Abstract
All inner ear organs possess extracellular matrix appendices over the sensory epithelia that are crucial for their proper function. The tectorial membrane (TM) is a gelatinous acellular membrane located above the hearing sensory epithelium and is composed mostly of type II collagen, and α– and β–tectorins. TM molecules self-assemble in the endolymph fluid environment, interacting medially with the spiral limbus and distally with the outer hair cell stereocilia. Here, we used immunogold labeling in freeze-substituted mouse cochleae to assess the fine localization of both tectorins in distinct TM regions. We observed that the TM adheres to the spiral limbus through a dense thin matrix enriched in α- and β–tectorin, both likely bound to the membranes of interdental cells. Freeze-etching images revealed that type II collagen fibrils were crosslinked by short thin filaments (4 ± 1.5 nm, width), resembling another collagen type protein, or chains of globular elements (15 ± 3.2 nm, diameter). Gold-particles for both tectorins also localized adjacent to the type II collagen fibrils, suggesting that these globules might be composed essentially of α- and β-tectorins. Finally, the presence of gold-particles at the TM lower side suggests that the outer hair cell stereocilia membrane has a molecular partner to tectorins, probably stereocilin, allowing the physical connection between the TM and the organ of Corti.
Keywords: extracellular matrix, electron microscopy, immunogold-labeling, tectorial membrane, tectorins
Introduction
The tectorial membrane (TM) is a gelatinous sheetlike structure that covers the sensory epithelium of vertebrate hearing organs. In the mammalian cochlea, the TM is medially attached to the spiral limbus surface and extends towards the organ of Corti. The tips of the tallest row of stereocilia of the outer hair cells (OHC) are anchored to the TM lower surface, via the membrane protein stereocilin, forming the TM imprints (Verpy et al. 2011). The inner hair cells (IHC) do not make imprints but interact with the TM Hensen’s stripe, a swelling region at the TM lower side. These physical connections between hair cells and TM enable the direct coupling of basilar membrane vibrations caused by sound waves and hair cell stereocilia deflections, triggering the mechano-electrical transduction machinery.
Different roles have been proposed for the TM, e.g., a rigid plate that slides back and forth as the basilar membrane vibrates, a secondary resonator that amplifies input to the hair bundle, an inertial mass, a structure for sound wave propagation, a mediator of OHC based cochlear amplification, and a regulator of electrokinetic motion (Richardson et al. 2008; Ghaffari et al. 2007; 2010; 2013; Lukashkin et al. 2010; Gavara et al. 2011; Sellon et al. 2014). Further investigations would clarify whether the TM performs one or more of these functions.
The TM is composed of four types of collagens (II, V, IX and XI), seven types of noncollagenous glycoproteins (α-tectorin, β-tectorin, carcinoembryonic antigen-related cell adhesion molecule 16 - CEACAM16, otogelin, otogelin-like, otoancorin and otolin) and two glycosaminoglycans (uronic acid and keratan sulfate) (Thalmann et al. 1986; 1987; 1993; Richardson et al. 1987; Thalmann 1993; Zwaenepoel et al. 2002; Deans 2010; Zheng et al. 2011; Kammerer et al. 2012; Oonk et al. 2014). During embryonic development, all these molecules self-assemble into the open fluid environment of the endolymph. This suggests that TM molecules possess specific sites of interaction, which determine its architecture and establish the connections to OHC stereocilia and the spiral limbus.
α-tectorin (Tecta) and β-tectorin (Tectb) are specific to inner ear organs and comprise a significant amount of the total TM mass (Richardson et al. 2008). Tecta is a 239 kDa glycoprotein that contains an NH2-terminal with similarities to entactin G1 domain (a large central segment with 4 von Willebrand factor type D repeats and 1 type C repeat), three repeat domains similar to trypsin inhibitors, and a carboxyl-terminal with a single zona pellucida (ZP) domain (Legan et al. 2005). Mutations in TECTA cause two types of hearing impairments in humans: non-syndromic autosomal recessive DFNB21 (Mustapha et al., 1999), and non-syndromic autosomal dominant DFNA8/12 (Legan et al. 1997; Verhoeven et al. 1998). Tectb is a small 36 kDa glycoprotein with a single zona pellucida domain (Goodyear and Richardson 2002). Thus far, no mutation in TECTB causing hearing loss has been described.
Mouse models have revealed the importance of tectorins during the formation of the TM matrix (Russell et al., 2007). Some authors suggest that tectorins may form filamentous proteins through their ZP domains (Legan et al. 1997). Legan and colleagues (2013) generated three mouse mutants to study human Tecta mutations linked to hearing impairments, finding common morphological features for all three mutants: reduced limbal zone, absence of striated-sheet matrix, disruption of collagen fibrils in the sulcal region, delamination of Kimura’s membrane, detachment of Hensen’s stripe, and altered morphology of the covernet (Legan et al. 2013). These results show the importance of individual tectorins for the shape and consequent function of the TM.
To date, localization of TM molecules has relied mostly on optical microscopy. In the present study, a detailed immunogold labeling investigation was performed on murine Tecta and Tectb at the ultrastructural level. In addition, high-resolution freeze-fracture replicas were used to understand the fine molecular architecture of the TM molecules and how they functionally correlate with their localization. We observed that the TM adheres to the spiral limbus through a dense thin matrix enriched in α- and β–tectorin. Gold-particles for both tectorins also localized adjacent to the type II collagen fibrils and at the TM lower side. The implications of these findings for the overall TM architecture will be discussed here.
Material and Methods
Animals
All rodents (rat, mouse and guinea pig) used in this work were handled following the NIH Guidelines for Animal Care and approved by the NIDCD Animal Protocol Committee (Protocol #1215).
Antibodies
Both mouse-generated antibodies for α-tectorin and β-tectorin were donated by Dr. Guy Richardson (University of Sussex). Donkey anti-mouse IgG conjugated with 10 nm colloidal gold particles (BBInternational) was used as the secondary antibody.
TEM
The animals were euthanized in an acrylic CO2 chamber, decapitated, with the inner ear removed and immediately immersed in a fixative solution containing 4% formaldehyde (EMS), 0.5% glutaraldehyde (EMS), 50 mM Hepes buffer (Sigma), 2 mM CaCL2, 1 mM MgCl2 and 140 mM NaCl for 2 h at room temperature. After 3 washes of 10 min in the same buffer, the organs of Corti were finely dissected, cryoprotected with graded glycerol baths (10%, 20%, 30%) and plunge-frozen into Freon 22 cooled by liquid nitrogen. The samples were then freeze-substituted with 1.5% uranyl acetate (Ted Pella) in anhydrous methanol for 24 h at −90°C (Leica AFS machine), embedded in Lowicryl acrylic resin (EMS) and polymerized at −45°C for 3 days under UV light.
SEM
Fixed organs of Corti were prepared with alternated baths of aqueous 1% osmium tetroxide (EMS) and 1% tannic acid (Sigma-Aldrich), twice, for 1 h each and 3 washes of 10 min each in between. Samples were then dehydrated in a graded series of ethanol, critical-point dried (Baltec CPD 050), placed on carbon tape covering an aluminum holder, coated with 4 nm of platinum and observed in a field-emission-gun SEM (Hitachi S4800), operated at 5 kV.
Immunogold labeling
Ultrathin sections (70 nm) were obtained with an ultramicrotome (Leica Ultracut) using diamond knives (Diatome). Sections were mounted on nickel grids (hexagonal thin-bar 300 mesh, EMS) and used for immunogold labeling. Grids were placed standing in plastic plates (Hiraoka Staining kit, NEM, Japan) and a solution containing 50 mM glycine (Fisher Scientific), 0.1% sodium borohydride (Sigma) and 0.1 M Tris buffer saline (TBS) was applied for 5 min at room temperature to block possible aldehyde residues. The sections were blocked with 10% normal goat serum (NGS) (Aurium) for 1 h at room temperature, incubated with primary antibodies (1–2 mg/mL) for 2 h, washed 3 × 10 min with 1% NGS/TBS, incubated with gold-conjugated secondary antibodies 1 h, washed 3 × 10 min in TBS and finally 3 × 10 min with distilled water. Sections were stained with aqueous 1% uranyl acetate for 15 min and observed in a JEOL 1010 TEM, operated at 80 kV. Digital images were acquired with a CCD camera (AMT, Woburn, MA) at 1024x1024 pixels of resolution.
Gold particle quantification
Gold particles from tectorin labeling were quantified in experiments performed on specimens of three 4-week old mice. We used ImageJ (NIH) software for the quantification. The original scale bars from the TEM images were used to calibrate the system. For each image, the threshold level was adjusted to highlight the gold particles from the background. Regions of interest were manually selected and the number of gold-particles per square-micron (gold/μm2) was automatically counted.
Freeze-etching
Samples were fixed as described for TEM, rinsed in distilled water and transferred to a thin gelatin layer (13% in distilled water, Sigma-Aldrich) on top of flat aluminum holder. Samples were slam frozen against the surface of a sapphire block cooled with liquid nitrogen in a Life Cell CF-100 freezing machine. Frozen samples were transferred to a freeze-fracture machine (Balzers BAF 300), fractured at −130°C and etched for 10 min at −100°C. Samples were then rotary-shadowed with platinum (4 nm) and carbon (10 nm). Replicas were cleaned in bleach, collected on copper grids and observed in a JEOL 1010, operated at 80 kV.
Results
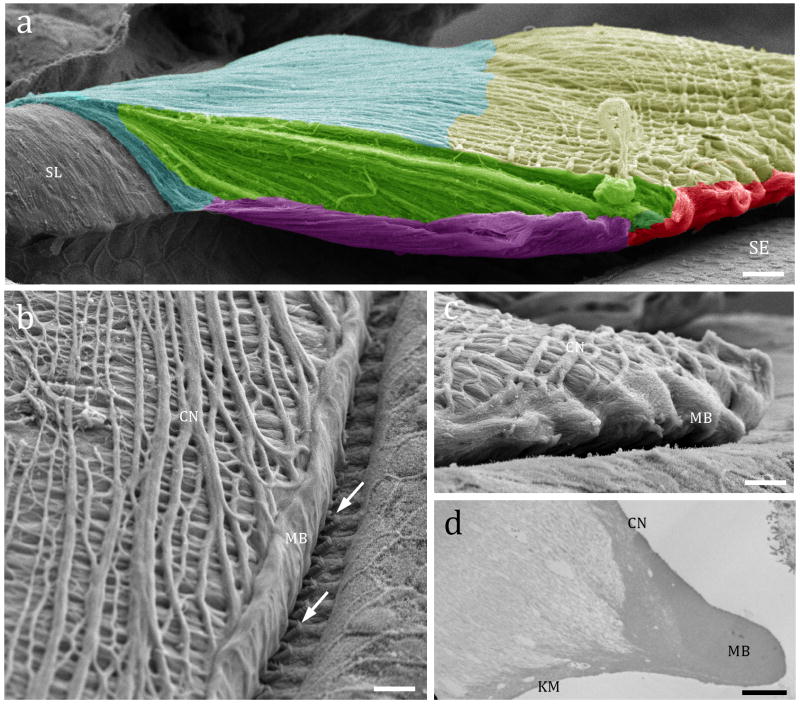
Figure 1a shows the overview of adult mouse TM anatomic regions that will be described in detail throughout the paper. The upper surface of the TM is composed of parallel-oriented collagen bundles extending from the spiral limbus towards the TM edge. The most superficial set of fibrillar bundles (the covernet) obliquely crosses the TM (Fig. 1b) and forms the marginal band at its edge (Figs. 1c and d).
Figure 1.
Tectorial membrane (TM) overview. Fig. 1a: SEM showing color-coded anatomical regions of an adult mouse (Myo15a−/−) TM attached to the spiral limbus (SL) and over the sensory epithelium (SE): blue= limbal region; yellow= cover net; green= striated sheet; red: marginal band; purple: Kimura’s membrane. Fig. 1b: SEM of an adult rat showing the TM upper side cover net (CN) composed of superficial sets of collagen bundles obliquely crossed (arrow), ending on the edge forming the marginal band (MB). Fig. 1c: Side view of an adult mouse TM underlying the sensory epithelium showing the edge of the marginal band (MB) and the collagen bundles of the covernet (CN). Fig. 1d: TEM of a similar region depicted in 1c showing a densely packed marginal band which continues its upside with the cover net bundles and downside by the Kimura’s membrane (KM). Scale bars: a = 15 μm; b and c = 10 μm; d = 2 μm.
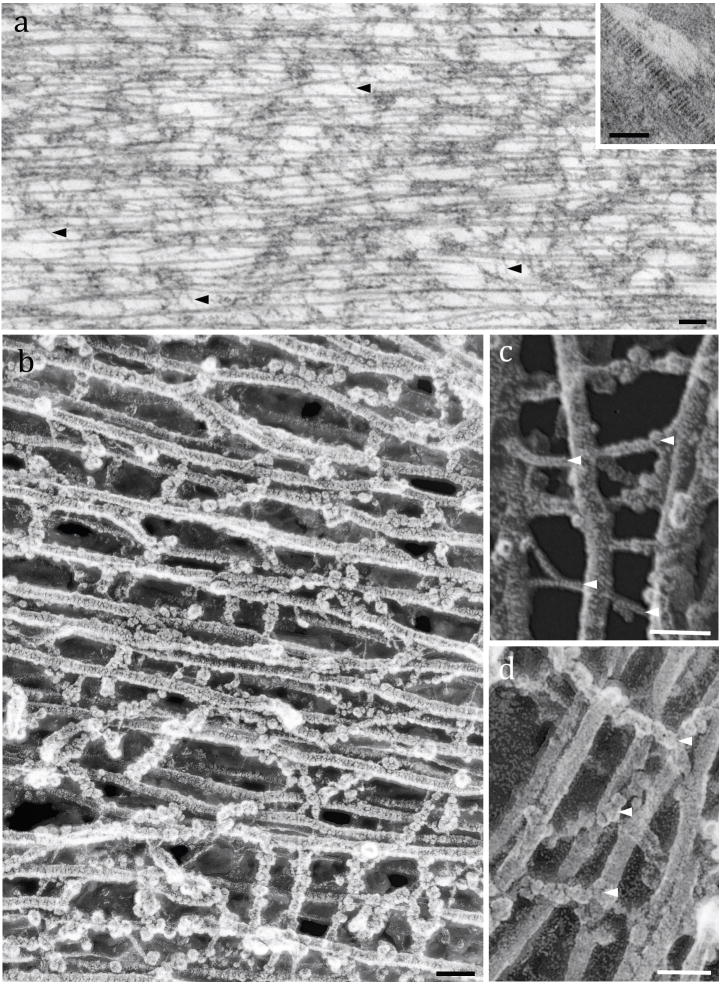
The body of the TM is organized in parallel-arranged type II collagen fibrils interwoven with all the previously mentioned proteins (Fig. 2a). TEM images showed the typical banding pattern of individual collagen fibrils (stained with uranyl acetate), which are spaced by electrondense, amorphous materials (Fig. 2a and inset). High-resolution freeze-etching images of adult rat TM revealed in detail the intricate 3D organization of the TM (Figs. 2b–d). Type II collagen fibrils (20 ± 1.7 nm width; n = 210) were crosslinked by thin linear filaments (4 ± 1.5 nm width; n = 205) (Fig. 2c) or globular-arranged filaments (15 ± 3.2 nm width; n = 200) (Fig. 2d).
Figure 2.
Fine structure of the TM striated sheet: Fig. 2a: TEM of a rat TM showing the parallel-arranged type II collagen fibrils interwoven by thin filaments (arrowheads). Inset: TEM of an individual collagen fibril with its typical banding pattern. Fig. 2b: High-resolution freeze-etching image of a guinea pig TM formed by collagen fibrils crosslinked by numerous filamentous materials. Fig. 2c: Detail image of collagen fibrils crosslinked by thin filaments (arrowheads). Fig. 2d: High magnification showing collagen fibrils connected by globular-arranged filaments. Scale bars: a = 100 nm; Inset, b, c and d = 50 nm
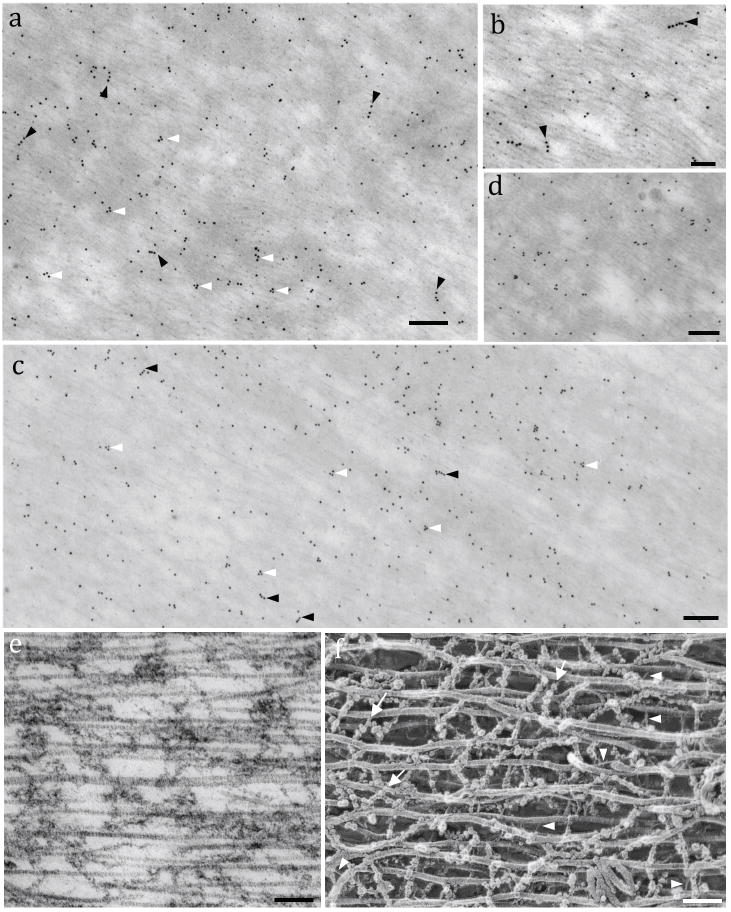
ImmunoEM for Tecta and Tectb revealed numerous gold particles localized among the collagen fibrils. α-tectorin displayed mostly triangular or linear patterns of labeling, probably due to restrictions in epitope exposure (Figs. 3a and b, arrowheads). β-tectorin labeling was found along the entire TM structure, but concentrated in regions with more amorphous aspects (Figs. 3c and d). β-tectorin was arranged in a linear pattern, resembling the rows of globules seen in freeze-etching images as well as the triangular shapes (Figs. 3e and 3f). The gold particle density was higher for β-tectorin (75/μm2; total = 1120 μm2) than α-tectorin (45/μm2; total = 1260 μm2) in our labeling study for the TM body. The pattern of the gold labeling suggests that those crosslinking structures could be tectorins.
Figure 3.
Localization of tectorins in the TM striated sheet. Fig. 3a: ImmunoEM for mouse α-tectorin reveals triangular (white arrowheads) and linear patterns (black arrowheads) of gold-particles. Fig. 3b: Higher magnification image of the α-tectorin gold labeling. Arrowheads indicate rows of gold particles. Fig. 3c: ImmunoEM for mouse β-tectorin positive labeling along the entire TM structure but concentrated in more amorphous regions, forming straight (black arrowheads) and triangular shapes (white arrowheads). Fig. 3d: Detail of the β-tectorin labeling in the striated sheet. Fig. 3e: TEM image of a rat TM showing the parallel collagen fibers and amorphous intermediate materials similar to such regions labeled for tectorins. Fig. 3f: Deep-etch image of guinea pig TM showing the collagen fibrils crosslinked by thin filaments (arrowheads) and chains of globules that resemble the structures that are labeled with antibodies to tectorins (arrows). Scale bars: a = 150 nm; b = 100 nm; c = 150 nm; d = 120 nm; e and f = 100 nm.
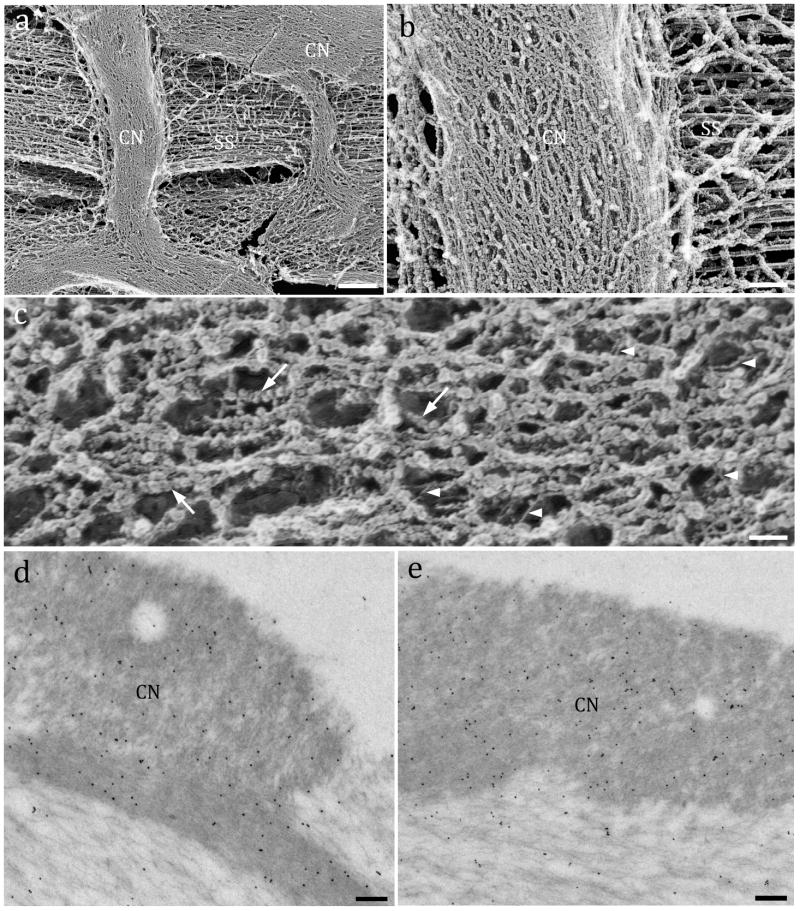
The development and function of the covernet remain elusive. Images of freeze-etched replicas of the covernet revealed a meshwork of densely packed filaments structurally distinct from the underlying fibrils (Figs. 4a and b). High magnification images indicated that the covernet filaments were formed by branched filament-interconnected globules (15 ± 5.5 nm in diameter; n = 110) and thinner fibrils (4.6 ± 0.9 nm width; n = 50) (Fig. 4c). ImmunoEM showed α-tectorin (Fig. 4d) and β-tectorin (Fig. 4e) highly enriched in the covernet (α-tectorin = 47 gold particles/μm2; total = 350 μm2; β-tectorin = 31 gold particles/μm2; total = 260 μm2), compared to the striated sheet area immediately below (α-tectorin = 15 gold-particles/μm2; total = 760 μm2; β-tectorin = 18 gold particles/μm2; total = 620 μm2). We suggest that the covernet globules are composed of tectorins based on the positive labeling for both glycoproteins.
Figure 4.
Fine structure and immunogold labeling of the covernet. Fig. 4a: Freeze-etching of a guinea pig TM showing covernet bundles (CN) above the striated sheet fibers (SS). Fig. 4b: Higher magnification image of the covernet bundle orthogonally crossing parallel collagen fibers (SS) underneath. Fig. 4c: Detail of the covernet fine structure showing branched filaments made of globular units (arrows) and interconnecting thin fibrils (arrowheads). Immunogold TEM for α-tectorin (Fig. 4d) and β-tectorin (Fig. 4e) showing an intense labeling in the covernet (electrondense upper regions) and lesser labeling at the area underneath. Scale bars: a = 2 μm; b = 150 nm; c = 25 nm; d and e = 100 nm.
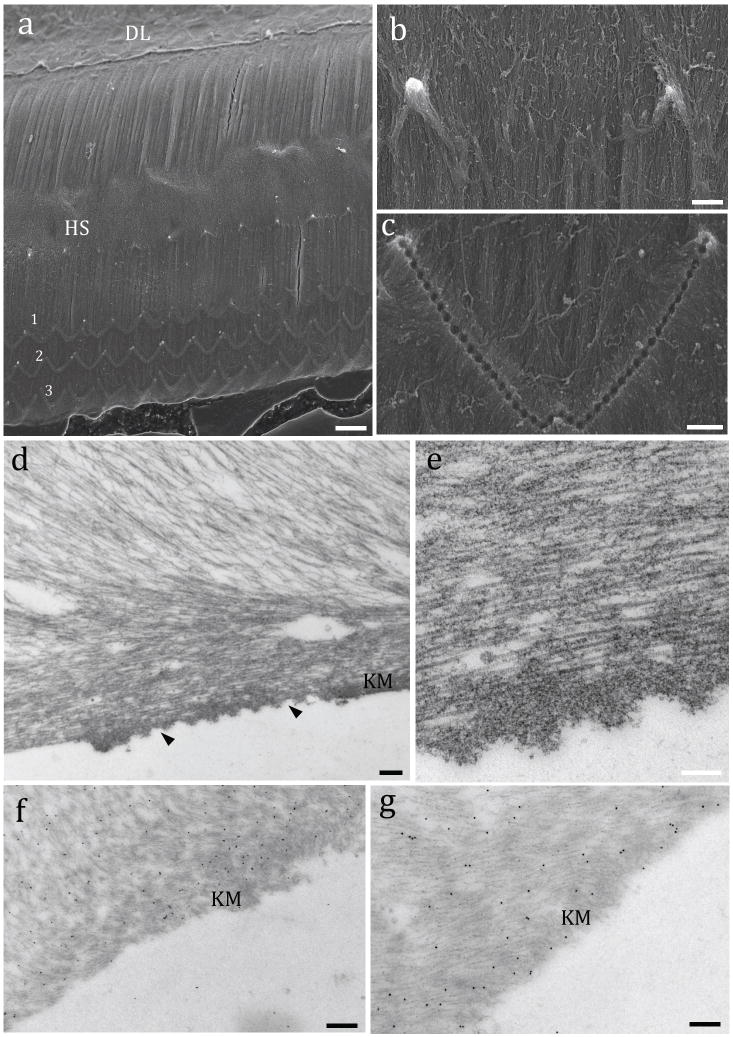
The lower surface of the TM displayed parallel-arranged collagen fibrils with two evident landmarks: the Hensen’s stripe, a deformation caused by the centripetal friction of IHC stereocilia against the TM during basilar membrane movements; and the imprints caused by the insertion of the OHC taller stereocilia at the Kimura’s membrane (Figs. 5a–c). TEM images of the imprint region showed that the collagen fibrils are embedded in a dense amorphous material (Figs. 5d and e). Immunogold-labeling revealed the presence of both Tecta (Fig. 5f) and Tectb (Fig. 5g) in the amorphous material of the Kimura’s membrane and adjacent to the collagen fibrils.
Figure 5.
Ultrastructure and immunogold labeling of the TM lower side. Fig 5a: Low magnification SEM image of the lower side of an adult mouse showing part of the dense lamina (DL), the superficial layer of parallel-arranged collagen bundles with the Hensen’s stripe (HS) and three rows of OHC stereocilia imprints (1, 2, 3). Fig. 5b: Detail of the Hensen’ stripe showing the ridge made by two IHCs. Fig. 5c: Detail of the W shaped imprints generated by the insertion of the tips of the tallest row of OHC stereocilia. Fig. 5d: TEM of the electron dense area of Kimura’s membrane (KM) with stereocilia imprints (arrowheads). Fig. 5e: A detail of the wavelike format of the amorphous material present at the OHC imprints below the fibrillar region of the striated sheet. Immunogold labeling indicated the presence of Tecta (Fig. 5f) and Tectb (Fig. 5g) in the amorphous material of Kimura’s membrane (KM) and upper regions. Scale bars: a = 5 μm; b = 8 μm; b = 6 μm; d = 150 nm; e, f and g = 100 nm.
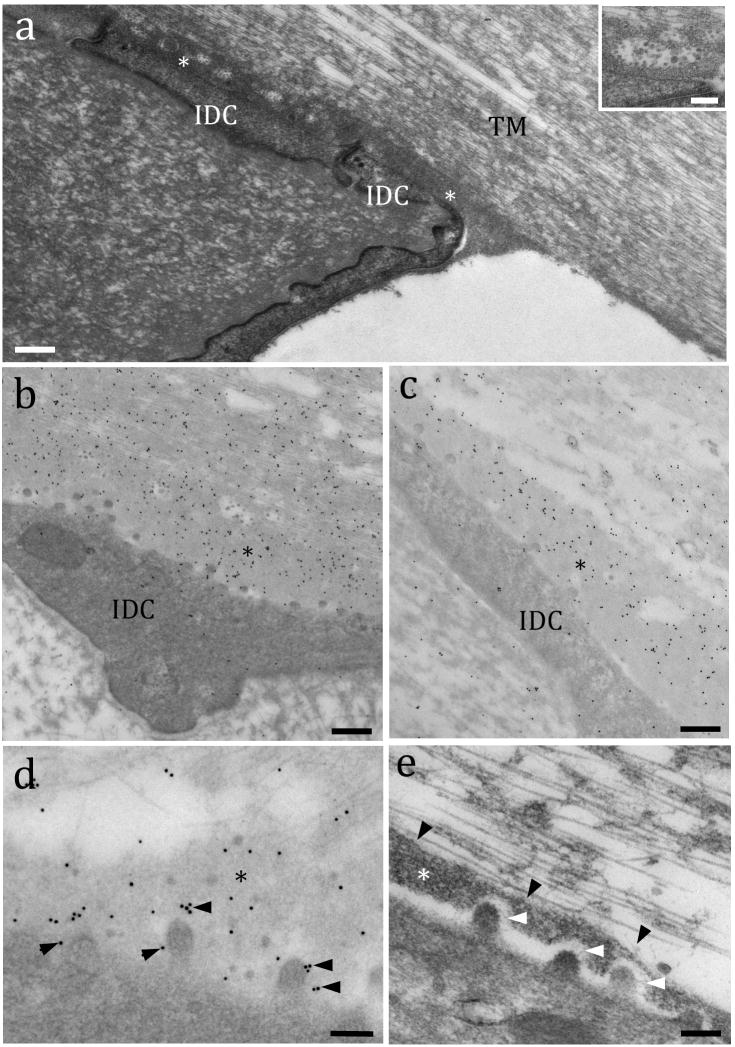
The TM undergoes constant pulling forces when the basilar membrane moves in response to recurrent sound vibrations. Adjacent to the surface of interdental cells, a dense thin layer or lamina (231 ± 68 nm high) provides a stable connection between the TM and the spiral limbus (Fig. 6a). Interestingly, this layer seemed to continue toward the lower TM side and to the inner sulcus surface (Fig. 6a). Numerous vesicles were frequently observed within this lamina (Fig. 6a insert), where immunogold labeling indicated a high concentration of α-tectorin (Fig. 6b) and β-tectorin (Fig. 6c). We observe labeling of α- and β-tectorin around the microvilli surface suggesting that interdental cells may express membrane receptors that interact directly or indirectly with tectorins (Fig. 6d). High magnification TEM images showed that thin filaments connecting the microvilli to the lamina and the lamina to the above collagen fibrils were compatible with the tectorins’ localization (Fig. 6e).
Figure 6.
The TM adhesion to the spiral limbus is mediated by a dense lamina rich in tectorins. Fig 6a: TEM from an adult rat showing the interface between the TM made of collagen parallel fibers and the spiral limbus (at its edge region) formed by a dense lamina layer of an extracellular matrix (*). IDC = interdental cells. Inset: Detail of an exocytotic vesicle found within the dense lamina filled with globular materials. Fig. 6b: Immunogold labeling shows a high concentration of α-tectorin (Fig. 6b) and β-tectorin (Fig. 6c) in the basal lamina (*). Fig 6d: Detail of the α-tectorin labeling close to the microvilli (arrowheads) in the dense lamina (*). Fig. 6e: High magnification TEM showing a detail of the molecular interaction (white arrowheads) between a microvillus and the dense lamina (*), and between the lamina and TM collagen fibrils (black arrowheads). Scale bars: a, b and c = 150 nm; d and e = 100 nm; inset in a = 50 nm.
Discussion
The coupling of the organ of Corti to the TM is fundamental for accurate sound perception. Genetic mutations in TM molecules can cause hearing loss, highlighting the significant contribution of its architecture and molecular composition to the TM’s normal function. This work focused on the localization of tectorins and its relationship to the collagen fiber network and on the site of attachment of the TM to the spiral limbus and to the OHC stereocilia.
All the secreted TM molecules interact and self-assemble in the endolymph and over the sensory epithelium during cochlear development. TM proteins possess specific interaction sites in order to correctly self-assemble and form its unique architecture. Moreover, membrane proteins of hair cell stereocilia must recognize proteins at the lower side of the TM while interdental cells must recognize TM proteins at the spiral limbus. The TM backbone is composed of type II collagen bundles associated with other fibrillar and non-fibrillar types of collagen. Virtually all collagen-based extracellular matrices such as cartilages, tendons and TM contain more than one type of collagen (Ricard-Blum and Ruggiero 2005). For instance, minor fibrillar types V and XI collagens nucleate and regulate the fibril diameter of type I and II collagens, respectively (Wenstrup et al. 2006; Kadler et al. 2008). At the molecular level, a hybrid molecule composed of both collagen V and XI chains has been described (Fichard et al. 1995). It is likely that the thin filaments we have observed crosslinking TM collagen II fibrils are type V and/or type XI collagens. Future experiments of immunogold labeling for those collagen types may confirm their fine localization and molecular partners. Freeze-etching replicas also revealed chains of globular structures connecting type II collagen fibrils, matching the tectorins’ localization. Gold-particles were often organized in rows or triangular figures adjacent to the collagen fibrils throughout the TM body, likely representing the tectorin epitopes available on the surfaces of the ultrathin sections.
The parallel arrangement of the type II collagen fibrils might influence the TM function as a mediator of OHC-based cochlear amplification and/or a regulator of electrokinetic motion during basilar membrane vibrations. Articular cartilages are resistant to distension and absorb the impact applied to the bones, and like the TM, they are highly hydrated gels composed of type II collagen fibrils. If TM is a semi-rigid anisotropic (Gavara et al. 2011) plate that slides back and forth while the basilar membrane vibrates, such collagen fibrils could be limiting TM stretching and dissipating the load energy transmitted by the basilar membrane.
We also detected both tectorins at the covernet region. Since tectorins are widespread in regions of connections (lower side-stereocilia and lamina-spiral limbus), we believe that tectorins are important for keeping the organization of the covernet. In TectaL1820F, G1824D/+ and TectaC1837G/+ mice, the covernet fibrils are still of large caliber but less tightly associated with the main body of the TM (Legan et al., 2013). We suggest that at least α-tectorin connects the covernet to the underlying collagen fibrils and provides the typical spiral distribution of the covernet bundles at the TM surface. This arrangement could be supporting some elements of the complex TM mechanical function, such as being a secondary resonator that amplifies input to the hair bundle or that accurately propagates the sound waves (Lukashkin et al., 2010).
Our results show that the site of contact of the TM to the spiral limbus consists of an amorphous layer rich in both α- and β-tectorins. The presence of gold particles close to the interdental cell membranes/microvilli suggests that tectorins might play a direct role in the interaction with interdental cells, in addition to its structural role. Legan et al. (2013) showed that the three mutants generated for different regions of α-tectorin present some structural distortion in the limbal zone: TM of TectaC 1619S/+ mice did not appear to extend fully across the surface of the spiral limbus; for Tecta1820F,G1824D/+ and Tecta 1837G/+ mice, the limbal zone was considerably reduced in its extent. This evidence strongly suggests that at least α-tectorin is involved in the connection of the TM to the spiral limbus. We cannot rule out the possibility of a redundant function for β-tectorin, although the TM was not found detached from the spiral limbus in knockout mice (Russell et al. 2007). Our TEM observations revealed secretory vesicles at the dense lamina suggesting that interdental cells may continuously provide de novo molecules to all regions of the TM, not only to the dense lamina.
In rodents, only the tallest stereocilia rows of OHCs are embedded in the lower side of the TM. The attachment of one row (out of three rows) of stereocilia is sufficient to synchronize the entire hair bundle during sound-induced deflection. Interciliary links (tip links, horizontal top connectors and ankle links) allow proper bundle movement by maintaining hair bundle cohesion. Two different antibodies have detected the protein stereocilin at the tips of the tallest stereocilia in P7 mice (Verpy et al. 2011). In Strc−/− mice, imprints were absent on the lower surface, indicating that stereocilin is crucial to the connection of the stereocilia to the TM. Our immunogold labeling showed a high concentration of both tectorins at the imprint region, suggesting that these proteins could be the partners for stereocilin. In the paper by Legan and colleagues (2013), they observed by TEM that in Tecta1820F,G1824D/+ and Tecta 1837G/+ mutant mice, the Kimura’s membrane was delaminated and Hensen’s stripe was no longer attached to the TM. Yet, in the TectaC1619S/+ mouse, imprints were not observed in apical low-frequency regions, only in basal regions of the cochlea (Legan et al. 2013). Our labeling, which shows high concentrations of α-tectorin (and β-tectorin) decorating the dense layer on the lower side of the TM is consistent with the observation that in the Tectb −/− mouse there is a noticeable absence of Hensen’s stripe; however, the Kimura’s membrane is apparently normal (Russell et al., 2007). The pattern of localization of α-tectorin and β-tectorin is consistent with the possibility that α-tectorin can compensate for β-tectorin absence in Kimura’s but not in Hensen’s stripe.
Many important questions still remain to be answered about the TM’s role in hearing. Its fine organization and overall format are directly involved with TM mechanics and functions. The fine localization of the remaining known TM components can help us understand the unique architecture of the TM and consequently fully elucidate its participation in hearing. Several mice mutants for stereocilia proteins have been generated and can give us hints about the relation between the TM and stereocilia. Additionally, protein turnover or plasticity of the TM in adult animals remains elusive. A complete and accurate picture of the TM’s development and maintenance is also crucial if we want to understand how the organ of Corti would reconnect to hair cells in case of injury, or cellular therapy necessary for any regeneration method.
Acknowledgments
We would like to Dr. Ronald Petralia (NIDCD, NIH) for the use of the TEM and critical reading of the manuscript, and Nicole Thompson (NIDCD) for the grammar review. Work was supported by NIH intramural research funds Z01-DC000002.
Footnotes
We declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PlosOne. 2010;5(9):e12765. doi: 10.1371/journal.pone.0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichard A, Kleman JP, Ruggiero F. Another look at collagenV and XI molecules. Matrix Biol. 1995;14:515–31. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- Gavara N, Manoussaki D, Chadwick RS. Auditory mechanics of the tectorial membrane and the cochlear spiral. Curr Opin Otolaryngol Head Neck Surg. 2011;19(5):382–7. doi: 10.1097/MOO.0b013e32834a5bc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari R, Aranyosi AJ, Freeman DM. Longitudinally propagating traveling waves of the mammalian tectorial membrane. Proc Natl Acad Sci USA. 2007;104(42):16510–5. doi: 10.1073/pnas.0703665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari R, Aranyosi AJ, Richardson GP, Freeman DM. Tectorial membrane travelling waves underlie abnormal hearing in Tectb mutant mice. Nat Commun. 2010;1:96–99. doi: 10.1038/ncomms1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari R, Page SL, Farrahi S, Sellon JB, Freeman DM. Electrokinetic properties of the mammalian tectorial membrane. Proc Natl Acad Sci USA. 2013;110(11):4279–4284. doi: 10.1073/pnas.1214744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J Neurobiol. 2002;53(2):212–27. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer R, Ruettiger L, Riesenberg R, Schaeuble C, Krupar R, Kamp A, Sunami K, Eisenried A, Hennenberg M, Grunert F, et al. Loss of the mammal-specific tectorial membrane component CEA cell adhesion molecule 16(CEACAM16) leads to hearing impairment at low and high frequencies. J Biol Chem. 2012;287:21584–21598. doi: 10.1074/jbc.M111.320481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Lukashkin AN, Verhoeven K, Van Camp G, Russell IJ, Richardson GP. A deafness mutation isolates a second role for the tectorial membrane in hearing. Nat Neurosci. 2005;8:1035–1042. doi: 10.1038/nn1496. [DOI] [PubMed] [Google Scholar]
- Legan PK, Goodyear RJ, Morin M, Mencia A, Pollard H, Olavarrieta L, Korchagina J, Modamio-Hoybjor S, Mayo F, Moreno F, Moreno-Pelayo M, Richardson GP. Three deaf mice: mouse models for TECTA-based human hereditary deafness reveal domain-specific structural phenotypes in the tectorial membrane. Human Molecular Genetics. 2013;201:1–18. doi: 10.1093/hmg/ddt646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashkin AN, Richardson GP, Russell IJ. Multiple roles for the tectorial membrane in the active cochlea. Hear Res. 2010;266(1–2):26–35. doi: 10.1016/j.heares.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Mustapha M, Weil D, Chardenoux S, Elias S, El-Zir E, Beckmann JS, Loiselet J, Petit C. An alpha-tectorin gene defect causes a newly identified autosomal recessive form of sensorineural pre-lingual non-syndromic deafness, DFNB21. Hum Mol Genet. 1999;8(3):409–12. doi: 10.1093/hmg/8.3.409. [DOI] [PubMed] [Google Scholar]
- Oonk AMM, Leijendeckers JM, Huygen PLM, Schraders M, del Campo M, del Castillo I, Tekin M, Feenstra I, Beynon AJ, Kunst HPM, Snik AFM, Kremer H, Admiraal RJC, Pennings RJE. Similar Phenotypes Caused by Mutations in OTOG and OTOGL. Ear & Hear. 2014;35(3):e84–91. doi: 10.1097/AUD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol. 2005;53(7):430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ, Duance VC, Bailey AJ. Polypeptide composition of the mammalian tectorial membrane. Hear Res. 1987;25:45–60. doi: 10.1016/0378-5955(87)90078-5. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Lukashkin AN, Russell IJ. The tectorial membrane: one slice of a complex cochlear sandwich. Curr Opin Otolaryngol Head Neck Surg. 2008;16(5):458–64. doi: 10.1097/MOO.0b013e32830e20c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Legan PK, Lukashkina VA, Lukashkin AN, Goodyear RJ, Richardson GP. Sharpened cochlear tuning in a mouse with a genetically modified tectorial membrane. Nat Neurosci. 2007;10(2):215–23. doi: 10.1038/nn1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon JB, Ghaffari R, Farrahi S, Richardson GP, Freeman DM. Porosity controls spread of excitation in tectorial membrane traveling waves. Biophys J. 2014;106(6):1406–13. doi: 10.1016/j.bpj.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann I, Thallinger G, Comegys TH, Thalmann R. Collagen – The predominant protein of the tectorial membrane. J Oto-Rhino-Laryngol. 1986;48:106–115. doi: 10.1159/000275855. [DOI] [PubMed] [Google Scholar]
- Thalmann I, Thallinger G, Comegys TH, Crouch EC, Barrett N, Thalmann R. Composition and supramolecular organization of the tectorial membrane. Laryng. 1987;97:357–367. [PubMed] [Google Scholar]
- Thalmann I, Machiki K, Calabro A, Hascall VC, Thalmann R. Uronic acid-containing glycosaminoglycans and keratan sulfate are present in the tectorial membrane of the inner ear: Functional implications. Arch Biochem Biophys. 1993;307:391–396. doi: 10.1006/abbi.1993.1605. [DOI] [PubMed] [Google Scholar]
- Thalmann I. Collagen of accessory structures of organ of Corti. Connect Tissue Res. 1993;29:191–201. doi: 10.3109/03008209309016826. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, Van Laer L, Kirschhofer K, Legan PK, Hughes DC, Schatteman I, Verstreken M, Van Hauwe P, Coucke P, Chen A, Smith RJ, Somers T, Offeciers FE, Van de Heyning P, Richardson GP, Wachtler F, Kimberling WJ, Willems PJ, Govaerts PJ, Van Camp G. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet. 1998;19(1):60–2. doi: 10.1038/ng0598-60. [DOI] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Michalski N, Goodyear RJ, Houdon C, Weil D, Richardson GP, Petit C. Stereocilin connects outer hair cell stereocilia to one another and to the tectorial membrane. J Comp Neurol. 2011;519:194–210. doi: 10.1002/cne.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- Zwaenepoel I, Mustapha M, Leibovici M, Verpy E, Goodyear R, Liu XZ, Nouaille S, Nance WE, Kanaan M, Avraham KB, Tekaia F, Loiselet J, Lathrop M, Richardson G, Petit C. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc Natl Acad Sci USA. 2002;99(9):6240–6245. doi: 10.1073/pnas.082515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Miller KK, Yang T, et al. Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with α-tectorin and is mutated in autosomal dominant hearing loss (DFNA4) Proc Natl Acad Sci USA. 2011;108:4218–4223. doi: 10.1073/pnas.1005842108. [DOI] [PMC free article] [PubMed] [Google Scholar]