ES)=0.46; FACIT-Fatigue: P<0.001, Std. Error=1.74, ES=0.64). Armodafinil alone did not show a statistically significant effect on fatigue levels (all Ps>0.40), nor did the drug influence the efficacy of CBT-I. Structural equation analysis revealed that reductions in insomnia severity were directly responsible for improving cancer-related fatigue.
Conclusions
CBT-I with and without armodafinil resulted in a clinically and statistically significant reduction of subjective daytime fatigue in cancer survivors with chronic insomnia. Armodafinil did not improve CRF and did not change the efficacy of CBT-I. Patients reporting CRF should be screened and, if indicated, treated for insomnia as part of a comprehensive fatigue management program.
Keywords: Cancer, Cancer-related fatigue, CBT-I, Armodafinil
Background
Fatigue is the most common side effect of cancer treatment [1]. Cancer-related fatigue (CRF) is conceptualized as a complex, multidimensional experience of reduced energy and increased need for rest that is not related to activity or relieved by sleep [2]. Post-treatment fatigue has been shown to negatively affect cognitive function [3], psychological well-being [4], and physical function [5]; reduce overall health-related quality of life [6]; and possibly influence survival rates [7]. Unfortunately, CRF is also one of the most persistent consequences of cancer treatment. A recent study by Jones et al. found that one-third of cancer survivors continued to report significant levels of CRF 6 years post-treatment, and that these survivors had higher rates of long-term disability [8].
Poor sleep is also very common in cancer survivors with fatigue. For example, in a sample of 114 disease-free breast cancer patients, 44% of fatigued patients met the diagnostic criteria for insomnia, defined as difficulty initiating or maintaining sleep occurring more than 3 times per week for at least 3 months. By comparison, only 16% of non-fatigued patients met these criteria [10]. Poor sleep, both prior to and after completing cancer treatment, has been shown to predict higher levels of fatigue, even after adjusting for mood disturbance and physical activity levels [11,10,12]. Cancer survivors with CRF are also more likely than survivors without CRF to have clinically significant insomnia symptoms, despite adequate sleep opportunity. The guidelines for the management of CRF also recommend treating coexisting symptoms, including insomnia, that are often associated with CRF [13].
The fact that the symptoms of CRF and insomnia often occur in parallel, suggest that they may have a similar underlying etiology. The causal mechanisms of CRF are not yet understood but likely involve a combination of biological and psychological factors [9]. Miller et al. describe some of the hypothesized neuroendocrine and immune mechanisms of cancer-related behavioral comorbidities[14]. To begin with, the cancer diagnosis and its treatment activate inflammation through tissue damage/destruction and/or psychological stress. This can lead to behavioural changes and an increase in proinflammatory cytokines, which can then disrupt the sleep-wake cycle causing disruption of the neuroendocrine system, and the hypothalamic-pituitary-adrenal (HPA) axis. These changes then impact central nervous system pathways that regulate behavior, which can produce the pathophysiological changes that underlie fatigue and impaired sleep, as well as depression and cognitive dysfunction. Understanding exactly how these processes interact is an area of great research interest and clinical importance.
Evidence is steadily accumulating that Cognitive Behavioral Therapy for Insomnia (CBT-I) is an effective intervention to address poor sleep in individuals diagnosed with cancer [16-19]. In addition to treating insomnia, CBT-I has also been shown to decrease levels of anxiety and depression related to cancer treatment [20,21]. However, studies examining the efficacy of CBT-I for improving CRF in cancer survivors are conflicting; hence, it is not clear whether the use of sleep management interventions will actually improve CRF [15]. In three previous RCTs of CBT-I in cancer, two reported improvements in both insomnia and fatigue [22,19], while the other improved insomnia only [23]. These trials, however, only included usual care and waitlist control groups. As such, it is still an imperative to evaluate interventions for insomnia in randomized clinical trials with active comparison groups to examine their efficacy in reducing CRF through associated improvement in insomnia.
Another parallel line of research is the use of wakefulness-promoting medications, such as modafinil or armodafinil, to treat CRF during and after cancer treatment; however, the results of these trials have not been consistent. In one study, modafinil was shown to elicit modest improvement in chemotherapy-related fatigue in patients with metastatic breast or prostate cancer [24]. By contrast, other studies have shown that modafinil was not significantly different from placebo for reducing fatigue in patients with lung cancer [25] or primary brain tumor [26]. Similarly, armodafinil (the R enantiomer of modafinil) was not significantly different than placebo in reducing fatigue in patients with multiple myeloma [27].
We recently published a four-arm placebo controlled randomized controlled trial of CBT-I +/− armodafinil compared to armodafinil alone and placebo alone in 96 cancer survivors with insomnia [28]. Both CBT-I groups experienced significant overall improvement in subjective insomnia severity and sleep quality. There were no differences between the two CBT-I groups, and there were no significant effects for the armodafinil only and placebo only groups. The objective of the present analysis was to address a secondary aim: examine the effect of CBT-I, with and without the addition of armodafinil, on levels of fatigue in cancer survivors.
Methods
As described in full detail previously [28] and briefly below, cancer survivors with chronic insomnia were recruited between September, 2008, and November, 2012. Subjects with any cancer type must have completed all chemotherapy and/or radiation not less than one month prior to study start, must have demonstrated no measurable disease, and were required to discontinue any prescribed or over the counter sleep medications for one week prior to beginning the baseline data collection as well as for the 11-week study period. Subjects who had ever taken modafinil or armodafinil, had received CBT-I therapy, or had an unstable medical or psychiatric illness were not eligible. Subjects were randomized to one of four groups (CBT-I + placebo, CBT-I + armodafinil 50 mg b.i.d., placebo b.i.d, or armodafinil 50 mg b.i.d.). All study personnel and subjects were blinded regarding medication (armodafinil, placebo) assignment but not CBT-I (yes, no) condition. The institutional review boards of the University of Rochester and the University of Pennsylvania approved the protocol, and subjects provided written informed consent. This trial was registered with ClinicalTrials.gov, number NCT01091974.
Treatments
All subjects received written sleep hygiene guidelines (e.g., keep bedroom cool and free of light, avoid naps, avoid using alcohol as a sleep aid, etc.) at the time of consent. The 7-week CBT-I intervention was provided on an individual basis and followed a published treatment manual [29]. CBT-I Sessions 1, 2 and 4 were in person (30-60 minutes in duration), and Sessions 3, 5, 6 and 7 (15-30 minutes in duration) were by phone. Subjects were instructed to take the study medication (armodafinil or placebo) in a split dose (7-9 am and 12-2 pm) for a total of 47 days. For titration purposes, a placebo capsule was substituted for the afternoon does of armodafinil on the first three days and on the last four days the medication was provided. We note that armodafinil is indicated for the promotion of wakefulness in several sleep disorders including Narcolepsy, Sleep Apnea Syndrome, and Shiftwork Disorder.
Assessments
Brief Fatigue Inventory (BFI): This 9-item instrument was designed to assess fatigue severity in cancer and non-cancer populations on a numerical rating scale ranging from 0-10, with 10 indicating the greatest severity or interference. The scale is reliable (α>0.9) and valid in multiple languages and diverse cancer populations [30]. A published Minimum Clinically Important Difference (MCID) has not been established. Half of the standard deviation (SD) is commonly used when more rigorous MCID studies have not been performed [31]. Based on a SD of 2.8 [30], we use a provisional MCID of half that number (1.4).
Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue): FACIT-Fatigue is a subscale of the FACIT-F instrument consisting of 13 questions directly related to the impact of fatigue on daily activities. The FACIT-Fatigue has good reliability (alpha>0.85) and test-retest reliability (ICC=0.89) with a MCID of ca. 2.7 [32].
Insomnia Severity Index (ISI): The ISI is a validated instrument for measuring insomnia. It ranges from 0 to 28, with 0-7, 8-14, 15-21, and 22-28 indicating absence, subthreshold, moderate and severe insomnia, respectively [33].
Sample Size
This is an analysis of a secondary aim from the trial that was designed to detect differences in the Insomnia Severity Index (ISI) change. For the 96 accrued subjects, an effect size of 0.9 could be detected with 80% power at an overall 0.05 significance level [28]. There were four comparisons; each comparison was tested at the 0.0125 level. For the analysis reported here, we used a linear model with Pre (pre-intervention) as a covariate, CBT-I (Yes/No) and armodafinil (Yes/No) as main effects, and CBT-I by armodafinil interaction. See the Statistical Analysis section for more details. For the 96 subjects, the effect size for the detectable interaction is 1.0, so that the detectable change in BFI is 2.2 and FACIT-Fatigue is 11.15. The effect size for the detectable main effects is 0.75, so that the detectable change in BFI is 1.6 and FACIT-Fatigue is 8.4. These simulation-based calculations used the observed sample sizes for the four groups and assumed a Pre-Post correlation of 0.7. There are six tests involved in the analysis of both outcomes (two for the interaction and four for the main effects), so we conservatively chose a significance level of 0.05/6=0.008 (Bonferroni adjustment). This is conservative because of the high correlation (0.8-0.9) between the two outcomes.
Statistical Analyses
Descriptive statistics were performed for demographic characteristics, clinical variables and baseline patient reported fatigue. The same analyses were performed on each of the two outcomes. Before performing the linear model analyses, we calculated descriptive statistics of pre-intervention (Pre), post-intervention (Post), and the change Post-Pre. One-sample T-tests were performed on the mean changes to identify those that were significantly nonzero. We started with a linear model (least squares estimation) with the post-intervention outcome as the response, pre-intervention response as a covariate, CBT-I (Yes/No) and armodafinil (Yes/No) as main effects, and a CBT-I by armodafinil interaction. The statistical significance of the interaction was assessed by Type III F tests, and if not statistically significant at the 0.05 level, was removed from the model. For both fatigue outcomes, the interaction was non-significant and was removed. The model without the interaction was refit, and the coefficients, standard errors, confidence intervals (CI), and hypothesis tests were calculated for the CBT-I and FACIT-Fatigue terms. As an exploratory analysis, we also assessed changes from Post-Intervention to Follow-up (the average of Weeks 23 and 24) using one-way ANOVA on the change scores (Post-Intervention - Follow-up). We tested whether there was an overall change or any differences among the four treatment arms. Analyses were done by intention to treat, although 29 (30%) of the 96 randomized eligible subjects did not provide post-intervention data. Only the one subject missing baseline was omitted from the analysis. The missing value patterns were examined through visual inspection and logistic regression of dropout versus treatment arm and relevant clinical demographic characteristics. We found that subjects who had surgery during the last three months were more likely to drop out after baseline; this characteristic was included in the multiple imputation (MI) procedure described below.
We also tested whether the rate of dropout depended on the previous BFI value and found no association. Assuming a Missing at Random (MAR) mechanism [34], Multiple imputation was performed using Pre (pre-intervention or baseline), Post (post-intervention), and Follow-up BFI and FACIT-Fatigue, as well as the nominal previous surgery variable. The Multiple Imputation by Chained Equations (MICE) procedure (50 iterations per imputed dataset) was used to generate fifty complete datasets [35,36]. The above analyses were performed on each dataset and the results combined using the method of Rubin [34] for the coefficient estimates and the combination of chi-squares method [37] for the Type III F tests. Since it is impossible to know whether the missing data mechanism is MAR or missing not at random (MNAR), we also performed sensitivity analyses where, during each MI, we added a range of offsets to the imputed values where the outcome was missing and assessed statistical significance [38].
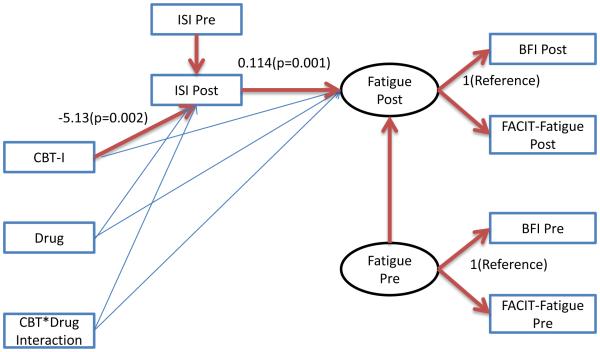
To explore the issue of whether the interventions affected fatigue directly, or the effect was a consequence of the intervention improving insomnia, we performed a mediation-like analysis using a structural equation model (SEM) to see if insomnia mediates the effect of the interventions on fatigue. Fatigue pre and post were defined as latent variables (LVs) that are measured by BFI and FACIT-Fatigue at Pre and Post, respectively. The interventions (CBT-I, Drug, and the interaction) entered the model as direct effects on ISI Post (adjusted for ISI Pre), as well as on Fatigue. Then the ISI Post (the primary sleep quality measure) was used as a predictor for Fatigue, defining the indirect effect. Maximum Likelihood estimation was used. (Figure 3) Analyses were performed using R Version 3 (with the car, mice, and miceadds packages), and Mplus Version 7.3 for the SEM.
Figure 3.
Path diagram for the structural equation model. Statistically significant paths are highlighted, with selected path coefficients marked. Paths that are not highlighted are not statistically significant, P>0.05.
Results
Details of the patient flow and demographics are provided in our published manuscript on the primary study aim of insomnia [28]. Of the 138 subjects who consented to screening, 114 were eligible and 96 were randomized, with 24, 23, 25, and 24 subjects randomized to CBT-I + Placebo (CBT-I+P), CBT-I + Armodafinil (CBT-I+A), Placebo, and Armodafinil, respectively. 88 Subjects began the intervention. Average compliance with the study medication, as determined by the returned study medication cards, was above 90% for all study arms and did not differ significantly by group. No serious related adverse events were reported. For the 96 randomized subjects, the mean(SD) age was 56(10) years, 88% were female, 68% had breast cancer, 90% were white, 95% were non-Hispanic, 80% received chemotherapy, and 74% received radiotherapy. At baseline, the mean(SD) BFI was 3.6(2.2), and FACIT-Fatigue was 31.6(11.1). There were no important or statistically significant differences in these characteristics among the four groups.
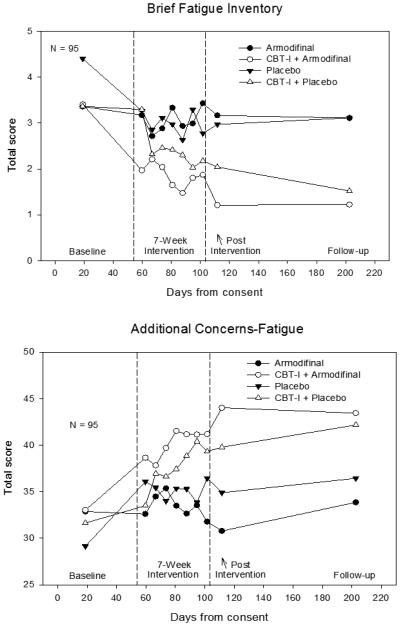
Means for all the assessments are plotted in Figure 1. MI estimates of the mean (95% CI) for Post-Pre change of the BFI and the FACIT-Fatigue for the four study conditions are provided in Table 1. The CBT-I by Drug interaction was not statistically significant (using MI) for either outcome, both Ps>0.05 (Table 2). The CBT-I effect (95% CI) for BFI was −1.00(−1.64,−0.37), P=0.0024, meaning that CBT-I led to a mean change one unit less than no CBT-I. The Drug effect (95% CI) was −0.11(−0.73,0.51), P=0.7304. The CBT-I effect (95% CI) for FACIT-Fatigue was 7.16(3.68,10.64), P<0.0001, meaning that CBT-I led to a mean change seven units higher than no CBT-I. The Drug effect (95% CI) was −1.27(−4.68,2.14), P=0.4584. The MNAR sensitivity analysis revealed that the CBT-I intervention would become statistically insignificant if the subjects that dropped out would have reported a reduction of fatigue, and we consider this to be a very unlikely reason for dropout, particularly since none of the recorded reasons for dropout were due to a reduction of fatigue. Indeed, examination of the dropout reasons revealed that none involved fatigue at the time of dropout, suggesting that MNAR is unlikely. There was no statistically significant change between Post-Intervention and Followup. P=0.294 (BFI), P=0.145 (FACIT-Fatigue).
Figure 1.
Mean fatigue outcomes by assessment. These are the raw (complete case) means.
Table 1.
Descriptive statistics for the two fatigue outcomes. N=Sample sixe for complete data only. P-values are associated with testing whether the mean change is statistically different than zero. LCB95 and UCB95 are the lower and upper 95% confidence limits, respectively. These results used multiple imputation.
| BFI Total |
FACIT-Fatigue |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm | N | Mean | Std. Error |
P-value | LCB9 5 |
UCB95 | Mean | Std. Error |
P-value | LCB9 5 |
UCB95 |
| CBT&Placebo | |||||||||||
| Pre | 24 | 3.37 | 0.44 | 2.44 | 4.29 | 31.63 | 2.35 | 26.75 | 36.50 | ||
| Post | 18 | 1.88 | 0.36 | 1.13 | 2.63 | 40.86 | 1.92 | 36.85 | 44.88 | ||
| Change | 18 | −1.48 | 0.36 | 0.0005 | −2.22 | −0.74 | 9.24 | 1.75 | <0.0001 | 5.57 | 12.90 |
|
| |||||||||||
| CBT & Drug | |||||||||||
| Pre | 23 | 3.41 | 0.47 | 2.43 | 4.39 | 33.01 | 2.37 | 28.06 | 37.96 | ||
| Post | 17 | 1.31 | 0.34 | 0.61 | 2.02 | 43.50 | 2.04 | 39.21 | 47.80 | ||
| Change | 17 | −2.10 | 0.37 | <0.0001 | −2.88 | −1.32 | 10.50 | 1.94 | <0.0001 | 6.40 | 14.59 |
|
| |||||||||||
| Drug | |||||||||||
| Pre | 24 | 3.36 | 0.37 | 2.58 | 4.14 | 32.86 | 1.94 | 28.82 | 36.90 | ||
| Post | 15 | 2.77 | 0.40 | 1.92 | 3.63 | 33.24 | 2.47 | 28.00 | 38.47 | ||
| Change | 15 | −0.59 | 0.38 | 0.1421 | −1.39 | 0.22 | 0.37 | 2.10 | 0.8609 | −4.12 | 4.87 |
|
| |||||||||||
| Placebo | |||||||||||
| Pre | 24 | 4.41 | 0.48 | 3.41 | 5.40 | 29.13 | 2.52 | 23.90 | 34.35 | ||
| Post | 17 | 2.97 | 0.46 | 2.01 | 3.94 | 35.02 | 2.53 | 29.74 | 40.29 | ||
| Change | 17 | −1.43 | 0.35 | 0.0007 | −2.17 | −0.70 | 5.89 | 1.56 | 0.0015 | 2.60 | 9.19 |
Table 2.
Linear model results. Both complete case (CC) and Multiple Imputation (MI) results are shown. LCB95 and UCB95 are the lower and upper 95% confidence limits, respectively.
| BFI Total |
FACIT-Fatigue |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Estimate | Std. Error |
P-value | LCB95 | UCB95 | Effect Size |
Estimate | Std. Error |
P-value | LCB95 | UCB95 | Effect Size |
|
|
|
|||||||||||
| CBT(Yes-No) | ||||||||||||
| CC | −1.11 | 0.34 | 0.0018 | −1.79 | −0.43 | −0.51 | 7.91 | 1.73 | <0.0001 | 4.45 | 11.37 | 0.71 |
| MI | −1.00 | 0.32 | 0.0024 | −1.64 | −0.37 | −0.46 | 7.16 | 1.74 | 0.0001 | 3.68 | 10.64 | 0.64 |
| Drug(Yes-No) | ||||||||||||
| CC | −0.16 | 0.34 | 0.6344 | −0.84 | 0.51 | −0.07 | −1.33 | 1.74 | 0.4478 | −4.80 | 2.14 | −0.12 |
| MI | −0.11 | 0.31 | 0.7304 | −0.73 | 0.51 | −0.05 | −1.27 | 1.70 | 0.4584 | −4.68 | 2.14 | −0.11 |
| Drug by CBT-I | ||||||||||||
| Interaction F Test | ||||||||||||
| CC | 0.1058 | 0.0398 | ||||||||||
| MI | 0.1057 | 0.0656 | ||||||||||
The SEM fit the data very well, Root Mean Square Error of Approximation=0.078 (less than 0.1 is ideal), and CFI=0.972 (which can be interpreted like an R^2 in a regression).[39] (Figure 3) The only statistically significant effects (path coefficients) were CBT-I on ISI Post, ISI Pre on ISI Post (expected), ISI Post on the Fatigue Post LV, and Fatigue Pre LV variable on Fatigue Post (expected). This implies that the positive effect of CBT-I on insomnia determined its positive effect on Fatigue, and there were no direct effects of any of the interventions on Fatigue.
There was no statistically significant overall change from Post-Intervention to Follow-up, P=0.480, and 0.378 for BFI and FACIT-Fatigue, respectively. There were also no statistically significant differences in mean change among the arms, P=0.295, and 0.145 for BFI and FACIT-Fatigue, respectively.
Discussion
We previously reported the primary outcome from this study, which was that a seven-week treatment program of CBT-I was effective in treating insomnia in cancer survivors [28]. Herein we show that CBT-I, both with and without armodafinil, results in a clinically and statistically significant reduction of fatigue in cancer survivors with insomnia. Specifically, CBT-I reduced the total score on the BFI by 1.0, (95% CI: 0.5,1.6). This is not inconsistent with a clinically significant change since the 95% confidence interval contains 1.4 (but the interval is somewhat wide because this was not a large study) CBT-I was also associated with a clinically important improvement in fatigue of 7.2 points (95% CI: 3.7,10.6) as measured by the FACIT-Fatigue scale. As shown by our SEM analyses, the reduction in insomnia severity was directly responsible for improving cancer-related fatigue. These findings are consistent with previous studies that have also shown CBT-I to result in decreased levels of cancer-related fatigue [20,21] and support the use of CBT-I as an effective intervention for fatigue related to cancer in survivors who also have insomnia.
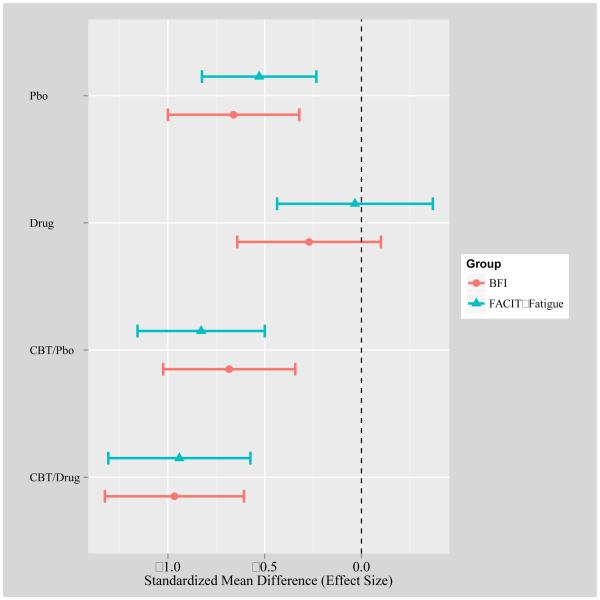
Armodafinil had no detectable effect on fatigue. This is consistent with other trials that have not demonstrated an effect of armodafinil or modafinil on fatigue in cancer patients [24-26]. Trials in populations other than cancer have reported a significant improvement in daytime fatigue with the use of armodafinil [40-42]. A possible explanation for this discrepancy is that fatigue associated with insomnia in cancer survivors is experientially different from the daytime sleepiness in these other populations. Another reason could be that the positive effect of armodafinil may be more likely to occur in cancer survivors with more severe fatigue at baseline [43]. It is also possible that the fixed dose of armodafinil may have been too low to see an effect, or our relatively small sample size may have reduced our ability to detect an effect for armodafinil, but we think that this is unlikely because the drug when provided without CBT-I appeared to be associated with increased fatigue (Figure 2). The failure of armodafinil to reduce fatigue should discourage the use of this drug for this purpose in oncologic practice.
Figure 2.
Post-Pre effect sizes with 95% confidence intervals using MI estimates. These were calculated by dividing the mean Post-Pre change by the Pre standard deviation. The FACIT-Fatigue values were multiplied by −1; Increased negative values imply improvement in fatigue.
We also found that improved nighttime sleep was directly responsible for the observed improvements in cancer-related fatigue. This is clinically relevant considering the high prevalence and burden of fatigue in cancer patients and suggests that patients reporting CRF should be screened, and if indicated, treated for insomnia as part of a comprehensive fatigue management program. Our findings are in contrast with a 2009 Cochrane review that found psychosocial interventions aimed at improving psychological distress, mood and sleep disturbances failed to improve CRF as a secondary outcome [44]. These studies, however, were broad in focus and did not necessarily target sleep for cancer survivors with clinical insomnia. We know that reductions in insomnia result in secondary improvement in mood and now have evidence that these sleep improvements can effectively reduce fatigue.
This study has several strengths, including the inclusion of patients with clinically diagnosed insomnia at baseline and the heterogeneity of the sample, increasing the likelihood that these findings may be generalizable to a variety of cancer survivors. However, the following limitations must be considered. First, the sample size was too small to detect a possibly clinically meaningful interaction between CBT-I and armodafinil reliably. From the power calculations, the detectable effect size (ES) was 0.75, larger than the ca. 0.5 difference in ES evident from the figure. Hence, the result might have changed with a larger sample size. Second, recruitment was a challenge in this trial, mainly due to refusal of patients to discontinue all current sleep medication and/or take the study medication, which negatively impacted sample size.
In conclusion, this study strongly supports the use of CBT-I for treatment of CRF in cancer survivors with insomnia. This positive effect is the result of the improvements in insomnia induced by this psychosocial intervention. Armodafinil did not have a detectable or clinically meaningful effect on CRF. There is a trend, however, suggesting that the drug alone (without CBT-I) actually made CRF worse.
Acknowledgments
Supported by NCI grants 5 R01 CA126968, R25 CA102618 and UG1 CA18961. Study medication was provided by Teva Pharmaceuticals USA.
Footnotes
Conflict of Interest / Disclosure
Dr. Perlis receives royalties for a CBT-I Manual and Demonstration DVD, served as an advisor to InsomniSolv, and research funding from Teva Pharmaceuticals. He has also provided expert testimony to Cantor Colburn, LLP. None of the remaining authors have disclosures to report.
Drs. Heckler and Roscoe have full control of the primary data, and agree to allow the journal to review it on request.
Literature Cited
- 1.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nature reviews Clinical oncology. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. doi:10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, Ancoli-Israel S, Reeve BB, Mustian K, O'Mara A, Lai JS, Fisch M, Cella D, National Cancer Institute Clinical Trials Planning M Recommendations for high-priority research on cancer-related fatigue in children and adults. Journal of the National Cancer Institute. 2013;105(19):1432–1440. doi: 10.1093/jnci/djt242. doi:10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, Reneman L, Schagen SB. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment--the role of fatigue. NeuroImage Clinical. 2015;7:547–554. doi: 10.1016/j.nicl.2015.02.005. doi:10.1016/j.nicl.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekse RJ, Hufthammer KO, Vika ME. Fatigue and quality of life in women treated for various types of gynaecological cancers: a cross-sectional study. Journal of clinical nursing. 2015;24(3-4):546–555. doi: 10.1111/jocn.12647. doi:10.1111/jocn.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, Springer BA, Pfalzer LA. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(10):1581–1591. doi: 10.1007/s00520-010-0986-7. doi:10.1007/s00520-010-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JA, Lasch KE, Barsevick AM, Piault-Louis E. Patients' experiences with cancer-related fatigue: a review and synthesis of qualitative research. Oncology nursing forum. 2011;38(3):E191–203. doi: 10.1188/11.ONF.E191-E203. doi:10.1188/11.ONF.E191-E203. [DOI] [PubMed] [Google Scholar]
- 7.Paiva CE, Paiva BS. Prevalence, predictors, and prognostic impact of fatigue among Brazilian outpatients with advanced cancers. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(4):1053–1060. doi: 10.1007/s00520-012-1625-2. doi:10.1007/s00520-012-1625-2. [DOI] [PubMed] [Google Scholar]
- 8.Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, McCready DR, Wong RK, Jiang H, Howell D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. Journal of cancer survivorship : research and practice. 2015 doi: 10.1007/s11764-015-0450-2. doi:10.1007/s11764-015-0450-2. [DOI] [PubMed] [Google Scholar]
- 9.Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M, Consortium G. I'm so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–1427. doi: 10.1007/s11136-010-9757-7. doi:10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012;2(3):231–238. doi: 10.1136/bmjspcare-2011-000172. doi:10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manage. 2013;45(2):213–222. doi: 10.1016/j.jpainsymman.2012.02.009. doi:10.1016/j.jpainsymman.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Pertl MM, Hevey D, Collier S, Lambe K, O'Dwyer AM. Predictors of fatigue in cancer patients before and after chemotherapy. Journal of health psychology. 2014;19(6):699–710. doi: 10.1177/1359105313477675. doi:10.1177/1359105313477675. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, Cimprich B, Cleeland C, Eisenberger MA, Escalante CP, Jacobsen PB, Kaldor P, Ligibel JA, Murphy BA, O'Connor T, Pirl WF, Rodler E, Rugo HS, Thomas J, Wagner LI. Cancer-related fatigue. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8(8):904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 14.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. doi:10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zee PC, Ancoli-Israel S, Workshop P. Does effective management of sleep disorders reduce cancer-related fatigue? Drugs. 2009;69(Suppl 2):29–41. doi: 10.2165/11531140-000000000-00000. doi:10.2165/11531140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. doi:23/25/6083 [pii] 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 17.Matthews EE, Berger AM, Schmiege SJ, Cook PF, McCarthy MS, Moore CM, Aloia MS. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncology nursing forum. 2014;41(3):241–253. doi: 10.1188/14.ONF.41-03AP. doi:10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 18.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(5):449–457. doi: 10.1200/JCO.2012.47.7265. doi:10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 19.Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, Douglas NJ, Engleman HM, Kelly HL, Paul J. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. doi:10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 20.Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, Campbell T. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatric disease and treatment. 2014;10:1113–1124. doi: 10.2147/NDT.S47790. doi:10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming L, Randell K, Harvey CJ, Espie CA. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psycho-oncology. 2014;23(6):679–684. doi: 10.1002/pon.3468. doi:10.1002/pon.3468. [DOI] [PubMed] [Google Scholar]
- 22.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61(6):664–675. doi: 10.1111/j.1365-2648.2007.04560.x. doi:10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 23.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005;23(25):6097–6106. doi: 10.1200/JCO.2005.12.513. doi:10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 24.Hovey E, de Souza P, Marx G, Parente P, Rapke T, Hill A, Bonaventura A, Michele A, Craft P, Abdi E, Lloyd A, investigators M. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(5):1233–1242. doi: 10.1007/s00520-013-2076-0. doi:10.1007/s00520-013-2076-0. [DOI] [PubMed] [Google Scholar]
- 25.Spathis A, Fife K, Blackhall F, Dutton S, Bahadori R, Wharton R, O'Brien M, Stone P, Benepal T, Bates N, Wee B. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(18):1882–1888. doi: 10.1200/JCO.2013.54.4346. doi:10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 26.Boele FW, Douw L, de Groot M, van Thuijl HF, Cleijne W, Heimans JJ, Taphoorn MJ, Reijneveld JC, Klein M. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro-oncology. 2013;15(10):1420–1428. doi: 10.1093/neuonc/not102. doi:10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berenson JR, Yellin O, Shamasunder HK, Chen CS, Charu V, Woliver TB, Sanani S, Schlutz M, Nassir Y, Swift RA, Andreu-Vieyra C, Vescio R. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014 doi: 10.1007/s00520-014-2486-7. doi:10.1007/s00520-014-2486-7. [DOI] [PubMed] [Google Scholar]
- 28.Roscoe JA, Garland SN, Heckler CE, Perlis ML, Peoples AR, Shayne M, Savard J, Daniels NP, Morrow GR. Randomized Placebo-Controlled Trial of Cognitive Behavioral Therapy and Armodafinil for Insomnia After Cancer Treatment. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.57.6769. doi:10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral tretament of insomnia: A Session-by-Session Guide. Springer; New York: 2005. [Google Scholar]
- 30.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. doi:10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 33.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. doi: 10.1002/pon.860. doi:10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons, Hoboke. 2002 [Google Scholar]
- 35.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 36.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3) [Google Scholar]
- 37.Enders CK. Applied Missing Data Analysis. The Guilford Press; New York: 2010. [Google Scholar]
- 38.van Buuren S. Chapman & Hall/CRC interdisciplinary statistics series. CRC Press; Boca Raton, FL: 2012. Flexible imputation of missing data. [Google Scholar]
- 39.Byrne BM. Multivariate applications series. Routledge Academic; New York: 2012. Structural equation modeling with Mplus : basic concepts, applications, and programming. [Google Scholar]
- 40.Brown JN, Howard CA, Kemp DW. Modafinil for the treatment of multiple sclerosis-related fatigue. The Annals of pharmacotherapy. 2010;44(6):1098–1103. doi: 10.1345/aph.1M705. doi:10.1345/aph.1M705. [DOI] [PubMed] [Google Scholar]
- 41.Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Medical science monitor : international medical journal of experimental and clinical research. 2010;16(8):RA177–186. [PubMed] [Google Scholar]
- 42.Saavedra-Velez C, Yusim A, Anbarasan D, Lindenmayer JP. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. The Journal of clinical psychiatry. 2009;70(1):104–112. doi: 10.4088/jcp.07r03982. [DOI] [PubMed] [Google Scholar]
- 43.Jean-Pierre P, Morrow GR, Roscoe JA, Heckler C, Mohile S, Janelsins M, Peppone L, Hemstad A, Esparaz BT, Hopkins JO. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520. doi: 10.1002/cncr.25083. doi:10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. The Cochrane database of systematic reviews. 2009;(1):CD006953. doi: 10.1002/14651858.CD006953.pub2. doi:10.1002/14651858.CD006953.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]