Abstract
Prescription narcotic use among living kidney donors is not well described. Using a unique database that integrates national registry identifiers for living kidney donors (1987-2007) in the United States with billing claims from a private health insurer (2000-2007), we identified pharmacy fills for prescription narcotic medications in periods 1-4 and >4 years post-donation, and estimated relative likelihoods of post-donation narcotic use by Cox regression. We also compared narcotic fill rates and medication possession ratios (MPRs, [days of medication dispensed]/[days observed], between donors and age- and sex-matched non-donors. Overall, rates of narcotic medication fills were 32.3 and 32.4 per 100 person-years in periods 1–4 and >4 years post-donation. After age and race adjustment, women were approximately twice as likely as men to fill a narcotic prescription in years 1–4 (adjusted hazard ratio, aHR, 2.28; 95% confidence interval, CI, 1.86–2.79) and >4 years (aHR 1.70; 95% CI 1.50–1.93). MPRs in donors were low (<2.5% days exposed), and lower than among age- and sex-matched non-donors. Prescription narcotic medication use is more common among women than men in the intermediate-term after live kidney donation. Overall, total narcotic exposure is low, and lower than among non-donors from the general population.
Keywords: Health Administrative Data, Health Outcomes, Living Kidney Donors, Narcotic Medication, Pharmacy Claims
Introduction
Each year, nearly 30,000 living kidney donations are performed around the world (1). Previous studies have shown that compared to healthy non-donors, long-term risks of many health outcomes including death, cardiovascular disease, acute kidney injury requiring dialysis, fractures, kidney stones resulting in surgical intervention, and major gastrointestinal bleeding are similar among living donors (2–6); however, recent work has also identified small absolute increases in risks of other outcomes after donation, including end-stage renal disease, gestational hypertension/preeclampsia, and gout (7–10). Quality of life surveys have generally found similar or better average quality of life scores among living donors compared with controls (11–16), but importantly, survey designs may be limited by sampling and response biases. Post-donation pain has been reported, with one randomized controlled trial of 122 donors finding that a laparoscopic nephrectomy approach was associated with less self-reported pain one month post-donation compared to open nephrectomy (17,18); however, in this study, these differences were no longer significant at 6- and 12-month assessments (18). Chronic pain, typically defined as persisting more than 6 months, has been reported in up to 25% of donors after open nephrectomy and 5% after laparoscopic nephrectomy in retrospective survey studies (19,20).
In the United States (U.S.) general population, the use of narcotic medications has increased dramatically in the last decade (21,22), and this pattern has correlated with rising rates of narcotic-related deaths, addictions, adverse drug events, and healthcare costs (21,23–25). Prescription narcotic medication use appears to be more prevalent among women than men (26–28). Previous studies have suggested that women are at higher risk than men for chronic pain conditions and co-morbid mood disorders, and may experience more severe pain and disability after surgeries and procedures (29–33). Narcotic medication use in women of reproductive age is of particular concern given associated risks of adverse pregnancy outcomes such as birth defects (34). A recent study by the Centers for Disease Control and Prevention (CDC) found that between 2008 and 2012, almost 40% of Medicaid-enrolled women (aged 15-44 years) filled an outpatient prescription for a narcotic medication each year (35).
Administrative medical and pharmacy claims offer a non-obtrusive measure of provider-reported diagnoses and prescribed health care that do not rely on patient self-report, and have been successfully used to study an array of health outcomes after living kidney donation that were difficult to examine by other methods given limited long-term donor follow-up by centers and lack of capture in national registries (2–10,36–42). For example, we previously found that post-donation depression, defined by a clinical diagnosis or use of an anti-depressant medication, was more than twice as likely among female donors compared to male donors (adjusted hazard ratio, aHR, 2.41), white donors compared to non-white donors (aHR 2.07), and those who experienced peri-operative complications compared to those who did not (aHR 2.46) (37).
In the current study, we sought to expand understanding of the breadth of pharmaceutical care required by living donors by examining use of prescription narcotic medications in the intermediate and longer-term (1–4 years and >4 years) after living kidney donation, as captured in linked national U.S. transplant registry and administrative claims databases. Our primary aim was to explore variation in narcotic medication use according to donor demographic traits; socio-economic and clinical characteristics were also explored in subsets with available information. We also compared prescription narcotic use among living kidney donors to that in age- and sex-matched non-donors from the general population.
Methods
Data Sources
We conducted a retrospective cohort study using linked healthcare databases in the U.S. to ascertain patient characteristics, pharmacy fill records, and descriptions of medical diagnoses. This study used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the OPTN, and has been described elsewhere (43). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. After approval from the Saint Louis University Institutional Review Board and HRSA, the unique OPTN identifiers for living kidney donors were linked using names and dates of birth to beneficiary identifiers from national private health insurer electronic databases. The insurer databases include information on provider-reported diagnostic billing claims as well as pharmacy claims for prescription medications. Analyses were performed using Health Information Portability and Accountability Act-compliant limited datasets, with all direct identifiers removed. These databases have been used for epidemiologic and health services research including study of living kidney donor outcomes (36–38,40–42). Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116).
Population and Covariates
We included living kidney donors who had donated between October 1987 and July 2007 and were enrolled in the insurance benefit plan at some point after donation during May 2000 to December 2007 (the period of available claims data). Persons who had not donated a kidney (non-donors) and were enrolled in the same insurance benefit plan at some point during the same time period (May 2000 to December 2007) were sampled as general population controls. All study participants were simultaneously enrolled in medical and pharmacy benefits with the insurer exclusively during the study period.
Baseline demographic information ascertained for living kidney donors from the OPTN at the time of donation included age, sex, and race as reported by the transplant center. The insurance records include information on age and sex, but not race; thus, information on race was not available for non-donors. Times from donation to start and end of captured insurance benefits were based on OPTN-reported donation dates and insurance enrollment records.
An index of neighborhood socio-economic status (SES) at the time of donation was computed from U.S. Census data linked by ZIP code, according to methods used by the Agency for Healthcare Research and Quality (44) (for details, please see Supplementary Appendix 1, Methods). SES index values range from 0 to 100, with higher values representing higher SES levels. Information on surgical approach (open vs. laparoscopic donor nephrectomy) was obtained from the OPTN, and was available for donations from 1999 onwards. Peri-operative surgical complications (during initial hospitalization or up to 6 weeks post-donation) were collected by the OPTN for those who donated from 2004 onwards.
Outcomes
The primary outcome was pharmacy fills for narcotic medications after living kidney donation during two post-donation time periods: 1-4 years post-donation and >4 years post-donation. Since most surgical patients receive a prescription for narcotic medications at the time of discharge (20), we limited our analysis to prescriptions filled after the first year post-donation. The median and maximum times from donation to the end of insurance benefits were 7.7 and 20.2 years, respectively.
In additional analyses, narcotic use was normalized to morphine equivalents (ME), according to conversion ratios as previously described (Supplemental Table 1) (45,46). Post-donation ME were aggregated for each donor and expressed as average mg ME exposure per enrollment day. We also computed medication possession ratios (MPRs), a metric quantifying the fraction of days of captured insurance enrollment for which narcotic medications were prescribed (40). MPRs are defined as [days of medication supplied over an observation window]/[days of observation], where the observation windows were defined as the period of captured insurance benefits for an individual during an observation period. To account for concomitant use of multiple agents, the MPR metric for any narcotic exposure aggregated fill days from different classes, even if prescription dates overlapped.
Statistical Analyses
Data management and analyses were performed with Statistical Analysis Software (SAS) for Windows software, version 9.3 (SAS Institute Inc., Cary, NC). In all outcome analyses, we interpreted two-tailed p-values <0.05 as statistically significant.
Demographic Correlates of Narcotic Use after Living Kidney Donation
Because the observation period for the insurance benefit plan inclusion varied between donors, Cox regression with censoring was used to estimate covariate-adjusted associations (adjusted hazards ratios, aHR) of baseline donor characteristics with the likelihood of narcotic medication fills in the periods 1-4 years and >4 years after donation. The study windows of interest began at 1 year and 4 years post-donation, respectively. Left-censoring was applied from the start of the study window to insurance benefit plan enrollment if a subject was not enrolled at the start of each risk window. For the 1-4 year analysis, right-censoring was applied at 4 years post-donation or the end of captured insurance benefits for those whose benefits ended earlier. Since claims data were available starting in May 2000, the 1-4 year analyses were limited to living kidney donors who had their nephrectomy performed in May 1996 or later (n=2,898). For the later analysis, right-censoring was applied at end of insurance benefits after year 4 post-donation or the end of study data (December 2007). Because collection of peri-operative surgical complications began in 2004, follow-up through 2007 was insufficient to assess the association between surgical complications and narcotic use >4 years post-donation.
Total narcotic exposure was categorized among living donors in each study period as no use and tertiles of ME among those with narcotic fills. We compared distributions of baseline clinical characteristics between living kidney donors with each tertile of ME exposure to donors with no narcotic use in each period.
Comparison of Narcotic Medication Use in Living Kidney Donors and General Non-Donors
To compare narcotic use among the donors to a general population sample, donors were matched 1:1 with general insurance beneficiaries (non-donors) by sex and age when benefits began. The maximum observation time, defined by insurance benefit plan duration in each matched pair, was limited to the shortest available in the pair. We compared narcotic prescription rates (per 100 person-years) and MPRs in donors and matched non-donors using the paired Wilcoxon signed-rank test. In donors and matched non-donors who filled a prescription for a narcotic medication, we assessed the frequency of each type of narcotic medication prescribed by post-donation time period and sex. Finally, we performed a qualitative assessment of the 20 most common primary medical diagnoses for healthcare services in the 7 days prior to narcotic prescription fills (including the date of the narcotic fill) in donors and matched non-donors, according to post-donation time period and sex. A schematic of the study design, including matching of donors and non-donors and ascertainment of study measures, is shown in Supplemental Figure 1.
Results
Baseline Characteristics of the Living Donor Sample
The integrated database captured 4,650 living kidney donors. The baseline characteristics of the study cohort have been previously described and were similar to that of all U.S. living kidney donors registered in the OPTN in the same period (Supplemental Table 2) (36). Among the donors, 76.3% were Caucasian, 13.1% were African-American, 8.2% were Hispanic, and 2.4% were other races (Table 1). The mean age at the time of donation was 37.2 years and 55% were women. Most living kidney donors (81.2%) were biologically related to their recipient. There were 3,384 living kidney donors with available information to determine SES, among whom the mean (standard deviation) SES score was 47.9 (6.1), comparable to the mean SES score of 47.2 (6.4) among all U.S. donors with linked SES information in the study period. Surgical type information was collected for 1,104 living kidney donors, among whom 59.4% had their donor nephrectomy performed laparoscopically. Of the 472 donors who donated after institution of OPTN collection of peri-operative surgical complication information, <1% experienced a re-operation and 5.3% had a peri-operative complication reported following nephrectomy (Supplemental Table 3).
Table 1. Adjusted associations of baseline characteristics with likelihood of pharmacy fill for a narcotic medication among privately-insured U.S. living kidney donors in periods 1–4 and >4 years post-donation.
| Baseline characteristics | Trait distributiona | Narcotic pharmacy fills Adjusted hazard ratiob (95% CI) | |
|---|---|---|---|
| All donors (n=4,650) | 1-4 years post-donationc | >4 years post-donationd | |
| Age at donation (per year) | 37.2 (10.0) | 0.99 (0.98-1.00) | 1.01 (1.00-1.01)e |
| Female sex | 54.6% | 2.28 (1.86-2.79)e | 1.70 (1.50-1.93)f |
| Race | |||
| Caucasian (non-Hispanic) | 76.3% | Reference | Reference |
| African-American | 13.1% | 0.90 (0.68-1.18) | 0.96 (0.80-1.16) |
| Hispanic | 8.2% | 0.85 (0.61-1.20) | 0.78 (0.60-1.01) |
| Other | 2.4% | 0.31 (0.14-0.70)e | 0.51 (0.29-0.88)e |
| Subgroups with additional information | |||
| SES index (n=3,384)g | 47.9 (6.1) | 1.00 (0.99-1.02)h | 1.01 (1.00-1.02)h |
| Surgical type (n=1,104) | |||
| Laparoscopy | 59.4% | 1.06 (0.82-1.36)h | 0.89 (0.66-1.91)h |
Data presented as percentages except for age and SES which are presented as mean (standard deviation).
Adjusted hazard ratios estimated in living kidney donors by multivariate Cox regression with left- and right-censoring.
Given the period of available claims data, the 1-4 year analyses were limited to living kidney donors who had their nephrectomy performed in May 1996 or later (n=2,898).
Median time from donation to the end of insurance benefits was 7.7 years (maximum 20.2 years).
p<0.05-0.0001.
p≤0.0001.
SES index values range from 0 to 100, with higher values for the composite score representing higher SES levels (see Appendix 1 for more details).
Adjusted for age, sex, and race.
Abbreviations: CI, confidence intervals; SES, socio-economic status; U.S., United States.
Demographic Correlates of Narcotic Use after Living Kidney Donation
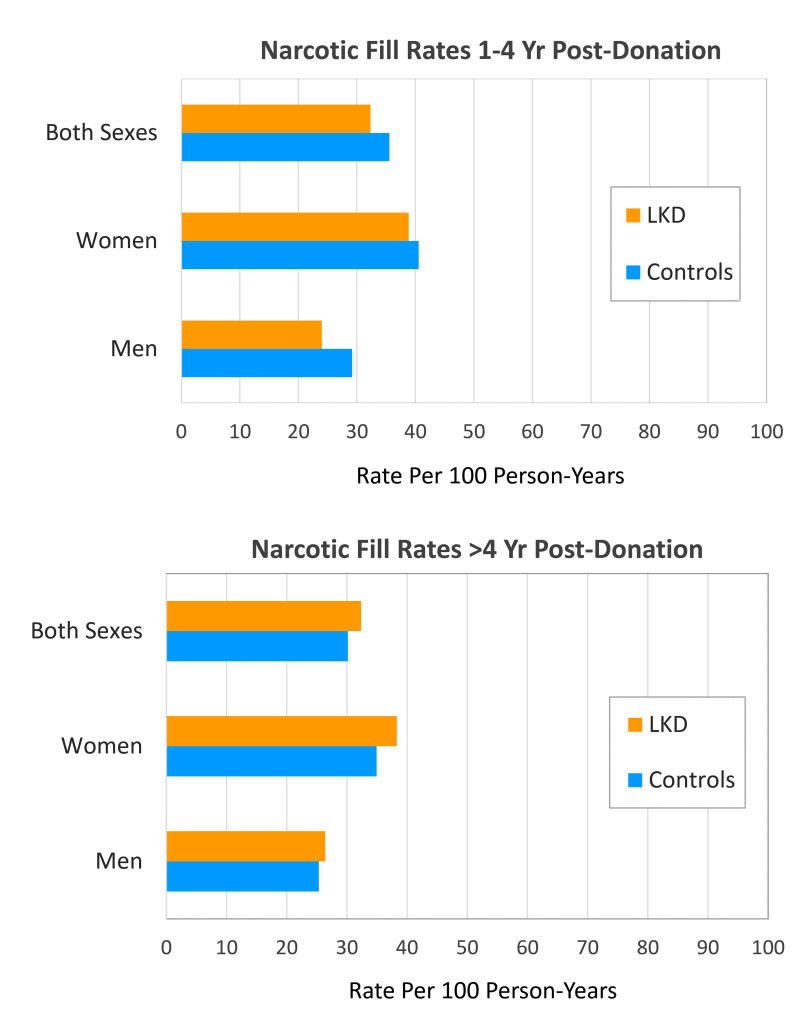
Overall, the rate of narcotic medication fills was 32.3 and 32.4 per 100 person-years in years 1-4 and >4 years after donation (Figure 1). After adjusting for age and race, female donors were approximately twice as likely as male donors to be prescribed a narcotic medication in years 1-4 post-donation (aHR 2.28; 95% confidence interval, CI, 1.86-2.79; p<0.0001) and >4 years post-donation (aHR 1.70; 95% CI 1.50-1.93; p<0.0001) (Table 1). Narcotic fills were less common in both periods among donors of “other” races compared with Caucasian donors, but did not differ significantly amongst African-American and Hispanic compared with Caucasian donors. Each year of donor age was associated with a 1% increase in the likelihood of narcotic fills after 4 years post-donation (aHR 1.01; 95% CI 1.00–1.01). Among the living kidney donors with available data to ascertain SES information, SES score was not significantly associated with narcotic use in the study periods. We also did not detect associations between surgical type (open vs. laparoscopic) or surgical complications with narcotic use post-donation in the smaller sub-samples with available information in the registry for these baseline factors (Table 1 and Supplemental Table 3).
Figure 1.
Prescription fill rates for narcotic medications in living kidney donors (LKD) and age- and sex-matched general non-donors, stratified by sex and follow-up period. *There were no significant differences in narcotic fill rates among LKD compared with matched non-donors.
Distribution of Donor Clinical Traits According to Post-donation Narcotic Use Level
Compared to living kidney donors with no narcotic use (of whom 54.9% were women), there were significantly higher proportions of women with first (70.6%; p<0.0001), second (63.2%; p<0.05), and third (68.4%; p<0.001) tertiles of narcotic use in the 1–4 years post-donation. Similarly, in the period >4 years post-donation, there were more women with second (62.7%; p<0.0001), and third (61.9%; p<0.0001) tertiles of prescription narcotic use compared to non-users (of which 49.2% were women). There were no significant differences in age, race, SES, surgical type, or reported surgical complications between non-users and those with any level of narcotic use (Table 2 and Supplemental Table 3).
Table 2. Distribution of clinical traits of living kidney donors according to post-donation narcotic use level (mg/day).
| Baseline characteristics All donors (n=4,650) | 1-4 years post-donation | >4 years post-donation | ||||||
|---|---|---|---|---|---|---|---|---|
| No Use | Tertile 1 (0.01-0.27) | Tertile 2 (0.28-1.03) | Tertile 3 (>1.03) | No Use | Tertile 1 (0.01-0.19) | Tertile 2 (0.20-0.71) | Tertile 3 (>0.72) | |
| Age at donation (years) | 38.1 | 37.9 | 37.8 | 37.3 | 36.8 | 36.4 | 38.4 | 36.5 |
| Female | 54.9% | 70.6%b | 63.2%c | 68.4%d | 49.2% | 53.5% | 62.7%b | 61.9%b |
| Race | ||||||||
| Caucasian | 73.9% | 75.9% | 77.8% | 77.7% | 76.4% | 79.2% | 81.1% | 80.6% |
| African-American | 13.2% | 15.3% | 13.5% | 12.4% | 13.3% | 12.7% | 11.9% | 12.9% |
| Hispanic | 9.1% | 7.7% | 8.2% | 8.2% | 7.9% | 6.7% | 6.2% | 5.2% |
| Other | 3.9% | 1.2% | 0.58% | 1.8% | 2.4% | 1.3% | 0.78% | 1.3% |
| SES (n=3,384) index | 48.1 | 48.3 | 48.2 | 47.4 | 47.6 | 48.6 | 48.5 | 47.2 |
| Surgical type (n=1,104) | ||||||||
| Laparoscopy | 60.4% | 60.6% | 62.8% | 53.1% | 49.0% | 44.4% | 45.6% | 34.9% |
Data presented as percentages except for age and SES which are presented as mean (standard deviation).
Morphine equivalent (ME) tertiles (mg/day) defined among those who filled narcotic prescriptions in the indicated period.
p<0.0001.
p<0.05-0.002.
p=0.001-0.0002.
Abbreviations: ME, morphine equivalent; SES, socio-economic status.
Comparison of Narcotic Use in Living Kidney Donors and General Non-donors
Narcotic fill rates were higher among female compared to male donors in both follow-up periods, but also varied by sex among matched non-donor controls. There were no statistically significant differences in the prescription fill rates for narcotic medications between living kidney donors and age- and sex-matched general non-donors overall or after stratification by sex (Figure 1). Specifically, in years 1–4 post-donation, narcotic fill rates per 100 person-years were as follows: all donors, 33.3; age- and sex- matched controls, 35.6; donor vs. control rate ratio 0.91 (95% CI 0.81–1.02); female donors, 38.8; age-matched female controls, 40.6; female donor vs. control rate ratio 0.96 (95% CI 0.83–1.11); male donors, 24.0; age-matched male controls, 29.2; male donor vs. control rate ratio 0.82 (95% CI 0.67–1.01). Beyond 4 years post-donation, narcotics fill rates per 100 person-years were as follows: all donors, 32.4; age- and sex-matched controls, 30.2; donor vs. control rate ratio 1.07 (95% CI 0.99–1.07); female donors, 38.3; age-matched female controls, 34.9; female donor vs. control rate ratio 1.10 (95% CI 0.98–1.22); male donors, 26.4; age-matched male controls, 25.3; male donor vs. control rate ratio 1.04 (95% CI 0.92–1.19).
In the matched-pairs comparisons, we also assessed MPRs, the fraction of days of captured insurance enrollment for which narcotic medications were prescribed. Overall, narcotic medication MPRs were lower in donors than in age- and sex-matched non-donors: 1.4% vs. 3.3% (p<0.001) in years 1–4 and 2.3% vs. 3.9% (p<0.05) in the period >4 years post-donation (Table 3). Among both donors and non-donors in both time periods, women had higher narcotic MPRs compared to men.
Table 3. Mean narcotic medication possession ratios (MPRs)a among living kidney donors compared to age- and sex-matched general non-donors, stratified by sex and follow-up period.
| Mean MPRs | ||||
|---|---|---|---|---|
| 1-4 years post-donation | >4 years post-donation | |||
| Donors | Non-donors | Donors | Non-donors | |
| Both sexes | 1.4% | 3.3%b | 2.3% | 3.9%c |
| Females | 1.7% | 4.3%c | 2.3% | 4.7%c |
| Males | 0.9% | 2.0%c | 2.2% | 3.0%c |
MPRs defined as [days of medication supplied over an observation window]/[days of observation] where the observation windows were defined as the period of captured insurance benefits for an individual.
p=0.001-0.0002.
p<0.05-0.002.
Abbreviations: MPR, medication possession ratio.
Patterns of types of narcotic medications prescribed to donors and general non-donors were similar (Supplemental Table 4), with the combination agent “hydrocodone and acetaminophen” representing approximately half of narcotic medications filled by donors and non-donors of both sexes in the earlier (48% to 61%) and later (43% to 52%) study periods. We also assessed primary medical diagnostic codes for healthcare services within 7 days prior to/concomitant with narcotic prescription fills in donors and matched general non-donors. Among women, spinal symptoms, joint or soft tissue complaints, and screening gynecological exams and mammograms represented the most common primary diagnoses for healthcare encounters prior to narcotic prescription fills among donors and non-donors (Table 4). Among female donors in the 1–4 year post-donation period, kidney donor status was the primary diagnosis preceding 1.9% of narcotic fills and urological conditions preceded 0.6% fills. Malignant neoplasm was the leading diagnosis preceding narcotic fills among non-donor women matched in the early period, and the 4th most common diagnosis among female non-donors matched in the later period; malignant neoplasm was not a leading diagnosis preceding narcotic use among female donors in the early period, but was the 15th most common primary diagnosis in the later period. Among men, spinal symptoms, joint or soft tissue complaints, and routine general medical exams were common diagnoses before narcotic fills in donors and non-donors (Supplemental Table 5). Among male donors in the 1–4 year post-donation period, kidney donor status was the primary diagnosis preceding 0.9% of narcotic fills. Similar to the pattern amongst women, malignant neoplasm preceded narcotic fills only amongst male donors in the period >4 years post-donation.
Table 4. Most common primary medical diagnoses within 7 days before prescription narcotic fills in female living kidney donors and general non-donors by follow-up period.
| 1-4 years post-donation | >4 years post-donation | ||||||
|---|---|---|---|---|---|---|---|
| Living kidney donors | General non-donors | Living kidney donors | General non-donors | ||||
| Diagnosis | Claims | Diagnosis | Claims | Diagnosis | Claims | Diagnosis | Claims |
| Spine | 7.9% | Malignant neoplasm | 11.1% | Spine | 9.5% | Spine | 8.0% |
| Screening gynecological exam or mammogram | 7.3% | Screening gynecological exam or mammogram | 4.9% | Joint or soft tissue | 8.0% | Screening gynecological exam or mammogram | 5.9% |
| Joint or soft tissue | 5.3% | Spine | 4.8% | Routine gynecological exam or mammogram | 7.5% | Joint or soft tissue | 4.5% |
| Gynecological | 5.2% | Joint or soft tissue | 3.3% | Gynecological | 5.0% | Malignant neoplasm | 3.4% |
| General symptoms | 4.8% | Infection | 3.3% | Gastric | 4.0% | Lipid disorder | 3.2% |
| Normal obstetrical follow-up | 2.7% | Gynecological | 3.1% | Respiratory | 3.0% | Gynecological | 3.2% |
| Routine medical follow-up | 2.6% | Gastric | 3.0% | Infection | 2.9% | Gastric | 3.1% |
| Gastric | 2.6% | Hypertension | 2.9% | Routine medical follow-up | 2.8% | Chest pain | 2.9% |
| Infection | 2.5% | Diabetes | 2.1% | Lipid disorder | 2.5% | Infection | 2.9% |
| Allergy | 1.9% | Respiratory | 2.1% | Chest pain | 2.3% | Hypertension | 2.6% |
| Chest pain | 1.9% | Normal obstetrical follow-up | 1.8% | General symptoms | 2.2% | Respiratory | 2.6% |
| Blood work | 1.9% | Routine medical follow-up | 1.7% | Psychiatric | 1.9% | Diabetes | 2.3% |
| Headache | 1.9% | Psychiatric | 1.7% | Hypertension | 1.8% | Routine medical follow-up | 2.2% |
| Kidney donor status | 1.9% | Chest pain | 1.7% | Allergy | 1.8% | Neuropathic | 2.2% |
| Neuropathic disorders | 1.5% | General symptoms | 1.6% | Malignant neoplasm | 1.8% | Urological or renal | 2.1% |
| Respiratory | 1.5% | Lipid disorders | 1.4% | Normal obstetrical follow-up | 1.8% | Thyroid | 2.0% |
| Lipid disorders | 1.4% | Thyroid | 1.0% | Breast disorders | 1.4% | Blood work | 1.8% |
| Psychiatric | 1.2% | Blood work | 1.0% | Neuropathic disorders | 1.4% | Psychiatric | 1.7% |
| Pregnancy complications | 0.6% | Pregnancy complications | 1.0% | Blood work | 1.3% | General symptoms | 1.5% |
| Urological | 0.6% | Headache | 0.9% | Dermatological | 1.2% | Headache | 1.1% |
Discussion
To date, limited information has been reported on the use of narcotic medications beyond the peri-operative period in living kidney donors. We examined a linkage of U.S. national transplant registry data for living kidney donors with pharmacy and medical claims from a private health insurer to examine patterns of prescription narcotic fills in the intermediate-term after living kidney donation. Based on this unique information source, we observed several key findings: 1) after age and race adjustment, women were approximately twice as likely as men to fill a narcotic prescription in years 1–4 and >4 years post-donation (with median end of follow-up 7.7 years). 2) Among the living kidney donors with available information, we did not detect correlations between narcotic medication use post-donation and SES, nephrectomy surgical technique, or post-operative complications. 3) Overall, narcotic medication fill rates were not significantly different in donors compared to age- and sex-matched general non-donors; however, donors had lower MPRs compared to non-donors in the same insurance plan, and absolute MPRs were approximately 2% or less in donors. Thus, while some prescription narcotic exposure was common, average use was not sustained. 4) The most common primary diagnoses for healthcare encounters prior to narcotic prescription fills among donors of both sexes (spinal symptoms, joint or soft tissue complaints, and screening or general medical exams represented) did not appear donation-related.
Post-operative pain following donor nephrectomy has been previously described using self-reported health-related quality of life questionnaires, such as the Short Form-36 Health Survey (SF-36) (16,18,47) or the Dartmouth COOP Functional Health Assessment Chart (48), focus groups, and in-person or telephone interviews (48). One observational cross-sectional study in the U.S. surveyed 2,455 living kidney donors from 1963 to 2005 who were at least 5 years from donation (mean follow-up time was 17 years) using the SF-36 questionnaire (16). In this study, 20% of donors had below average scores (where lower scores indicate worse health) for the SF-36 component on bodily pain compared to age- and sex-standardized scores of the general population. Limitations of self-reported pain include response and recall bias. Use of national administrative data may offer an alternative or complementary, objective method of assessing pain after donation. In the current study, we found that prescription narcotic use patterns were similar to or lower than that of age- and sex-matched general controls.
The gender differences in narcotic medication use amongst living kidney donors observed in the current study are comparable to previous reports of higher rates of prescription narcotic use in women compared to men in the general population (27,28). We also observed similar gender variation among non-donor general controls sampled in the current study. There are many potential explanations for these gender differences. As previously stated, women have been shown to experience higher risks of chronic medical and co-morbid mood disorders which may increase risks of chronic pain, more severe pain, and more disability due to pain (29,30,32,49). Using similar methodology, we previously reported that female donors were more than twice as likely as male donors to be diagnosed with depression after donation (aHR 2.41; 95% CI 1.89–3.06) (37). In the current study, psychiatric conditions, such as depression or anxiety, were among the top 20 most common primary diagnoses in the 7 days preceding narcotic prescription fills among female donors and non-donors, and also among male donors, although these conditions comprised <2% of the primary diagnoses preceding narcotic medication use.
Particular concern regarding narcotic use amongst women of reproductive age was highlighted in a recent CDC report showing that approximately 40% of Medicaid-enrolled women and almost one-third of privately insured women had filled an outpatient prescription for a narcotic medication (35). In addition to the increased risk of narcotic-associated death (50), overdose (24), and other neurological complications (51), women using narcotic medications also face increased risk of pregnancy-related complications such as birth defects (34). A recent study using commercial insurance beneficiaries in the U.S. found that between 2005 and 2011, 14% of women were dispensed a narcotic medication at some point during pregnancy (52), although use did appear to be decreasing slightly over time, from 15% in 2005 to 13% in 2011. In our study, normal obstetrical follow-up was the primary diagnosis within 7 days for 2.7% and 1.8% of narcotic fills among female donors in the 1–4 and >4 year periods, respectively, while pregnancy complications preceded <1% of narcotic fills among donors in the earlier period. We did not assess for pregnancy-related complications following narcotic medication use; however, a recent study by Garg et al. found that the absolute risks of maternal and fetal pregnancy complications were small in living kidney donors and matched general non-donors of similar baseline health (9).
Our study has strengths including the use of a novel linkage of large databases to investigate post-donation use of pharmaceutical agents. Our pharmacy claims database is able to capture details of dispensed prescriptions including dose, circumventing some of the limitations of self-reported medication use such as response or recall bias. However, there are also important limitations to our study. Due to the observational nature of our study design, we are able to describe associations but are not able to prove causality. Unmeasured factors, including patient behaviors, may affect the findings. We relied on administrative data from a private insurance plan, and thus, uninsured living kidney donors were not captured. Although electronic pharmacy claims and fill records have been found to be highly accurate records of physician prescribing, we were unable to account for illicit drug use or over-the-counter medication use (53). The degree of narcotic exposure may be underestimated if patients engaged in “pharmacy shopping” behaviors and filled narcotic prescriptions outside of their insurance benefits. Additionally, we lacked information on pre-donation narcotic use, although we suspect that such use was low given the rigorous medical and psychosocial screening inherent in living donor selection. Since claims data were available starting in May 2000, the 1-4 year analyses were limited to living kidney donors who had their nephrectomy performed in May 1996 or later. Importantly, the primary finding of approximately two-fold higher narcotic use among women compared to men was robust across assessment periods, and other patterns were similar in the earlier and later period analyses. Only subsets of living kidney donors had information from which we could examine potential associations between narcotic use and SES, nephrectomy surgical technique, and surgical complications. In those for whom we did have available information, there did not appear to be associations, although sample sizes may be insufficient to detect differences. Further, capture of early complications by the national transplant registry likely suffers from under-reporting (39). Lastly, our non-donor comparisons used general (rather than “healthy”) controls, as we lacked sufficient capture durations to allow for screening and selection of non-donors free of baseline co-morbidity. While general population comparisons do not address risk attributable to donation, as long as the type of comparison is explicit, general population experience can provide one relevant benchmark for framing post-donation outcomes (40,54).
Our study, therefore, provides insight into within-donor comparisons of narcotic use according to factors such as sex, as well as comparisons to the average general population as one commonly used benchmark (14,36,40,55). While we were unable to address the risk of narcotic medication use attributable to donor nephrectomy itself, we examined patterns of primary healthcare diagnoses in the 7 days preceding narcotic prescription fills, and found that the majority of healthcare visits were related to diagnoses such as spinal, joint, and soft tissue complaints. Reassuringly, these appear unlikely to be related to the nephrectomy procedure itself. Kidney donor status and urological conditions were among the 20 most common primary diagnoses preceding prescription narcotics fills among women in the early period, but together preceded <3% of fills. Importantly, however, assessment of primary diagnoses for medical encounters does not exclude the possibility that narcotics were prescribed for other conditions not coded at an encounter, such as chronic pain due to nephrectomy. Better understanding of the outcomes of donors with either past or current chronic pain, as assessed by prescription narcotic fills and other metrics is needed to improve donor management and informed consent processes. Attention to possible risks of pregnancy-related complications among female donors of childbearing age that are prescribed narcotic medications after donation warrants particular attention.
In conclusion, we found that there are gender differences in the use of narcotic medications in living kidney donors, with women having approximately twice the exposure as men. Overall, the rate of narcotic medication fills was 32.3 and 32.4 per 100 person-years in periods 1–4 and >4 years after donation, and rates were not significantly different in donors compared to age- and sex-matched general non-donors. Proportions of days covered by narcotic medications (MPRs) were low among donors, at approximately 2% or less in both periods, and MPRs were lower among donors compared to non-donors in the same insurance plan. Integration of national transplant registry and administrative claims data can advance understanding of the breadth of pharmaceutical care required after living kidney donation. Further research is needed to define relationships of prescription narcotic use in this unique patient population with long-term medical and psychosocial outcomes, including possible outcome variation by sex and other demographic traits.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK096008 and K24-DK101828. NNL was supported by the Clinical Investigator Program at Western University and by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) postdoctoral fellowship award. AXG was supported by the Dr. Adam Linton Chair in Kidney Health Analytics. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Footnotes
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
Conflict of Interest Statement: The authors have no disclosures relevant to the content of this article.
References
- 1.Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009 May;75(10):1088–98. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 2.Garg AX, Meirambayeva A, Huang A, Kim J, Prasad GVR, Knoll G, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012 Jan;344:e1203. doi: 10.1136/bmj.e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam N, Huang A, Feldman LS, Gill JS, Karpinski M, Kim J, et al. Acute dialysis risk in living kidney donors. Nephrol Dial Transplant. 2012 Aug;27(8):3291–5. doi: 10.1093/ndt/gfr802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg AX, Pouget J, Young A, Huang A, Boudville N, Hodsman A, et al. Fracture risk in living kidney donors: a matched cohort study. Am J Kidney Dis. 2012 Jun;59(6):770–6. doi: 10.1053/j.ajkd.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Thomas SM, Lam NN, Welk BK, Nguan C, Huang A, Nash DM, et al. Risk of kidney stones with surgical intervention in living kidney donors. Am J Transplant. 2013 Nov;13(11):2935–44. doi: 10.1111/ajt.12446. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SM, Lam NN, Huang A, Nash DM, Prasad GV, Knoll GA, et al. Risk of serious gastrointestinal bleeding in living kidney donors. Clin Transplant. 2014 May;28(5):530–9. doi: 10.1111/ctr.12344. [DOI] [PubMed] [Google Scholar]
- 7.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014 Feb 12;311(6):579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Oyen O, et al. Long-term risks for kidney donors. Kidney Int. 2013 Nov 27;86(1):162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 9.Garg AX, Nevis IF, McArthur E, Sontrop JM, Koval JJ, Lam NN, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015 Jan 8;372(2):124–33. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam NN, McArthur E, Kim SJ, Prasad GVR, Lentine KL, Reese PP, et al. Gout after living kidney donation: A matched cohort study. Am J Kidney Dis. 2015 Mar 25; doi: 10.1053/j.ajkd.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Clemens K, Boudville N, Dew MA, Geddes C, Gill JS, Jassal V, et al. The long-term quality of life of living kidney donors: a multicenter cohort study. Am J Transplant. 2011 Mar;11(3):463–9. doi: 10.1111/j.1600-6143.2010.03424.x. [DOI] [PubMed] [Google Scholar]
- 12.Clemens KK, Thiessen-Philbrook H, Parikh CR, Yang RC, Karley ML, Boudville N, et al. Psychosocial health of living kidney donors: a systematic review. Am J Transplant. 2006 Dec;6(12):2965–77. doi: 10.1111/j.1600-6143.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- 13.Mjøen G, Stavem K, Westlie L, Midtvedt K, Fauchald P, Norby G, et al. Quality of life in kidney donors. Am J Transplant. 2011 Jun;11(6):1315–9. doi: 10.1111/j.1600-6143.2011.03517.x. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009 Jan 29;360(5):459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroencke S, Fischer L, Nashan B, Herich L, Schulz KH. A prospective study on living related kidney donors' quality of life in the first year: choosing appropriate reference data. Clin Transplant. 2012;26(4):E418–27. doi: 10.1111/j.1399-0012.2012.01691.x. [DOI] [PubMed] [Google Scholar]
- 16.Gross CR, Messersmith EE, Hong BA, Jowsey SG, Jacobs C, Gillespie BW, et al. Health-related quality of life in kidney donors from the last five decades: results from the RELIVE study. Am J Transplant. 2013 Nov;13(11):2924–34. doi: 10.1111/ajt.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen MH, Mathisen L, Oyen O, Edwin B, Digernes R, Kvarstein G, et al. Postoperative pain and convalescence in living kidney donors-laparoscopic versus open donor nephrectomy: a randomized study. Am J Transplant. 2006 Jul;6(6):1438–43. doi: 10.1111/j.1600-6143.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 18.Andersen MH, Mathisen L, Veenstra M, Oyen O, Edwin B, Digernes R, et al. Quality of life after randomization to laparoscopic versus open living donor nephrectomy: long-term follow-up. Transplantation. 2007 Jul 15;84(1):64–9. doi: 10.1097/01.tp.0000268071.63977.42. [DOI] [PubMed] [Google Scholar]
- 19.Owen M, Lorgelly P, Serpell M. Chronic pain following donor nephrectomy--a study of the incidence, nature and impact of chronic post-nephrectomy pain. Eur J Pain. 2010 Aug;14(7):732–4. doi: 10.1016/j.ejpain.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Mathuram Thiyagarajan U, Bagul A, Nicholson ML. Pain management in laparoscopic donor nephrectomy: a review. Pain Res Treat. 2012 Jan;2012:201852. doi: 10.1155/2012/201852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manchikanti L, Helm S, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Physician. 2012 Jul;15(3 Suppl):ES9–38. [PubMed] [Google Scholar]
- 22.Betses M, Brennan T. Abusive prescribing of controlled substances--a pharmacy view. N Engl J Med. 2013 Sep 12;369(11):989–91. doi: 10.1056/NEJMp1308222. [DOI] [PubMed] [Google Scholar]
- 23.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004 Dec;112(3):372–80. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Bohnert ASB, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011 Apr 6;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 25.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med. 2012 Sep;13(9):1162–73. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 26.Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Vander Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015 May 18;30(5):597–604. doi: 10.1007/s11606-014-3143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010 Dec;100(12):2541–7. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008 Sep 15;138(3):507–13. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009 May;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijnhoven HAH, de Vet HCW, Picavet HSJ. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006 Oct;22(8):717–24. doi: 10.1097/01.ajp.0000210912.95664.53. [DOI] [PubMed] [Google Scholar]
- 31.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007 Nov;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs D, Krebs E, Bair M, Damush T, Wu J, Sutherland J, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010 Feb;11(2):232–9. doi: 10.1111/j.1526-4637.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 33.Manubay J, Davidson J, Vosburg S, Jones J, Comer S, Sullivan M. Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J Addict Med. 2015;9(1):46–52. doi: 10.1097/ADM.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broussard CS, Rasmussen SA, Reefhuis J, Friedman JM, Jann MW, Riehle-Colarusso T, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011 Apr;204(4):314.e1–11. doi: 10.1016/j.ajog.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Ailes EC, Dawson AL, Lind JN, Gilboa SM, Frey MT, Broussard CS, et al. Opioid prescription claims among women of reproductive age - United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2015 Jan 23;64(2):37–41. [PMC free article] [PubMed] [Google Scholar]
- 36.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010 Aug 19;363(8):724–32. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lentine KL, Schnitzler MA, Xiao H, Axelrod D, Davis CL, McCabe M, et al. Depression diagnoses after living kidney donation: linking U.S. Registry data and administrative claims. Transplantation. 2012 Jul 15;94(1):77–83. doi: 10.1097/TP.0b013e318253f1bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lentine KL, Vijayan A, Xiao H, Schnitzler MA, Davis CL, Garg AX, et al. Cancer diagnoses after living kidney donation: linking U.S. Registry data and administrative claims. Transplantation. 2012 Jul 27;94(2):139–44. doi: 10.1097/TP.0b013e318254757d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lentine KL, Segev DL. Better understanding live donor risk through big data. Clin J Am Soc Nephrol. 2013 Oct;8(10):1645–7. doi: 10.2215/CJN.08530813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lentine KL, Schnitzler MA, Garg AX, Xiao H, Axelrod D, Tuttle-Newhall JE, et al. Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. Am J Nephrol. 2014 Jan;40(2):174–83. doi: 10.1159/000365157. [DOI] [PubMed] [Google Scholar]
- 41.Lentine KL, Schnitzler MA, Xiao H, Axelrod D, Garg AX, Tuttle-Newhall JE, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014 Feb 15;97(3):316–24. doi: 10.1097/01.TP.0000436731.23554.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lentine KL, Schnitzler MA, Garg AX, Xiao H, Axelrod D, Tuttle-Newhall JE, et al. Race, relationship and renal diagnoses after living kidney donation. Transplantation. 2015 doi: 10.1097/TP.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.2004 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994-2003. Department of Health and Human Services, Health Resources and Services Administration, Healthcare; [Google Scholar]
- 44.Agency for Healthcare Research and Quality. Chapter 3: Creation of New Race -Ethnicity Codes and SES Indicators for Medicare Beneficiaries - Chapter 3 [Internet] [cited 2015 Mar 31];2008 Available from: http://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators3.html.
- 45.Lentine KL, Yuan H, Tuttle-Newhall JE, Xiao H, Chawa V, Axelrod D, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015 Jan 15;99(1):187–96. doi: 10.1097/TP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 46.Lentine KL, Lam NN, Xiao H, Tuttle-Newhall JE, Axelrod D, Brennan DC, et al. Associations of pre-transplant prescription narcotic use with clinical complications after kidney transplantation. Am J Nephrol. 2015 Mar 27;41(2):165–76. doi: 10.1159/000377685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jowsey SG, Jacobs C, Gross CR, Hong BA, Messersmith EE, Gillespie BW, et al. Emotional well-being of living kidney donors: findings from the RELIVE Study. Am J Transplant. 2014 Nov;14(11):2535–44. doi: 10.1111/ajt.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lennerling A, Forsberg A. Donors self-reported experiences of live kidney donation--a prospective study. J Ren Care. 2012 Dec;38(4):207–12. doi: 10.1111/j.1755-6686.2012.00320.x. [DOI] [PubMed] [Google Scholar]
- 49.Edlund MJ, Austen MA, Sullivan MD, Martin BC, Williams JS, Fortney JC, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014 Nov;155(11):2337–43. doi: 10.1016/j.pain.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowell D, Kunins HV, Farley TA. Opioid analgesics--risky drugs, not risky patients. JAMA. 2013 Jun 5;309(21):2219–20. doi: 10.1001/jama.2013.5794. [DOI] [PubMed] [Google Scholar]
- 51.Reid MC, Henderson CR, Papaleontiou M, Amanfo L, Olkhovskaya Y, Moore AA, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010 Jul;11(7):1063–71. doi: 10.1111/j.1526-4637.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bateman BT, Hernandez-Diaz S, Rathmell JP, Seeger JD, Doherty M, Fischer MA, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014 May;120(5):1216–24. doi: 10.1097/ALN.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003 Jan;10(2):67–71. [PubMed] [Google Scholar]
- 54.Lam NN, Lentine KL, Levey AS, Kasiske BL, Garg AX. Long-term medical risks to the living kidney donor. Nat Rev Nephrol. 2015 May 5; doi: 10.1038/nrneph.2015.58. [DOI] [PubMed] [Google Scholar]
- 55.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tydén G, Groth CG. Kidney donors live longer. Transplantation. 1997 Oct 15;64(7):976–8. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.